Abstract
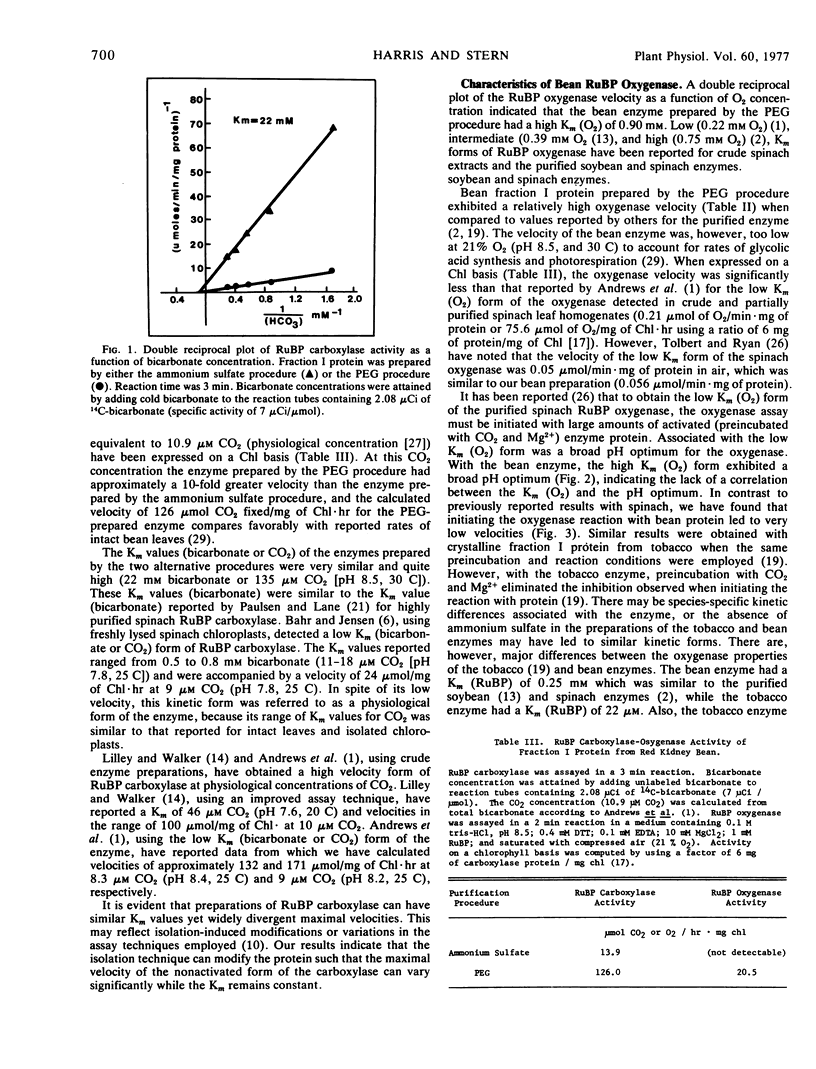
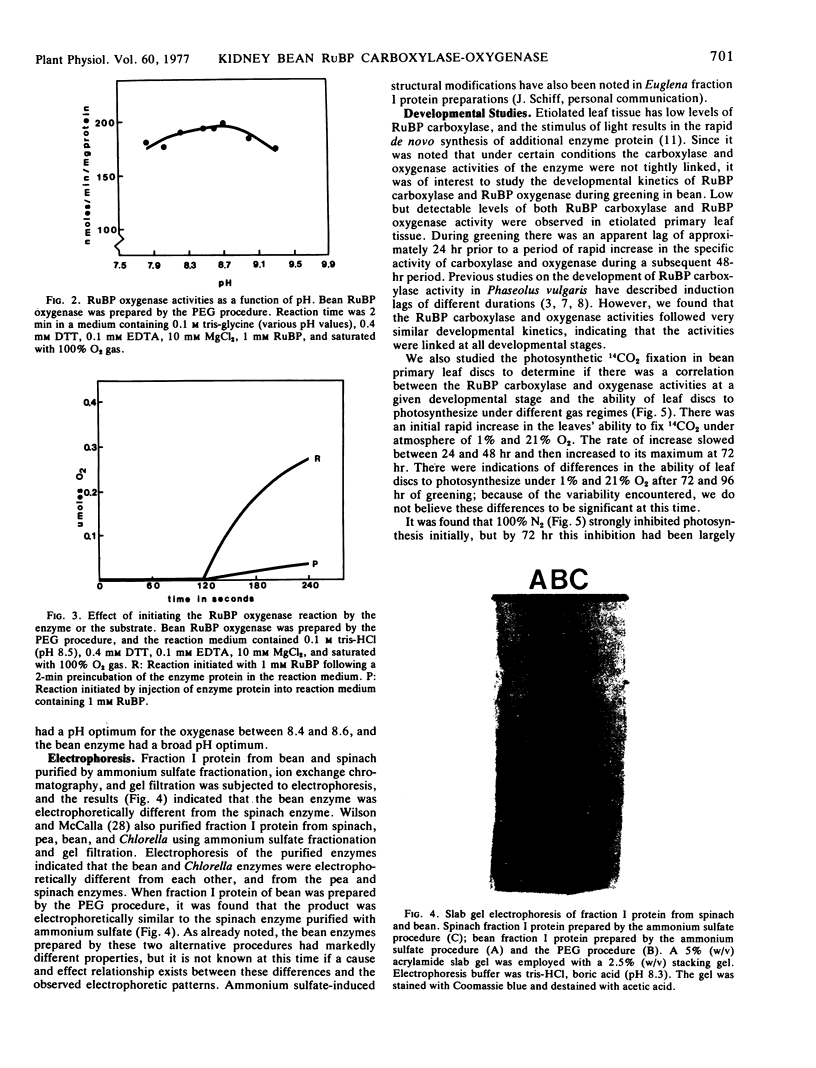
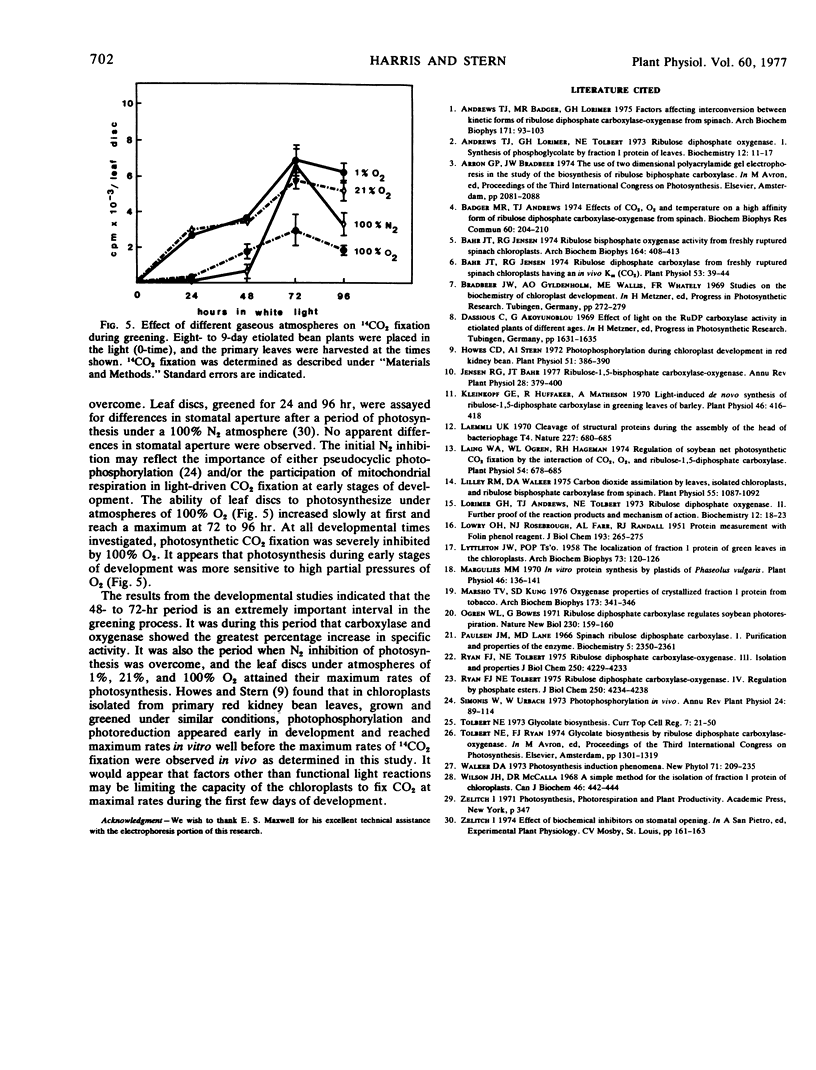
Purification of ribulose-1,5-bisphosphate carboxylase from primary leaves of Phaseolus vulgaris var. Red Kidney with ammonium sulfate precipitation, ion exchange chromatography, and gel filtration resulted in the complete loss of detectable oxygenase activity and the retention of a low velocity and a high Km form of both the carboxylase and oxygenase. The polyethylene glycol-6000-purified ribulose-1, 5-bisphosphate oxygenase displayed a broad pH optimum (7.9-9.4) and a high Km for O2 and ribulose 1,5-bisphosphate (0.90 mm and 0.25 mm, respectively). Initiation of the oxygenase reaction with protein rather than ribulose 1,5-bisphosphate resulted in reduced activity. The enzymes prepared by the two purification procedures were electrophoretically different.
