Summary
Biotin synthetic pathways are readily separated into two stages, synthesis of the seven carbon α, ω-dicarboxylic acid pimelate moiety and assembly of the fused heterocyclic rings. The biotin pathway genes responsible for pimelate moiety synthesis vary widely among bacteria whereas the ring synthesis genes are highly conserved. Bacillus subtilis seems to have redundant genes, bioI and bioW, for generation of the pimelate intermediate. Largely consistent with previous genetic studies we found that deletion of bioW caused a biotin auxotrophic phenotype whereas deletion of bioI did not. BioW is a pimeloyl-CoA synthetase that converts pimelic acid to pimeloyl-CoA. The essentiality of BioW for biotin synthesis indicates that the free form of pimelic acid is an intermediate in biotin synthesis although this is not the case in E. coli. Since the origin of pimelic acid in Bacillus subtilis is unknown, we carried out 13C-NMR studies to decipher the pathway for its generation. Our data provided evidence for the role of free pimelate in biotin synthesis and the involvement of fatty acid synthesis in pimelate production. Cerulenin, an inhibitor of the key fatty acid elongation enzyme, FabF, markedly decreased biotin production by B. subtilis resting cells whereas a strain having a cerulenin-resistant FabF mutant produced more biotin. In addition, supplementation with pimelic acid fully restored biotin production in cerulenin-treated cells. These results indicate that pimelic acid originating from fatty acid synthesis pathway is a bona fide precursor of biotin in B. subtilis.
Graphical abstract
Abbreviated Summary
Free pimelic acid is a key intermediate of biotin synthesis in Bacillus subtilis. Labeling and inhibitor studies show that pimelic acid is made by the fatty acid synthetic pathway.
Introduction
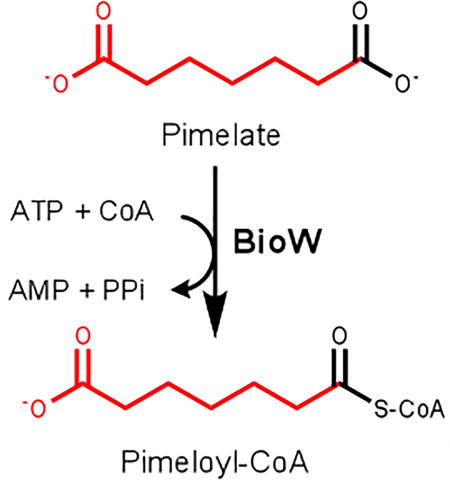
Biotin is a cofactor required for function of key metabolic enzymes that carry out carboxylation and decarboxylation reactions in fatty acid synthesis, amino acid metabolism and gluconeogenesis (Tong, 2013). Biotin consists of two fused rings, an ureido moiety and a sulfur-containing ring with an attached valeric acid side-chain (Fig. 1). The side chain is attached to a conserved lysine residue of biotin dependent enzyme subunit that enables to act like a “swinging arm” to perform carboxylation reactions (Fig 1). Since its discovery in 1901 as a growth factor for yeast (Wildiers, 1901), biotin biosynthetic pathways with diverse sets of genes have been identified in different bacteria, archaea, fungi and plants (Alban, 2011, Rodionov, 2002). However the only fully understood biotin biosynthesis pathway is that of Escherichia coli (Lin and Cronan, 2011). Biotin synthetic pathways are readily separated into two stages. Four well-conserved genes, bioF, bioA, bioD and bioB, encode the enzymes involved in the second stage of biotin biosynthesis in which the fused rings are assembled. These enzymes have been very well characterized (Lin and Cronan, 2011). In contrast the initial stage of biotin synthesis pathway varies widely depending on the enzymes responsible for synthesis of a thioester of pimelic acid, a seven carbon α, ω-dicarboxylic acid (IUPAC name, heptanedioic acid (Fig. 1).
Fig. 1. Biotin synthesis in B. subtilis.
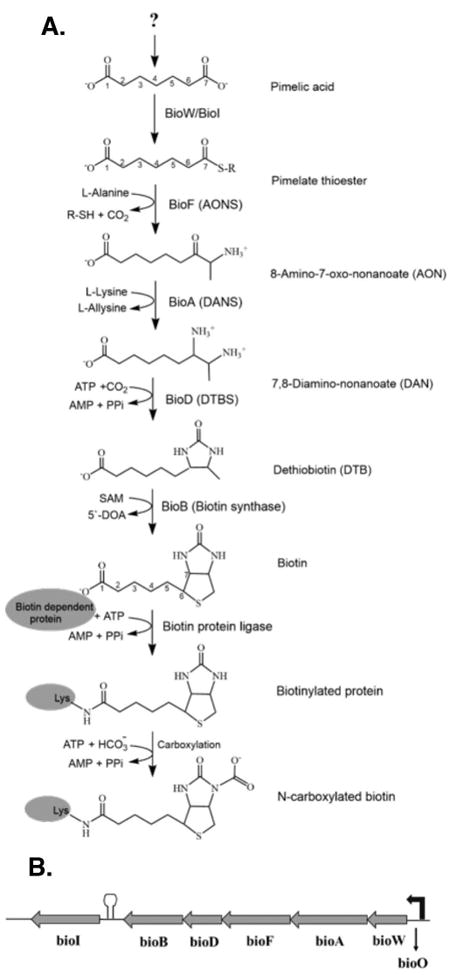
A. The question mark indicates the unknown source of pimelic acid. BioW and BioI have both been predicted to generate the pimelate thioester intermediate in the initial stage of the pathway. The R group in the pimelate thioester intermediate indicates a carrier molecule, expensive either coenzyme A (CoA) or acyl carrier protein (ACP). The second stage of the pathway involves BioF, BioA, BioD and BioB for the completion of the fused heterocyclic rings of biotin. The seven carbons of pimelate make up most of the valeric acid side chain of biotin. Biotin protein ligase attaches biotin to a conserved lysine residue of a biotin dependent protein subunit. Biotin acts a cofactor after being carboxylated by the carboxylase subunit of a biotin- dependent enzyme. The intermediates, 8-amino-7-oxo-nonanoate and 7, 8-diaminononanate, are often called by their informal names which are 7-keto-8-aminopelargomic acid (KAPA) and 7, 8-diaminopelargomic acid (DAPA), respectively.
B. The B. subtilis bio operon consists of bioW, bioA, bioF, bioD, bioB and bioI. The arrow at the right indicates the promoter while the hairpin structure denotes the terminator upstream of bioI (Perkins et al., 1996). The bio genes are transcribed from a promoter that is regulated in a biotin-dependent manner by the BirA, biotin protein ligase/repressor which binds to bioO, the operator sequence located upstream of bioW (Bower et al., 1996, Henke and Cronan, 2014).
The role of this unusual molecule as a biotin precursor was discovered many years ago as a compound that bypassed the biotin requirement for growth of Corynebacterium diphtheriae (Mueller, 1937a, Mueller, 1937c, Mueller, 1937b). Later work showed that pimelic acid supplementation bypassed the biotin requirements of a subset of naturally biotin-requiring bacteria and fungi. Conversion of 14C-pimelic acid into biotin and synthesis of biotin vitamers from pimelic acid in cell-free extracts of various bacteria indicated that pimelate was a direct precursor of biotin (Izumi et al., 1972) and was probably incorporated intact (Eisenberg, 1962). Seminal in vitro studies by Eisenberg and coworkers (Eisenberg, 1973, Eisenberg and Star, 1968) demonstrated that E. coli extracts could condense pimeloyl-CoA with alanine to give the first intermediate in biotin ring formation and that BioF catalyzed this reaction. However, the source of pimelate and the identity of its thioester derivative in E. coli remained unknown (the high pimeloyl-CoA concentrations required suggested it may not be the physiological thioester). Recent work from this laboratory showed that synthesis of the acyl carrier protein (ACP) thioester of pimelate (pimeloyl-ACP) proceeds by a modified fatty acid synthesis pathway (Agarwal et al., 2012, Lin and Cronan, 2012, Lin et al., 2010). Genetic and cross-feeding studies had placed the E. coli bioC and bioH biotin auxotrophs at the beginning of the pathway and hence the encoded proteins were thought to somehow account for pimelate synthesis. The BioH sequence suggested it could be an acyltransferase, an esterase or a thioesterase and these activities could be incorporated into a variety of hypothetical pimelate synthetic pathways. The puzzle was BioC which appeared to be an S-adenosyl-L-methionine-dependent methyl transferase. However, isotopic labelling studies had shown that none of the biotin carbon atoms were derived from methionine (Ifuku et al., 1994, Sanyal et al., 1994) and that the pimelate moiety was synthesized by head-to-tail condensations of acetate units (as in fatty acid synthesis) with one carboxyl coming from CO2 and the other from acetate. These data ruled out free pimelate (a symmetrical molecule) as an intermediate in E. coli. It was found that the role of BioC is to facilitate biotin synthesis by deceiving fatty acid synthesis into making the pimelate moiety. BioC methylates the free carboxyl of a small portion of the key fatty acid precursor, malonyl-ACP (Lin and Cronan, 2012). Methylation eliminates the charge of the free carboxyl of malonyl-ACP and the resulting malonyl-ACP methyl ester is able to access the hydrophobic active site usually occupied by an acetyl-thioester. Two cycles of the fatty acid synthesis pathway results in pimeloyl-ACP methyl ester (Lin et al., 2010). BioH then cleaves the methyl ester which prevents further elongation (Agarwal et al., 2012). Hence, the methyl ester used for pimelate synthesis and the protective groups used in organic synthesis share the property that both are removed upon completion of the synthesis. However, they are used oppositely, in that the methyl group facilitates desired reactions whereas protective groups prevent undesired reactions. Pimeloyl-ACP then reacts with alanine in the BioF reaction to give 8-amino-7-oxononanoate (Fig. 1). Although many bacteria use the BioC-BioH pathway (or variations in which other esterases replace BioH), there are biotin prototrophic bacteria that lack BioC-encoding genes and a esterase known to cleave pimeloyl-ACP methyl ester. In this regard the most puzzling of these are the Bacilli. The bio operons of B. subtilis and closely related bacilli (e.g., B. licheniformis, B. amyloliquefaciens, etc.) lack bioC and bioH and instead have bioW and bioI. In contrast B. cereus and closely related bacteria (e.g., B. anthracis, B. thuringiensis, etc.) encode BioC and BioH proteins within their biotin operons that are quite similar to and can functionally replace the E. coli proteins.