Etiolated primary leaf tissue exhibited low rates of both carboxylase and oxygenase. Similar developmental kinetic activity was observed for both reactions during greening. Photosynthetic 14CO2 fixation was inhibited 95% by 100% N2 gas during the first 24 hours of greening, but the inhibition was rapidly overcome by 48 to 72 hours of light exposure.
Full text
PDF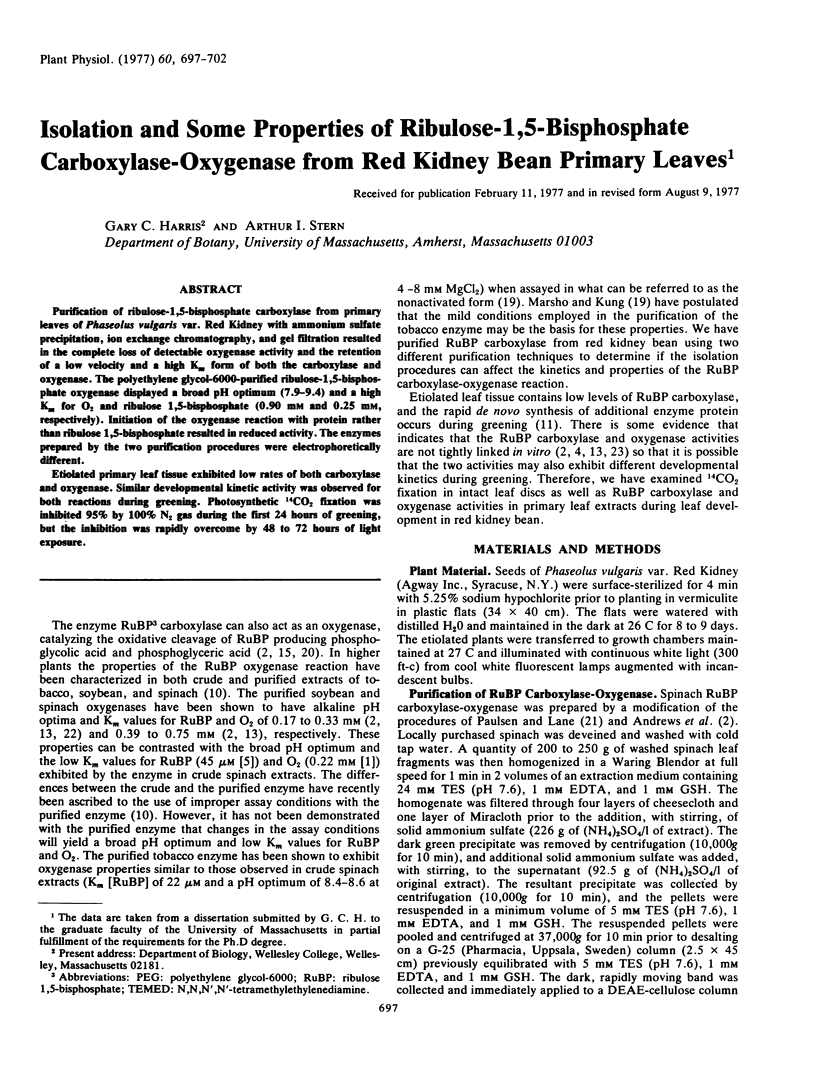


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews T. J., Badger M. R., Lorimer G. H. Factors affecting interconversion between kinetic forms of ribulose diphosphate carboxylase-oxygenase from spinach. Arch Biochem Biophys. 1975 Nov;171(1):93–103. doi: 10.1016/0003-9861(75)90011-9. [DOI] [PubMed] [Google Scholar]
- Badger M. R., Andrews T. J. Effects of CO2, O2 and temperature on a high-affinity form of ribulose diphosphate carboxylase-oxygenase from spinach. Biochem Biophys Res Commun. 1974 Sep 9;60(1):204–210. doi: 10.1016/0006-291x(74)90192-2. [DOI] [PubMed] [Google Scholar]
- Bahr J. T., Jensen R. G. Ribulose Diphosphate Carboxylase from Freshly Ruptured Spinach Chloroplasts Having an in Vivo Km[CO(2)]. Plant Physiol. 1974 Jan;53(1):39–44. doi: 10.1104/pp.53.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr J. T., Jensen R. G. Ribulose bisphosphate oxygenase activity from freshly ruptured spinach chloroplasts. Arch Biochem Biophys. 1974 Oct;164(2):408–413. doi: 10.1016/0003-9861(74)90049-6. [DOI] [PubMed] [Google Scholar]
- Howes C. D., Stern A. I. Photophosphorylation during Chloroplast Development in Red Kidney Bean: II. Photophosphorylation and Photoreduction Appear Concomitantly but Initially are Uncoupled. Plant Physiol. 1973 Feb;51(2):386–390. doi: 10.1104/pp.51.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinkopf G. E., Huffaker R. C., Matheson A. Light-induced de Novo Synthesis of Ribulose 1,5-Diphosphate Carboxylase in Greening Leaves of Barley. Plant Physiol. 1970 Sep;46(3):416–418. doi: 10.1104/pp.46.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LYTTLETON J. W., TS'O P. O. The localization of fraction I protein of green leaves in the chloroplasts. Arch Biochem Biophys. 