The B. subtilis bioW and bioI genes are, respectively, the first and last genes of the biotin operon (Bower et al., 1996) (Fig. 1B). The intervening genes, bioAFDB, are readily identified due to the strong conservation of their protein sequences across diverse bacteria, plants and fungi. B. subtilis BioW has long been known to be a pimeloyl-CoA synthetase (Bower et al., 1996, Ploux et al., 1992) and this atypical acyl-CoA synthetase has been studied in detail in recent enzyme mechanism and x-ray crystal analyses (Manandhar and Cronan, 2013, Estrada et al., 2017). BioI is characterized as a cytochrome P450 due to its sequence homology and characteristic P450 spectral properties (Green et al., 2001, Stok and De Voss, 2000). BioI has been shown to cleave carbon-carbon bonds of free fatty acids to mixtures of dicarboxylic acids that include pimelate (Stok and De Voss, 2000). BioI also been reported to cleave the carbon-carbon bonds of acyl-ACPs that copurified with preparations of BioI expressed in E. coli (Stok and De Voss, 2000) to give pimeloyl-ACP, albeit at sub-stoichiometric levels. Crystal structures of B. subtilis BioI in a complex with acyl-ACPs have been solved that show the catalytic heme located above carbons 7 and 8 of the acyl chain poised to cleave the C7-C8 bond to give pimeloyl-ACP (Cryle and Schlichting, 2008). The prior work plus the elegant crystal structures argued strongly that BioI provided the pimelate moiety required for B. subtilis biotin synthesis. Hence, B. subtilis seemed to have redundant systems operating in the first stage of the pathway. However two considerations weakened this argument. First early genetic studies concluded that BioI function was not strictly required for biotin synthesis (Bower et al., 1996) and bacteria and archaea exist that encode BioW but lack genes that encode a recognizable BioI.
In the studies presented here we found that disruption of bioI failed to result in biotin auxotrophy whereas disruption of bioW resulted in a growth requirement for biotin. The auxotrophy of bioW mutant strains plus the pimeloyl-CoA synthetase activity of BioW argued that free pimelic acid must be a biotin precursor. We report isotopic labelling studies that demonstrate this to be the case and that the pimelate moiety is synthesized by the head-to-tail condensations of acetate units characteristic of fatty acid synthesis.
Results
Expression of either B. subtilis BioW or BioI bypasses the biotin auxotrophy of an E. coli ΔbioC ΔbioH strain
The B. subtilis bioW and bioI coding sequences were inserted into the araBAD promoter vector pBAD322 (Cronan, 2006) and the plasmid was introduced into strain STL25, a ΔbioC ΔbioH derivative of strain MG1655. Complementation of the E. coli ΔbioC bioH strain was tested in biotin-free minimal medium supplemented with pimelic acid. The biotin auxotrophy of the mutant was successfully rescued by the induction of bioW gene expression with arabinose but only in the presence of pimelic acid (Fig. 2A) as previously reported (Lin et al., 2010, Bower et al., 1996). In contrast, basal level expression of bioI without arabinose induction was able to bypass the ΔbioC ΔbioH mutations even in the absence of pimelic acid, consistent with results obtained by Bower et al. (Bower et al., 1996) (Fig. 2B).
Fig. 2. Complementation of the biotin requirement of the E. coli ΔbioC bioH strain STL25 with B. subtilis bioW or bioI.
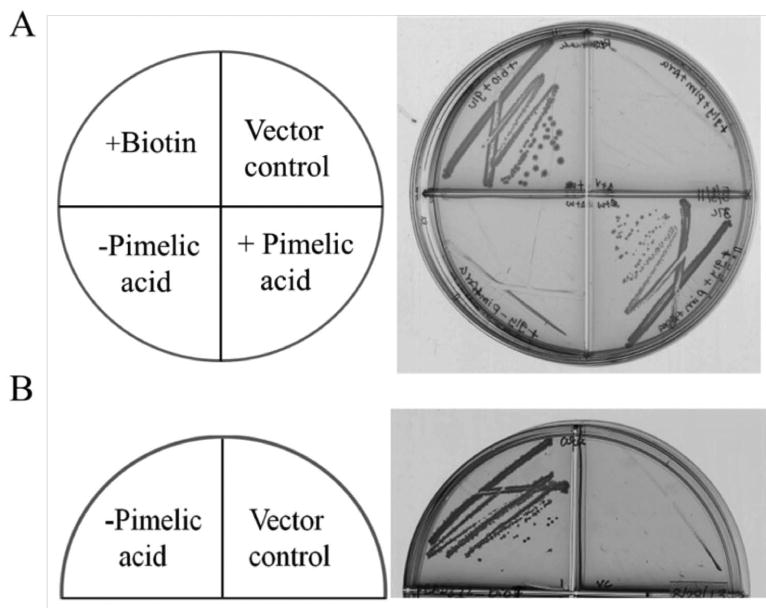
A. B. subtilis bioW allowed growth of the E. coli ΔbioC bioH strain in biotin-free minimal media only upon supplementation with pimelic acid. Expression of bioW was induced from the PBAD promoter of vector pBAD322 by arabinose. Vector control indicates the empty vector induced with arabinose. Pimelic acid (0.1 mM) was used for growth supplementation.
B. B. subtilis bioI allowed growth of the E. coli ΔbioC bioH strain without supplementation with either pimelic acid or arabinose. B. subtilis bioI was inserted into vector pBAD322.
bioW is essential for biotin synthesis whereas bioI is dispensable
B. subtilis seems to contain two genes that serve the same purpose, generation of the pimelate thioester intermediate. In order to determine the importance of bioW and bioI, we constructed chromosomal gene disruptions. Vector pMUTIN4 was used to inactivate the chromosomal bioW gene by single-crossover homologous recombination and has an IPTG-inducible promoter (Pspac) positioned to drive expression of the downstream genes (Vagner et al., 1998). Disruption of bioW caused biotin auxotrophy in biotin-free media (Fig. 3A). Growth was rescued by expression of bioW from an IPTG-inducible promoter at an ectopic chromosomal site (the amyE locus) (Fig. 3A) demonstrating that the biotin auxotrophy is due to bioW inactivation and is not a polarity effect. Also, supplementation with dethiobiotin or 8-amino-7-oxononanoate rescued growth of the ΔbioW strain indicating that induction of the Pspac promoter prevented polar effects on expression of the downstream genes (data not shown).
Fig 3. Growth phenotypes of B. subtilis bioW and bioI mutant strains.
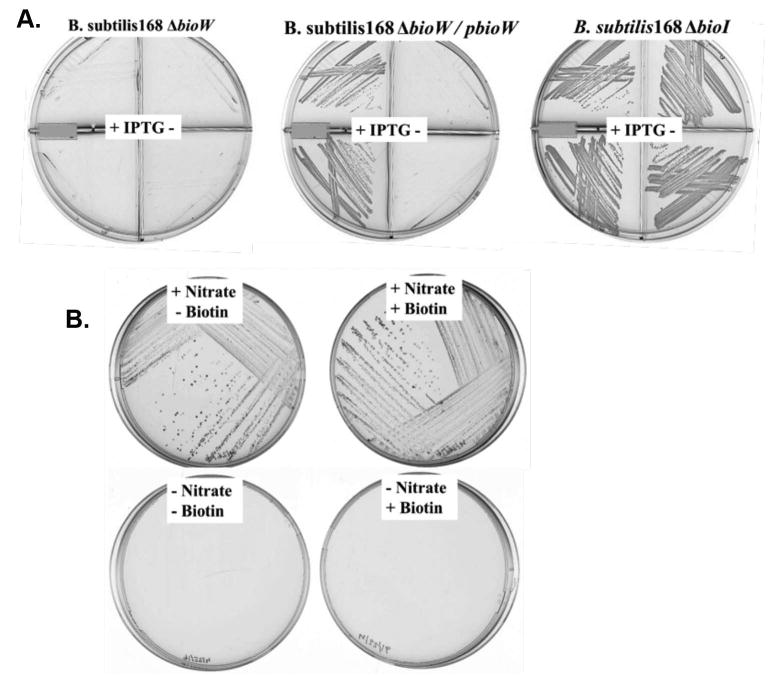
A. Phenotypes of B. subtilis bioW and bioI deletion mutants. Deletion of the chromosomal bioW through single crossover recombination by integration of recombinant vector pMUTIN4 blocked growth in biotin-free minimal media. Two separate colonies were streaked into upper and lower sectors of the plates sectioned by plastic walls. IPTG (1 mM) was used (left sectors, note the plus signs) to induce the expression of downstream genes whereas no IPTG was present in the right hand sectors (minus signs). Expression of bioW from the Phyper-spank promoter of vector pDR111 inserted at an ectopic site (the amyE locus) restored growth only when promoter activity was induced with IPTG. No change in growth was observed when the chromosomal bioI gene was partially deleted and disrupted by insertion of the kanamycin cassette of plasmid pDG780. The bioW strain was MM43 whereas the ΔbioI strain was MM57. The derivative of MM43 with an ectopic bioW was strain MM59 (Perkins et al., 1996).
B. Anaerobic growth of B. subtilis. B. subtilis 168 was streaked and incubated on biotin-free minimal medium plates with nitrate as the terminal electron acceptor using the BD Gaspak EZ Anaerobe Pouch System. Growth was dependent on nitrate but not biotin indicating that B. subtilis synthesizes biotin anaerobically and therefore the oxygen-dependent cytochrome P450 enzyme, BioI is not required. The nitrate-dependent growth indicates that the conditions were anaerobic.
In contrast, disruption of bioI failed to cause biotin auxotrophy. The strain grew as well in biotin-free minimal media as the wild type strain (Figure 3A). It should be noted that the internal fragment deleted from bioI encodes several residues that position the acyl chain for attack by heme moiety (Cryle Schlichting, 2008). In the first studies of the B. subtilis bio operon insertion of an antibiotic resistance cassette into a site early in the BioI coding sequence resulted in strains that grew (albeit very slowly) in biotin-free minimal media. Moreover, a ΔbioW derivative of strain BI304 (Perkins et al., 1996, Bower, 2000) encoding a modified bio operon in which the terminator upstream of bioI was replaced with a constitutive strong promoter resulting in overexpression of BioI (Perkins et al., 1996) failed to grow in biotin-free media. However upon expression of a bioW gene integrated at the ectopic amyE locus this strain grew well (data not shown).
To provide a further test of the nonessential nature of BioI in B. subtilis biotin synthesis we tested the ability of the wild type strain 168 to grow anaerobically with nitrate as electron acceptor (Nakano and Zuber, 1998). The cytochrome P450 reaction requires molecular oxygen (Makris et al., 2002) and thus if BioI played an essential role in biotin synthesis growth should be blocked under these conditions. Two different anaerobic systems were tested, the BD Gaspak EZ Anaerobe Pouch System and a Coy anaerobic chamber containing 10% H2 - 90% N2. Growth proceeded in both systems regardless of the presence of biotin demonstrating that oxygen is not required for biotin synthesis (Figure 3B). Nitrate was required for growth confirming that the culture conditions were anaerobic.
Free pimelic acid is a bona fide precursor of biotin synthesis in B. subtilis
The origin of pimelic acid in bacteria lacking the E. coli pathway remains a mystery. We carried out 13C-NMR experiments to test for the presence of free pimelic acid in vivo in B. subtilis and also to begin to deduce a pimelate synthesis pathway. These experiments were analogous to previous studies in E. coli by Sanyal, Ifuku and coworkers (Ifuku et al., 1994, Sanyal et al., 1994). In both studies, E. coli was grown in the presence of [1-13C]acetate or [2-13C]acetate and the labelling patterns of biotin extracted from the media was analyzed by 13C nuclear magnetic resonance (NMR). The authors ruled out free pimelic acid as a biotin precursor in E. coli because the labelling patterns of biotin (and dethiobiotin) demonstrated that one of the pimelate carboxyl groups was fixed during synthesis possibly by a thioester bond. (If free pimelate was an intermediate, the biotin carbon atoms derived from the pimelate carboxyl groups would show the same labeling patterns due to the symmetry of pimelate.) Given that BioW is essential for biotin synthesis and activates free pimelate we expected a different result. Free pimelic acid is a rotationally symmetric molecule and thus the carbon atoms derived from the carboxyl groups would have the same labelling pattern.
In this work we used B. subtilis strain BI274 which has an engineered bio operon driven by a phage SP01 promoter resulting in overproduction of biotin up to 1-2 mg/L of growth medium (Bower, 2000). B. subtilis cannot grow on acetate as sole carbon source because of the lack of a glyoxylate cycle and thus had to be grown on a more complex carbon source. In order to prevent dilution of the supplemented 13C-acetate by unlabelled acetate produced from the glucose provided as carbon source, we used a ΔlipM derivative of strain BI274 called strain MM194. The loss of LipM blocks lipoic acid synthesis which inactivates the essential lipoic acid-requiring enzymes, pyruvate dehydrogenase and branched-chain dehydrogenase (Martin et al., 2011). However, loss of these enzymes can be bypassed by supplementation with acetate and branched chain fatty acid precursors which endogenous CoA ligases convert to their CoA esters (Martin et al., 2011).
Strain MM194 was grown with either [1-13C]acetate or [2-13C]acetate as the sole acetate source to allow maximum incorporation of the 13C label into biotin (the branched chain precursors were also added) (Fig. 4A). We then attempted to use the methods developed for E. coli to extract and purify biotin from the culture medium in sufficient amounts and purity to perform 13C nuclear magnetic resonance (NMR) analyses. However, although the recovery of biotin was good (Fig. 4B), the resulting samples were much too impure for NMR. Several additional purification steps were unsuccessful in ridding the samples of intensely colored contaminants. Finally we found that a mutant (F43A) derivative of shwanavidin, a dimeric avidin from Shewanella denitrificans (Meir et al., 2012) provided the needed purification. In native shwanavidin phenylalanine-43 acts to shield bound biotin from solvent and its substitution with alanine results in a precipitous drop in the affinity of the protein for biotin to 10-8 M (Meir et al., 2012). Expression in E. coli of a synthetic gene encoding the mutant shwanavidin gave abundant protein that was refolded and immobilized on agarose. The impure biotin samples were bound to the shwanavidin F43A column which was washed with three column volumes of 100 mM Na2HPO4 containing 150 mM NaCl (pH 7.2) to remove the colored contaminants and biotin was eluted with water heated to 70°C. The elution conditions were those used to release biotinylated nucleic acids from streptavidin (Holmberg et al., 2005). Shwanavidin like other avidins is very heat tolerant and thus the column could be reused multiple times.
Fig. 4. Acetate supplementation is required for growth and biotin production by strain MM194.
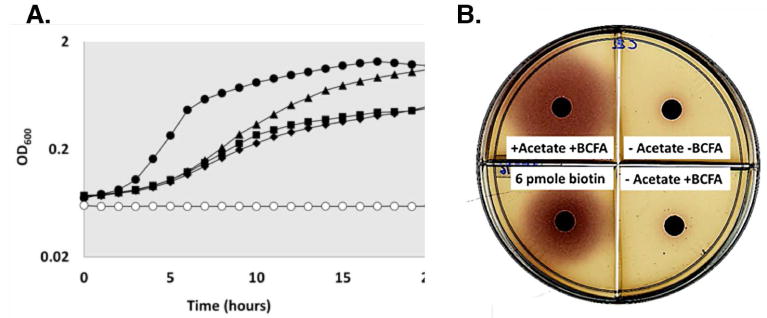
A. Growth of strain MM194 with 1 mM sodium acetate and 0.1 mM each of the branched-chain fatty acid BCFA) precursors isovaleric acid, isobutyric acid and 2-methylbutyric acid. Strains BI274 and MM194 were grown overnight in minimal medium supplemented with yeast extract. Cells were harvested, washed twice in Spizizen salts and subcultured in a 96 well plate to a final OD600 of 0.012 in 300 μL of minimal media containing different growth supplements: (solid circles) BI274 without sodium acetate and BCFA precursors, (triangles) MM194 with sodium acetate and BCFA precursors, (squares) MM194 with BCFA precursors only, (diamonds) MM194 without acetate and BCFA precursors and (open circles) minimal media lacking cells. Strain BI274 grew without acetate and BCFA precursors whereas strain MM194 required both acetate and BCFA precursors for growth.