1958 Jan;73(1):120–126. doi: 10.1016/0003-9861(58)90246-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laing W. A. Regulation of Soybean Net Photosynthetic CO(2) Fixation by the Interaction of CO(2), O(2), and Ribulose 1,5-Diphosphate Carboxylase. Plant Physiol. 1974 Nov;54(5):678–685. doi: 10.1104/pp.54.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley R. M., Walker D. A. Carbon dioxide assimilation by leaves, isolated chloroplasts, and ribulose bisphosphate carboxylase from spinach. Plant Physiol. 1975 Jun;55(6):1087–1092. doi: 10.1104/pp.55.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer G. H., Andrews T. J., Tolbert N. E. Ribulose diphosphate oxygenase. II. Further proof of reaction products and mechanism of action. Biochemistry. 1973 Jan 2;12(1):18–23. doi: 10.1021/bi00725a004. [DOI] [PubMed] [Google Scholar]
- Marsho T. V., Kung S. D. Oxygenase properties of crystallized fraction 1 protein from tobacco. Arch Biochem Biophys. 1976 Mar;173(1):341–346. doi: 10.1016/0003-9861(76)90268-x. [DOI] [PubMed] [Google Scholar]
- Ogren W. L., Bowes G. Ribulose diphosphate carboxylase regulates soybean photorespiration. Nat New Biol. 1971 Mar 31;230(13):159–160. doi: 10.1038/newbio230159a0. [DOI] [PubMed] [Google Scholar]
- Paulsen J. M., Lane M. D. Spinach ribulose diphosphate carboxylase. I. Purification and properties of the enzyme. Biochemistry. 1966 Jul;5(7):2350–2357. doi: 10.1021/bi00871a025. [DOI] [PubMed] [Google Scholar]
- Ryan F. J., Tolbert N. E. Ribulose diphosphate carboxylase/oxygenase. III. Isolation and properties. J Biol Chem. 1975 Jun 10;250(11):4229–4233. [PubMed] [Google Scholar]
- Ryan F. J., Tolbert N. E. Ribulose diphosphate carboxylase/oxygenase. IV. Regulation by phosphate esters. J Biol Chem. 1975 Jun 10;250(11):4234–4238. [PubMed] [Google Scholar]
- Wilson J. H., McCalla D. R. A simple method for the isolation of fraction I protein of chloroplasts. Can J Biochem. 1968 May;46(5):441–444. doi: 10.1139/o68-066. [DOI] [PubMed] [Google Scholar]