B. Bioassay of biotin produced by strain MM194 plus acetate or BCFA precursors or both. Supernatants from the cultures were collected after growth on acetate and BCFA precursors and 10 μl of each was spotted into sterile paper disks on minimal agar plates containing the E. coli biotin auxotroph NRD25 (ΔbioABFCD:Cm). Production of biotin with acetate and BCFA precursors supplementation agrees with the growth dependence on these substrates (panel A). Known biotin amounts were used to quantitate biotin in each batch of the minimal plates used for bioassay.
As shown in Fig. 5B, carbons 3, 10, 12 and 14 were enriched when [1-13C]acetate was fed whereas when [2-13C]acetate was the acetate source carbons 7, 11 and 13 were enriched (Fig. 5C). Simultaneous 13C enrichment from [1-13C]acetate of the biotin carbon atoms derived from the pimelate carboxyl groups, carbons 3 and 14, provides strong evidence for the existence of free pimelate, a symmetrical molecule. In addition, the alternating pattern of enrichment of carbons from [1-13C]acetate and [2-13C]acetate indicates that pimelate synthesis involves the head-to-tail chain elongations characteristic of fatty acid synthesis. The enrichments of the 13C-biotin samples used for NMR analysis were verified by high-resolution electrospray ionization mass spectroscopy (Figure 6). The biotin samples labelled with [1-13C]acetate and [2-13C]acetate contained four and three 13C atoms, respectively demonstrating that all of the acetate was derived from the supplements. The lack of dilution of the 13C-acetate supplements by 12C-acetate produced from the glucose carbon source indicates that the ΔlipM mutation completely inactivated the pyruvate dehydrogenase of strain MM194.
Fig 5. 13C-NMR spectra of biotin from [1-13C]acetate or [2-13C]acetate.
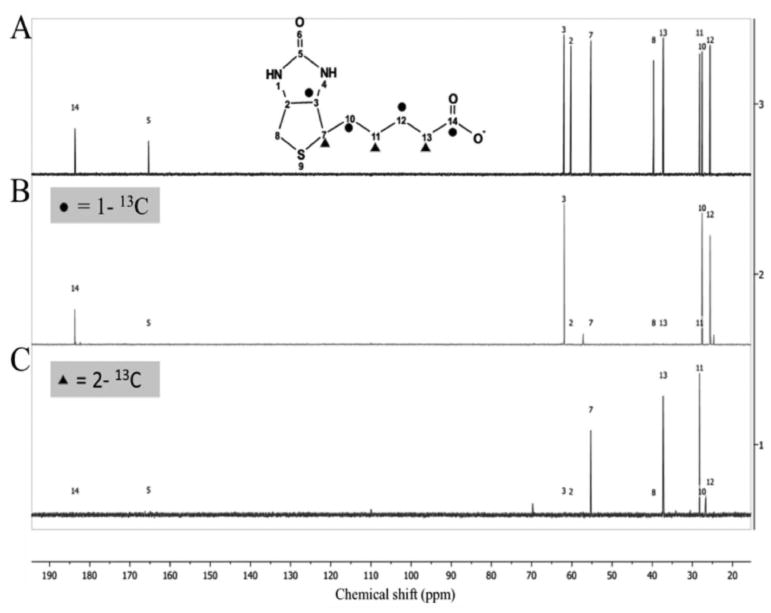
A. Natural abundance 13C spectrum of commercial biotin gives peaks with chemical shifts corresponding to each of the biotin carbon atoms (Ifuku et al., 1994, Sanyal et al., 1994). Note that our NMR program, Ifuku and Sanyal each use different biotin atom numbering systems. Our C14 is C1 of Ifuku and C10 of Sanyal whereas our C3 is C7 of Ifuku and C3 of Sanyal.
B. Spectrum of 13C-enriched carbons from [1-13C]acetate. Carbons 3, 10, 12 and 14 of biotin (solid circles) are enriched with 13C. Carbon atoms 3 and 14 are derived from the carboxyl carbons of pimelate
C. Spectrum of 13C-enriched carbons from [2-13C]acetate. Carbons 7, 11 and 13 of biotin (solid triangles) are enriched with13C.
Fig. 6.
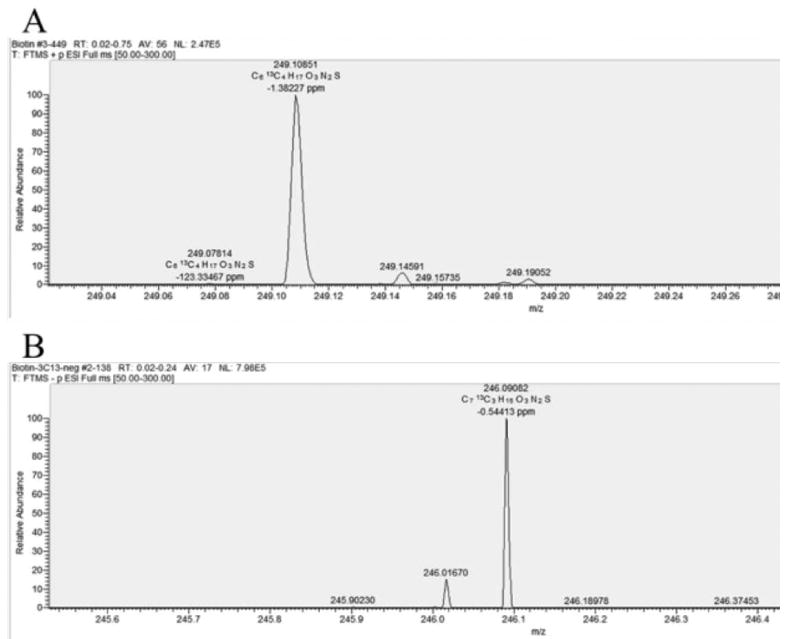
Electrospray ionization mass spectroscopy identification of 13C-biotin from A. [1-13C]acetate (calculated mass of 249.109) in the positive ESI mode and B. [2-13C]acetate (calculated mass 246.090) in the negative ESI mode. The monoisotopic mass of biotin is 244.088.
Synthesis of pimelate depends on fatty acid synthesis in B. subtilis
In order to further explore the pimelate link between fatty acid and biotin synthesis, we carried out resting cell assays with fatty acid enzyme inhibitors and monitored the effects on biotin synthesis. We tested the effects of cerulenin (Schujman et al., 2001), platensimycin and platencin, inhibitors of fatty acid elongation enzymes, FabF/FabB and FabH/FabF, respectively (Yao and Rock, 2016). Biotin production was determined by bioassay. Resting cell assays (cells starved for nitrogen) were used to avoid the difficulties of comparing inhibitor-free growing cultures to inhibited cultures that were unable to grow due to lack of lipid synthesis.
Cerulenin at 10 μg/ml was found to inhibit the production of biotin by resting cells of strain BI274 by more than 70% (Fig. 7) whereas 40 μg/ml cerulenin inhibited biotin production by more than 80% (Fig. 8) indicating that the generic fatty acid elongation enzyme FabF, is required for biotin synthesis. Biotin synthesis was restored to 50% (at 10 μg/ml cerulenin) in the FabF [I108F] cerulenin-resistant derivative of strain BI274 (Fig. 7) which is relatively insensitive to low concentrations of cerulenin (Schujman et al., 2001). In addition, pimelic acid supplementation of cerulenin-treated resting cell cultures resulted in restoration of biotin synthesis to levels comparable to that of the untreated cultures whereas supplementation with glutaric acid had no effect (Fig. 7). Therefore the effects of cerulenin on biotin synthesis were due to blockage of pimelate synthesis.
Fig. 7.
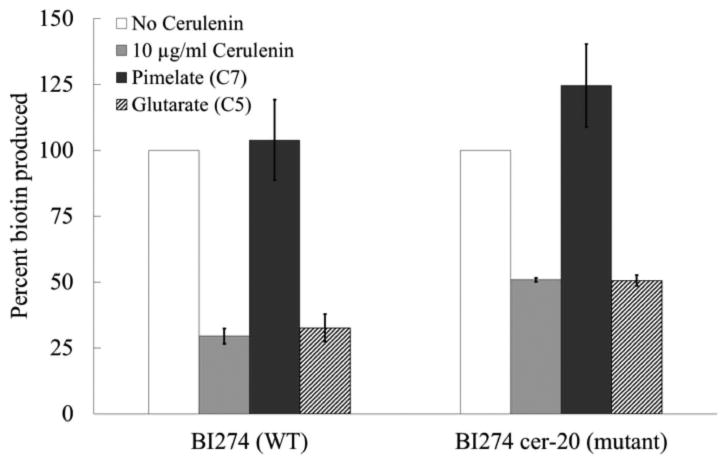
Biotin production by untreated (white bars) and cerulenin-treated (light grey bars) resting cell cultures of BI274 (WT) and BI274 cer-20 (cerulenin-resistant mutant) strains. The cells were grown in 400 mL of minimal media overnight. The cells were harvested, washed and transferred to nitrogen-limited medium in 25 mL culture volumes and allowed to incubate for 30 min at 37°C before treatment with 10 μg/ml of cerulenin (light grey bars). Samples (2 mL) were taken at the end of every hour up to 6 h and either 0.1 mM pimelate (dark grey bars) or 0.1 mM glutarate (stippled bars) was added to the cerulenin-treated cultures and incubated for one additional hour before taking the final sample. Supernatants were harvested by centrifugation and filtered through a 0.22 μm membrane. Ten microliters of each sample was spotted onto paper disks placed on top of minimal medium plates containing a biotin auxotrophic strain of E. coli and a redox indicator (2,3,5-triphenyltetrazolium chloride) as in Fig. 4. The percent biotin production was calculated by comparing the amount of biotin produced with that of the untreated culture. The error bars represent standard errors. In the seven repetitions of this experiment, 100% biotin production ranged from 6-25 pmol in the bioassays. The final biotin measurements for the antibiotic-treated cultures were determined after 6 h in each experiment
Fig. 8.
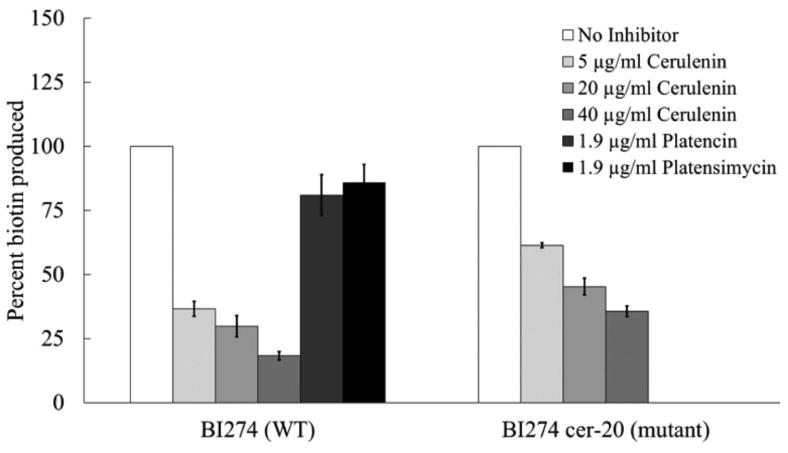
Biotin production by resting cells of the wild type strain BI274 and BI274 cer-20 its FabF (I108F) derivative. The methods were the same as in Fig. 7 and Experimental Procedures. Resting cells were treated with 5, 20 or 40 μg/ml of cerulenin in both panels whereas (left panel only) either 1.9 μg/ml of platencin or 1.9 μg/ml of platensimycin was added. The biotin contents of the supernatants were determined by bioassay. The error bars represent standard errors.
We also tested two other fatty acid synthesis inhibitors that to our knowledge had not been tested on B. subtilis. These were a dual FabH/FabF inhibitor, platencin, (Wang et al., 2007) which inhibited biotin production by 19% when added at 1.9 μg/ml and platensimycin a FabF specific inhibitor, (Wang et al., 2007) which inhibited biotin production by 15% at the same concentration (Fig. 8). Note that the platencin and platensimycin experiments were limited by the expense of the antibiotics coupled with the relative insensitivity of B. subtilis to these molecules.
Discussion
The initial stage of B. subtilis biotin synthesis is markedly different from that of E. coli in that both the enzymes and thioester moieties differ (CoA in B. subtilis, ACP in E. coli). Although the presence of bioI and bioW in B. subtilis indicated a redundancy in the generation of the pimelate thioester intermediate, our data shows that bioW is the only bio gene essential for generation of the pimeloyl-CoA substrate of BioF. Bower and coworkers (Bower et al., 1996) reported that inactivation of bioW resulted in a biotin-requiring phenotype. However, growth was only partially restored by addition of dethiobiotin or 7, 8-diaminononanate (Fig. 1) indicating polar effects on the downstream genes. In our bioW disruption construct we included a promoter oriented to transcribe the downstream genes. This was successful in that supplementation with late intermediates in the pathway completely restored growth. Moreover, complementation of the ΔbioW mutation by expression of wild type bioW from an ectopic site further demonstrated the absence of polar effects on the downstream genes.
Our 13C-NMR data provide the first definitive evidence for the presence of free pimelic acid in B. subtilis cells. Following the rationale of Sanyal, Ifuku and coworkers (Sanyal et al., 1994, Ifuku et al., 1994) that eliminated free pimelic acid as a biotin precursor in E. coli, we conclude that the opposite situation is the case for B. subtilis. In the E. coli studies, free pimelic acid was ruled out as the precursor because upon incorporation into biotin the carbon atoms derived from the carboxyl groups of pimelate were not labelled symmetrically and thus are metabolically distinct. In contrast, our data showed that feeding experiments with [1-13C]acetate enriched both of the biotin carbon atoms derived from the pimelate carboxyl groups indicating that free pimelic acid, a symmetrical molecule, is the precursor. In addition, our data also indicate fatty acid synthesis to be responsible for pimelate synthesis. This conclusion is based on the alternate pattern of assimilation of labelled carbons from [1-13C]acetate and [2-13C]acetate which indicate a head-to-tail configuration identical to the fatty acid chain elongation process. Resting cell assays with FabF inhibitors decreased biotin production indicating that fatty acid synthesis is involved in pimelate and hence, biotin synthesis. The effect of cerulenin on biotin production was more pronounced than with the other two inhibitors tested (note that B. subtilis is naturally resistant to two other fatty acid synthesis inhibitors, thiolactomycin and triclosan). We expect that the efficiency of cerulenin in inhibiting biotin production is probably due to its covalent interaction with FabF whereas platencin and platensimycin bind non-covalently (Jayasuriya et al., 2007). Moreover, platencin and platensimycin are poor inhibitors of B. subtilis growth. The concentrations reported to inhibit Staphylococcus aureus were ineffective with B. subtilis. It should be noted that even inhibitors that almost completely block fatty acid synthesis (e.g., cerulenin) might be expected to have less effect on biotin synthesis due to the fact that any residual fatty acid synthesis capacity could suffice for biotin synthesis given that fatty acids are made in great excess (ca. 104) over biotin. Note that we made numerous attempts to develop an in vitro B. subtilis cell extract system that would synthesize dethiobiotin starting from malonyl-CoA. A successful system would have allowed us to test intermediates and to overcome any cell membrane impermeability to inhibitors as was done using E. coli extracts (Lin et al., 2010). However, this approach was unsuccessful, at best we saw only traces of dethiobiotin synthesis. Indeed, prior workers reported that B. subtilis extracts were almost totally deficient in synthesis of fatty acids that lacked terminal branching (Butterworth Bloch, 1970) which may have precluded synthesis of the straight chain pimelate molecule,.
The ability of B. subtilis BioI to support robust growth of E. coli ΔbioC ΔbioH strains in the absence of biotin indicated that the gene encodes a functional enzyme that almost certainly generates pimeloyl-ACP, a known intermediate in the E. coli pathway, that readily explains the ΔbioC ΔbioH bypass. In B. subtilis the bioI transcript is 8-fold less abundant than the upstream bio operon transcript due to transcription termination (Perkins et al., 1996). However, deletion of the terminator had only a very modest effect (<2-fold) on biotin production (Perkins et al., 1996) and did not allow biotin synthesis in a ΔbioW strain. Hence, BioI does not play a detectable role in B. subtilis biotin synthesis even when overproduced and thus presents an engima. Bower and coworkers reported that a ΔbioI mutation caused a bradytrophic phenotype in biotin-free media (Bower et al., 1996). However, we found that disrupting bioI failed to cause any growth defect. The ability of B. subtilis to grow anaerobically in biotin-free media further supported the results of our genetic data. If BioI, a cytochrome P450, was important for biotin synthesis B. subtilis would depend on oxygen for growth in biotin-free media. We hypothesize that acquiring bioW and making bioI extraneous during the course of evolution might have enabled B. subtilis to survive in environments where oxygen is scarce and other electron acceptors are available. If so, bioW acquisition seems likely to have been a recent occurrence because the BioI sequence has not degenerated into an inactive protein. Consistent with this notion bioW is reported to have its own dedicated transcript in addition to the transcript that encodes bioW and the other genes of the operon (Perkins et al., 1996). It should be noted that other bacteria are known that encode a BioW but lack a BioI. Some of these are Bacillus (now Lysinibacillus) sphaericus, S. aureus, Aquifex aeolicus and Chlamydia trachomatis. The methanogenic archeon, Methanococcus jannaschii also encodes a BioW but lacks BioI consistent with it strictly anaerobic lifestyle.
The mechanism of the B. subtilis pimelate synthesis remains to be determined. The most straightforward pathway would be if the fatty acid synthetic pathway could accept a malonyl-thioester as a primer in place of the thioesters of the usual acetyl or branched chain precursors. If so, when the pimelate chain was complete, it would be cleaved from ACP by a (presumably) specific pimeloyl-ACP thioesterase and BioW would convert the free pimelate to pimeloyl-CoA. Work to be published elsewhere argues that pimeloyl-CoA but not pimeloyl-ACP is the substrate of B. subtilis BioF. This pathway would be wasteful of ATP since an already activated pimeloyl carboxyl is cleaved and then reactivated, but since biotin synthesis is a very low demand pathway (Feng et al., 2013) there should be negligible effects on B. subtilis physiology.
Experimental procedures
Materials and strains
Sodium acetate (1-13C and 2-13C, each 99% enriched) was purchased from Cambridge Isotope Laboratories, Inc. Cerulenin, platencin, platensimycin and 8-amino-7-oxononanoate were purchased from Cayman Chemical. All other chemicals were purchased from Fisher Scientific or Sigma Aldrich. The bacterial strains are given in Table 1. The B. subtilis strains are all derivatives of strain 168 whereas the E. coli strains are derivatives of the wild type K-12 strains MC1061 or MG1655. The B. subtilis biotin overproducing strain BI274 and BI304 (derivatives of strain PY79 with constitutive expression of the bio operon) (Bower, 2000) was from the American Type Culture Collection (accession 55575 and 55573, respectively). The cer-20 derivative of B. subtilis 168 that is partially resistant to cerulenin (Schujman et al., 2001) was from the Bacillus Genetics Stock Center (accession 1A577).
Table 1. Bacterial strains.
| B. subtilis | ||
| 168 | Wild type | |
| MM43 | ΔbioW of 168, downstream reading promoter | This work |
| MM57 | ΔbioI of 168 | This work |
| MM59 | MMX with ectopic bioW | This work |
| PY79 | Wild type | (Bower et al., 1996) |
| BI274 | PY79 bio operon driven by strong constitutive SPO1-15 promoter | (Perkins et al., 1996, Bower, 2000) |
| BI304 | PY79 bio operon driven by strong constitutive promoter SPO1-15 and containing SPO1-15 in place of the terminator upstream of bioI [P15 bioWAFDB (Δt) P15 bioI] | (Perkins et al., 1996) |
| MM194 | ΔlipM of BI274 | This work |
| Cer-20 | Cerulenin-resistant derivative of 168 | (Schujman et al., 2001) |
| NM57 | ΔlipM∷Km | (Martin et al., 2011) |
| E. coli | ||
| NRD25 | Δ(bioABFCD)∷Cm | (Choi-Rhee and Cronan, 2005b) |
| ER90 | ΔbioF bioC bioDa | (Choi-Rhee and Cronan, 2005a) |
| STL25 | ΔbioC ΔbioH | (Lin et al., 2010) |
The ΔbioF lesion is polar on bioC and bioD.
General growth media
E. coli and B. subtilis strains were grown in LB media. The minimal medium for E. coli contained M9 salts, 0.4% glucose, 1 μg/ml thiamine and 0.1% Casamino acids. General defined media for B. subtilis was Spizizen salts consisting of (NH4)2SO4; 0.2 %, K2HPO4; 1.4%, KH2PO4; 0.6%, sodium citrate · 2H2O; 0.1%, MgSO4 · 7H2O; 0.02%] (Spizizen, 1958), plus trace elements [MgCl2·6H2O, CaCl2, FeCl2·6H2O, MnCl2·4H2O, ZnCl2, CuCl2·2H2O, CoCl2.6H2O NaMoO4.2H2O], 0.5% glucose, 0.04% potassium glutamate and 1 mM MgSO4 · 7H2O. Supplements required for growth included 0.01% tryptophan, 1 mM sodium acetate, 0.1 mM each of branched chain fatty acid (BCFA) precursors (isobutyric acid, isovaleric acid and 2-methylbutyric acid). Genetic complementation experiments requiring expression of Pspac and PBAD promoters were performed in minimal media with 0.5% glycerol as the carbon source. Either isopropyl β-D-1-thiogalactopyranoside (IPTG) (1 mM) or 0.2% arabinose was used for induction of the respective promoters. Antibiotics were used in the following concentrations (in μg/ml): chloramphenicol (5), erythromycin sulfate (1), lincomycin hydrochloride (25), spectinomycin sulfate (100), sodium ampicillin (100) and kanamycin sulfate (50).
Construction of bioW and bioI deletion mutant strains
A 432 bp internal bioW fragment was amplified by PCR using primers: 5′ GAG ATC GAA TTC CCA TTC AGC CAT TGC 3′ and 5′ ATA ATG CGG CCG CTC ATT GCT GTA ATA CG 3′. The PCR product was digested with EcoRI and NotI and inserted into pMUTIN4 (Vagner et al., 1998). The resultant recombinant plasmid was used to transform B. subtilis 168 to allow single crossover recombination of the nonreplicating plasmid into the chromosome. The transformants were selected on plates containing erythromycin and lincomycin. To disrupt bioI fragments of 570 bp and 627 bp corresponding to the 5′ and 3′ region of bioI, respectively, were amplified with primers 5′ ATC AGG GAT CCA AAT GAA GGC TAG TTT AAG 3′, 5′ CAC TCC GAA TTC TGC TCC CTA TCT TCC 3′ (5′ region) and 5′ CGG AC GTC GAC CAA CGG TCA ATC TCA TC 3′, 5′ AAG CAC GGG CCC TTC ATA GTC TGA AAT AAG C 3′(3′ region) and inserted into the BamHI-EcoRI (5′) and SalI-ApaI (3′) sites that bracket the kanamycin cassette of pDG780 (Guérout-Fleury et al., 1995). The recombinant plasmid encodes a bioI in which 75 internal codons were replaced with the kanamycin cassette. This plasmid was used to transform B. subtilis 168 and the recombinants resulting from a double-crossover recombination were selected on kanamycin-containing LB plates. Transformation of B. subtilis was carried out by the procedure of Dubnau and Davidoff-Abelson (Dubnau and Davidoff-Abelson, 1971).
For ectopic expression of bioW, the coding region was inserted into the SalI-SphI sites of pDR111. The recombinant plasmid was introduced into the bioW mutant strain by a double crossover recombination at the amyE locus. The gene disruption constructs were verified by colony PCR, restriction digestion and sequencing of the PCR products. The amyE phenotype was assayed on colonies grown for 16 h on LB starch plates by flooding the plates with 1% I2-KI solution (Sekiguchi et al., 1975).
Growth of strain MM194 and purification of biotin from culture supernatants
Strain MM194 was constructed by transformation of strain BI274 with the genomic DNA of NM57 (JH642 ΔlipM∷Km) (Martin et al., 2011) with selection for ΔlipM mutants on kanamycin plates. The mutants were verified by the growth phenotype on minimal media and colony PCR. Strain MM194 was cultured overnight in LB and subcultured at the final OD600 of 0.02 in minimal medium I which contained 0.05% yeast extract and 0.02% Bacto-Tryptone and allowed to grow at 37°C for 6-9 h until an OD600∼2 was reached. Finally, the MMI culture was transferred to 1 L of minimal medium II which lacked yeast extract and tryptone but contained sodium acetate and BCFA at a final OD600 of 0.02 and allowed to grow overnight at 37°C for 15-18 h.
The cells were removed from 10-20 L cultures by centrifugation and the supernatant was filtered through Millipore 0.22 μm membranes. The supernatant was treated with activated charcoal and the charcoal was collected and extracted with an ethanol-ammonium hydroxide mixture as described by Ogata (Ogata, 1970). This extract was concentrated to a volume of 2 mL using a Buchi Rotavapor R-210. N-hydroxysuccinimide-activated agarose (Pierce Thermo-Fisher Scientific) was used to covalently immobilize the F43A mutant derivative of shwanavidin, a dimeric avidin from Shewanella denitrificans (Meir et al., 2012) following the manufacturer recommended protocol. Shwanavidin (20 mg) was coupled to 150 mg of agarose in coupling buffer (100 mM Na2HPO4, 150 mM NaCl at pH 7.2) by mixing at 4 °C overnight. The shwanavidin-coupled agarose was washed twice in coupling buffer and any remaining N-hydroxysuccinimide groups were quenched with 1M Tris-HCL (pH 7.4). Multiple loadings of 200-400 nmol biotin in the charcoal extract samples were allowed to bind to the shwanavidin-agarose complex by mixing at 4°C overnight. The agarose was then washed three times with coupling buffer. Most of the colored impurities were found in the flow-through and wash fractions with some loss of biotin. The bound biotin was then eluted with heated water (70°C) (Holmberg et al., 2005). Biotin was determined by bioassay throughout the process of purification. The biotin samples obtained (∼1 mg from 10 L cultures) were analyzed by Agilent 600 MHz NMR at the Carl R. Woese Institute for Genomic Biology, University of Illinois at Urbana-Champaign. D2O was used as solvent (pH ∼13) and number of scans was 16000nt. The biotin carbon atoms were assigned based on the 13C NMR shifts that had been independently determined by Sanyal and coworkers and Ifuku and coworkers (Sanyal et al., 1994, Ifuku et al., 1994).
Shwanavidin purification
A synthetic gene encoding the F43A mutant derivative of Shewanella denitrificans shwanavidin was inserted into the NdeI-HindIII sites of vector pET28b to encode a protein with a N-terminal hexahistidine tag. The recombinant plasmid was transformed into expression strain BL21 (DE3) Tuner (Novagen) with selection for kanamycin resistance to obtain strain MM200. Strain MM200 was grown overnight in LB medium and subcultured into 1 L of LB to allow growth up to OD600 of 0.8. The culture was induced with 1 mM IPTG for 3 h at 37°C. The cells were harvested after centrifugation and washed with 10 mM Tris-HCl, 100 mM NaCl and 1 mM EDTA (pH 7.5). Cell pellets were suspended in 50 mM Tris-HCl, 200 mM NaCl, 1% Triton X-100, 8% sucrose and 1mM phenylmethylsulfonyl fluoride at pH 8. Cells were lysed by passage through a French press and the lysate was centrifuged at 18,000 rpm for 30 min. The insoluble inclusion bodies were washed in the same buffer without Triton X-100 and centrifuged again to remove the buffer. The inclusion bodies were then dissolved in 6 M guanidine hydrochloride and 50 mM Tris-HCl (pH 7.5) to a concentration of 10 mg /mL and stirred slowly at 4°C for 4-6 h to allow equilibration. The solubilized protein was added dropwise into folding buffer (1/4 -1/2 of induced culture volume) of 50 mM Tris-HCl, 150 mM NaCl, 5 mM EDTA and 0.1 mM phenylmethylsulfonyl fluoride at pH 7.5 at 4°C while stirring and allowed to equilibrate overnight. The solution was centrifuged to remove insoluble debris and dialyzed against 50 mM NaH2PO4, 500 mM NaCl and 10% glycerol (pH 8). The refolded protein was further purified using an ÄKTA protein purification system and a 5 mL HisTrap HP column. The pure protein was eluted with 150 mM imidazole in 50 mM NaH2PO4, 500 mM NaCl and 10% glycerol (pH 8). The protein was concentrated using 3K Amicon concentrator (EMD Millipore) and flash frozen at -80 °C in 50 mM NaH2PO4, 500 mM NaCl and 10% glycerol (pH 8).
Mass spectral analyses
Purified biotin samples were analyzed with Agilent LC/MS (Agilent Technologies, Santa Clara, CA). The LC separation was performed on an Agilent Eclipse XDB-C18 (4.6 × 50mm, 5μm) with mobile phase A (0.1% formic acid in water) and mobile phase B (0.1% formic acid in acetontrile). The flow rate was 0.4 mL/min. The linear gradient was as follows: 0-2 min, 100% A; 6-17.5 min, 0% A. For high resolution mass spectra, the samples were analyzed by using the Q-Exactive MS system (Thermo, Bremen, Germany) of the Metabolomics Laboratory of the Carver Biotechnology Center, University of Illinois at Urbana-Champaign.
Bioassay of biotin production
E. coli strain NRD25 (Choi-Rhee and Cronan, 2005b) which lacks the entire bio operon and strain ER90 (ΔbioF bioC bioD) which retains a functional bioB (Choi-Rhee and Cronan, 2005a) were grown overnight in defined media containing 1 nM biotin. The cells were harvested by centrifugation, washed three times in M9 salts and allowed to starve in 100 mL of defined media containing 0.5 units of avidin for 5 h at 37°C. The biotin-starved cells were washed three times in 1mL of M9 salts and mixed with melted 1.5% agar containing defined media and 0.01% 2,3,5-triphenyltetrazolium chloride, a redox indicator that develops a red color upon cellular respiration (Lin et al., 2010, Cleary and Campbell, 1972). Five ml of medium was poured into plates quarter-sectored by plastic walls and sterile 6 mm disks (BD BBL blank test discs) were placed on top of the solidified agar. After spotting 10 μl of sample onto the disks the plates were incubated at 30°C overnight. The amounts of biotin in the samples were quantitated by measuring the diameter of the red zone produced by known amounts of commercial biotin used as controls for each batch of minimal plates.
Resting cell assays
Strain BI274 cer-20 was constructed by transforming strain BI274 with the genomic DNA of the original cerulenin resistant strain and selection on LB plates containing 10 μg/ml cerulenin. The FabF mutation conferring increased cerulenin resistance (I108F) (Schujman et al., 2001) was verified by sequencing. Strain BI274 and BI274 cer-20 were grown in LB media for 7-8 h at 37°C. The cells were subcultured to 400 mL of defined minimal media to a final OD600 of approximately 0.02 and incubated at 37°C for 16 h. The cells were harvested by centrifugation at 8000 rpm for 10 min and washed twice with Spizizen salts. Cells were transferred to 25 mL nitrogen-deficient minimal medium (Spizizen salts lacking ammonium sulfate) to a final OD600 of approximately 3 to 5. Aliquots were taken at several time points after adding antibiotics and the amount of biotin released into the supernatant was determined by bioassay with E. coli strain ER90 (ΔbioF bioC bioD) (Choi-Rhee and Cronan, 2005a). The amount of biotin produced was quantitated by comparison to known amounts of control biotin assayed on the same batch of plates.
Acknowledgments
This work was supported by National Institutes of Health Grant AI15650 from the National Institute of Allergy and Infectious Diseases. We thank Michelle Goettge for assistance with the NMR analyses and Dr. Zhong (Lucas) Li for the mass spectral analyses.
Footnotes
Author Contributions: MM designed and performed experiments, interpreted data and wrote the first draft of the manuscript. JC designed experiments, interpreted data and wrote the final draft of the manuscript with the assistance of MM.
Conflict of Interest: The authors declare that they have no conflicts of interest.
References
- Agarwal V, Lin S, Lukk T, Nair SK, Cronan JE. Structure of the enzyme-acyl carrier protein (ACP) substrate gatekeeper complex required for biotin synthesis. Proc Natl Acad Sci USA. 2012;109:17406–17411. doi: 10.1073/pnas.1207028109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alban C. Biotin (Vitamin B8) Synthesis in Plants. Adv Bot Res. 2011;59:39–66. [Google Scholar]
- Bower S, Perkins JB, Yocum RR, Howitt CL, Rahaim P, Pero J. Cloning, sequencing, and characterization of the Bacillus subtilis biotin biosynthetic operon. J Bacteriol. 1996;178:4122–4130. doi: 10.1128/jb.178.14.4122-4130.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower SG, Perkins John B, Pero Janice G, Yocum Rogers R. Biotin biosynthesis in Bacillus subtilis. Hoffmann, Roche LA (CH), pp 2000 [Google Scholar]
- Butterworth PH, Bloch K. Comparative aspects of fatty acid synthesis in Bacillus subtilis and Escherichia coli. Eur J Biochem. 1970;12:496–501. doi: 10.1111/j.1432-1033.1970.tb00878.x. [DOI] [PubMed] [Google Scholar]
- Choi-Rhee E, Cronan JE. Biotin synthase is catalytic in vivo, but catalysis engenders destruction of the protein. Chem Biol. 2005a;12:461–468. doi: 10.1016/j.chembiol.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Choi-Rhee E, Cronan JE. A nucleosidase required for in vivo function of the S-adenosyl-L-methionine radical enzyme, biotin synthase. Chem Biol. 2005b;12:589–593. doi: 10.1016/j.chembiol.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Cleary PP, Campbell A. Deletion and complementation analysis of biotin gene cluster of Escherichia coli. J Bacteriol. 1972;112:830–839. doi: 10.1128/jb.112.2.830-839.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan JE. A family of arabinose-inducible Escherichia coli expression vectors having pBR322 copy control. Plasmid. 2006;55:152–157. doi: 10.1016/j.plasmid.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Cryle MJ, Schlichting I. Structural insights from a P450 Carrier Protein complex reveal how specificity is achieved in the P450BioI ACP complex. Proc Natl Acad Sci USA. 2008;105:15696–15701. doi: 10.1073/pnas.0805983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D, Davidoff-Abelson R. Fate of transforming DNA following uptake by competent Bacillus subtilis: I. Formation and properties of the donor-recipient complex. J Mol Biol. 1971;56:209–221. doi: 10.1016/0022-2836(71)90460-8. [DOI] [PubMed] [Google Scholar]
- Eisenberg MA. The incorporation of 1,7 C14 pimelic acid into biotin vitamers. Biochem Biophys Res Commun. 1962;8:437–441. doi: 10.1016/0006-291x(62)90292-9. [DOI] [PubMed] [Google Scholar]
- Eisenberg MA. Biotin: biogenesis, transport, and their regulation. Adv Enzymol Relat Areas Mol Biol. 1973;38:317–372. doi: 10.1002/9780470122839.ch7. [DOI] [PubMed] [Google Scholar]
- Eisenberg MA, Star C. Synthesis of 7-oxo-8-aminopelargonic acid, a biotin vitamer, in cell-free extracts of Escherichia coli biotin auxotrophs. J Bacteriol. 1968;96:1291–1297. doi: 10.1128/jb.96.4.1291-1297.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada P, Manandhar M, i, Dong SH, Deveryshetty J, Agarwal V, Cronan JE, Nair SK. Structure and function of the pimeloyl-coA synthetase biow defines a new fold for adenylate-forming enzymes. Nature Chem Biol. 2017 doi: 10.1038/nchembio.2359. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Zhang H, Cronan JE. Profligate biotin synthesis in alpha-proteobacteria - a developing or degenerating regulatory system? Mol Microbiol. 2013;88:77–92. doi: 10.1111/mmi.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AJ, Rivers SL, Cheesman M, Reid GA, Quaroni LG, Macdonald IDG, Chapman SK, Munro AW. Expression, purification and characterization of cytochrome P450 Biol: a novel P450 involved in biotin synthesis in Bacillus subtilis. J Biol Inorgan Chem. 2001;6:523–533. doi: 10.1007/s007750100229. [DOI] [PubMed] [Google Scholar]
- Guérout-Fleury AM, Shazand K, Frandsen N, Stragier P. Antibiotic-resistance cassettes for Bacillus subtilis. Gene. 1995;167:335–336. doi: 10.1016/0378-1119(95)00652-4. [DOI] [PubMed] [Google Scholar]
- Henke SK, Cronan JE. Successful conversion of the Bacillus subtilis BirA Group II biotin protein ligase into a Group I ligase. PLoS One. 2014;9:e96757. doi: 10.1371/journal.pone.0096757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg A, Blomstergren A, Nord O, Lukacs M, Lundeberg J, Uhlén M. The biotin-streptavidin interaction can be reversibly broken using water at elevated temperatures. Electrophoresis. 2005;26:501–510. doi: 10.1002/elps.200410070. [DOI] [PubMed] [Google Scholar]
- Ifuku O, Miyaoka H, Koga N, Kishimoto J, Haze S, Wachi Y, Kajiwara M. Origin of carbon atoms of biotin. 13C-NMR studies on biotin biosynthesis in Escherichia coli. Eur J Biochem. 1994;220:585–591. doi: 10.1111/j.1432-1033.1994.tb18659.x. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Morita H, Sato K, Tani Y, Ogata K. Synthesis of biotin-vitamers from pimelic acid and coenzyme A by cell-free extracts of various bacteria. Biochim Biophys Acta. 1972;264:210–213. doi: 10.1016/0304-4165(72)90133-x. [DOI] [PubMed] [Google Scholar]
- Jayasuriya H, Herath KB, Zhang C, Zink DL, Basilio A, Genilloud O, Diez MT, Vicente F, Gonzalez I, Salazar O, Pelaez F, Cummings R, Ha S, Wang J, Singh SB. Isolation and structure of platencin: A FabH and FabF dual inhibitor with potent broad-spectrum antibiotic activity. Angew Chem Int Ed Engl. 2007;46:4684–4688. doi: 10.1002/anie.200701058. [DOI] [PubMed] [Google Scholar]
- Lin S, Cronan JE. Closing in on complete pathways of biotin biosynthesis. Mol Biosyst. 2011;7:1811–1821. doi: 10.1039/c1mb05022b. [DOI] [PubMed] [Google Scholar]
- Lin S, Cronan JE. The BioC O-methyltransferase catalyzes methyl esterification of malonyl-acyl carrier protein, an essential step in biotin synthesis. J Biol Chem. 2012;287:37010–37020. doi: 10.1074/jbc.M112.410290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Hanson RE, Cronan JE. Biotin synthesis begins by hijacking the fatty acid synthetic pathway. Nat Chem Biol. 2010;6:682–688. doi: 10.1038/nchembio.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris TM, Davydov R, Denisov IG, Hoffman BM, Sligar SG. Mechanistic enzymology of oxygen activation by the cytochromes P450. Drug Metab Rev. 2002;34:691–708. doi: 10.1081/dmr-120015691. [DOI] [PubMed] [Google Scholar]
- Manandhar M, Cronan JE. Proofreading of noncognate acyl adenylates by an acyl-coenzyme A ligase. Chem Biol. 2013;20:1441–1446. doi: 10.1016/j.chembiol.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin N, Christensen QH, Mansilla MC, Cronan JE, de Mendoza D. A novel two-gene requirement for the octanoyltransfer reaction of Bacillus subtilis lipoic acid biosynthesis. Mol Microbiol. 2011;80:335–349. doi: 10.1111/j.1365-2958.2011.07597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meir A, Bayer EA, Livnah O. Structural adaptation of a thermostable biotin-binding protein in a psychrophilic environment. J Biol Chem. 2012;287:17951–17962. doi: 10.1074/jbc.M112.357186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller JH. Pimelic acid as a growth accesory factor for the strain of the Diptheria Bacillus. Science. 1937a;85:1935–1936. doi: 10.1126/science.85.2212.502. [DOI] [PubMed] [Google Scholar]
- Mueller JH. Pimelic acid as a growth accessory for the diphtheria bacillus. J Biol Chem. 1937b;119:121–131. doi: 10.1126/science.85.2212.502. [DOI] [PubMed] [Google Scholar]
- Mueller JH. Studies on cultural requirements of bacteria. Pimelic acid as a growth stimulant for C. diphtheriae. J Bacteriol. 1937c;34:163–178. doi: 10.1128/jb.34.2.163-178.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano MM, Zuber P. Anaerobic growth of a “strict aerobe” (Bacillus subtilis) Annu Rev Microbiol. 1998;52:165–190. doi: 10.1146/annurev.micro.52.1.165. [DOI] [PubMed] [Google Scholar]
- Ogata K. Microbial synthesis of dethiobiotin and biotin. Meth Enzymol. 1970;18:390–394. [Google Scholar]
- Perkins JB, Bower S, Howitt CL, Yocum RR, Pero J. Identification and characterization of transcripts from the biotin biosynthetic operon of Bacillus subtilis. J Bacteriol. 1996;178:6361–6365. doi: 10.1128/jb.178.21.6361-6365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploux O, Soularue P, Marquet A, Gloeckler R, Lemoine Y. Investigation of the first step of biotin biosynthesis in Bacillus sphaericus. Purification and characterization of the pimeloyl-CoA synthase, and uptake of pimelate. Biochem J. 1992;287:685–690. doi: 10.1042/bj2870685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov Da. Conservation of the biotin regulon and the BirA regulatory signal in eubacteria and archaea. Genome Res. 2002;12:1507–1516. doi: 10.1101/gr.314502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal I, Lee SL, Flint DH. Biosynthesis of pimeloyl-CoA, a biotin precursor in Escherichia coli, follows a modified fatty acid synthesis pathway: 13C-labeling studies. J Am Chem Soc. 1994;116:2637–2638. [Google Scholar]
- Schujman GE, Choi KH, Altabe S, Rock CO, De Mendoza D. Response of Bacillus subtilis to cerulenin and acquisition of resistance. J Bacteriol. 2001;183:3032–3040. doi: 10.1128/JB.183.10.3032-3040.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi J, Takada N, Okada H. Genes affecting the productivity of alpha-amylase in Bacillus subtilis Marburg. J Bacteriol. 1975;121:688–694. doi: 10.1128/jb.121.2.688-694.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. Transformation of biochemically deficient strains of Bacillus subtilis by deoxyribonucleate. Proc Natl Acad Sci USA. 1958;44:1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stok JE, De Voss JJ. Expression, purification, and characterization of BioI: A carbon–carbon bond cleaving cytochrome P450 involved in biotin biosynthesis in Bacillus subtilis. Arch Biochem Biophys. 2000;384:351–360. doi: 10.1006/abbi.2000.2067. [DOI] [PubMed] [Google Scholar]
- Tong L. Structure and function of biotin-dependent carboxylases. Cell Mol Life Sci. 2013;70:863–891. doi: 10.1007/s00018-012-1096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagner V, Dervyn E, Ehrlich SD. A vector for systematic gene inactivation in Bacillus subtilis. Microbiology. 1998;144:3097–3104. doi: 10.1099/00221287-144-11-3097. [DOI] [PubMed] [Google Scholar]
- Wang J, Kodali S, Lee SH, Galgoci A, Painter R, Dorso K, Racine F, Motyl M, Hernandez L, Tinney E, Colletti SL, Herath K, Cummings R, Salazar O, Gonzalez I, Basilio A, Vicente F, Genilloud O, Pelaez F, Jayasuriya H, Young K, Cully DF, Singh SB. Discovery of platencin, a dual FabF and FabH inhibitor with in vivo antibiotic properties. Proc Natl Acad Sci USA. 2007;104:7612–7616. doi: 10.1073/pnas.0700746104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildiers E. Nouvelle substance indispensable au developpement de la levure. La cellule. 1901;18:313–316. [Google Scholar]
- Yao J, Rock CO. Bacterial fatty acid metabolism in modern antibiotic discovery. Biochim Biophys Acta. 2016 doi: 10.1016/j.bbalip.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


