Abstract
A mammalian receptor for bacterial lipopolysaccharide (LPS), Toll-like receptor 4 (TLR4), plays a beneficial role in controlling bacterial infections, but is also a main driver of aberrant inflammation in lethal sepsis. As a result, investigation of TLR4 signaling has been a major area of research. Despite this focus, our understanding of the mechanisms that regulate TLR4 activities remains primitive. Nowhere is our knowledge of TLR4 biology more lacking than at the receptor-proximal level, where many factors act in concert to regulate LPS signaling. Several recent studies have begun filling these gaps in our knowledge. In this review, we discuss the importance of these receptor proximal activities in the spatiotemporal regulation of TLR4 signaling, and suggest interesting areas for future research.
Introduction
Detection of microbes by the innate immune system is mediated by specialized proteins called pattern recognition receptors (PRRs). Signaling via PRRs induces the activation of intracellular signal transduction networks that promote inflammatory gene expression to mediate immediate and long-term host defense [1]. One of the best-studied PRRs is Toll-like receptor 4 (TLR4), a receptor for bacterial lipopolysaccharide (LPS) [2]. TLR4 is a critical driver of immune responses to bacterial infections, and its dysregulation is thought to promote aberrant cytokine production in bacterial sepsis [3]. Thus, understanding the TLR4 signaling pathway will inform discussions of the basic mechanisms underlying inflammation and clinical care.
Genetic analysis has revealed an array of co-receptors, adaptors, and signaling pathways that work with TLR4 to regulate immunity to bacterial infections. Despite this increasing knowledge of the genes required for TLR4 signaling, we still lack a basic understanding of how TLR4 pathway components interact in space and time to regulate host defense. In particular, the mechanisms that link LPS detection with the execution of receptor trafficking and signal transduction have only recently become the focus of attention. In this review, we will discuss the receptor-proximal signaling events that unleash the powerful inflammatory activities of TLR4, and discuss how these recent advances may shape future research.
Proteins that regulate LPS-inducible movement and signaling activities of TLR4
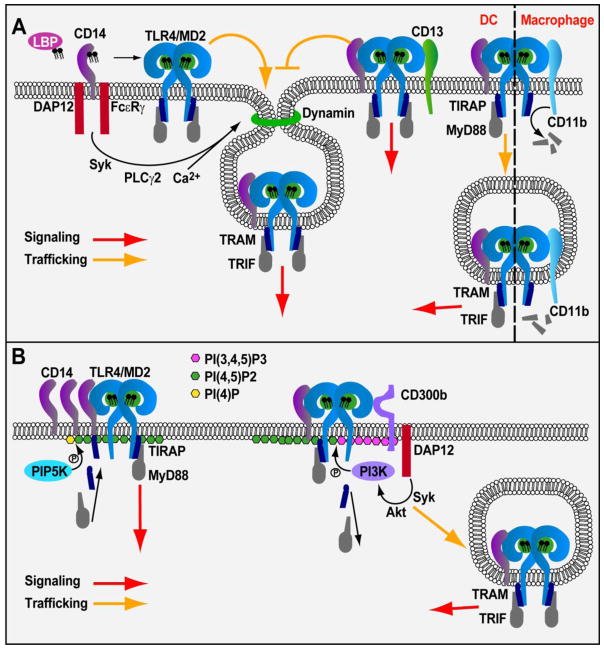
TLR4 first encounters LPS in the extracellular space, either upon interaction with intact bacteria or upon exposure to soluble LPS aggregates. Upon LPS detection, TLR4 rapidly induces the assembly of a Supramolecular Organizing Center (SMOC) known as the myddosome [4–6]. The myddosome minimally consists of the adaptor proteins MyD88 and TIRAP, and several serine threonine kinases of the IRAK family. This organizing center is the principle subcellular site where signals from TLR4 promote NF-κB and AP-1 activation, leading to inflammatory gene expression. Subsequently, TLR4 is internalized into endosomes, where it promotes IRF3-dependent Type-I interferon (IFN) production through the adaptor proteins TRAM and TRIF [8, 9]. Importantly, prior to signaling, several receptor-proximal proteins interact with TLR4 and regulate LPS-binding, and this process of TLR4 endocytosis. LPS-Binding Protein (LBP) binds to LPS-aggregates or bacteria, and somehow facilitates the extraction of LPS monomers by the extracellular protein CD14 (Fig. 1A) [10]. CD14 then transfers LPS to TLR4 and its associated factor MD-2 (Fig. 1A) [11]. LPS-binding promotes TLR4/MD-2 dimerization, which is a necessary step in myddosome- and TRIF-dependent responses [12]. Subsequent to TLR4 dimerization, this receptor is delivered to endosomes by a process dependent on the aforementioned LPS receptor CD14 [13]. CD14-dependent TLR4 endocytosis occurs via a SyK/PLCγ2-dependent process termed “inflammatory endocytosis”, which is regulated by the Immunoreceptor Tyrosine-based Activating Motif (ITAM) containing adaptors DAP12 and FcεRγ, dynamin GTPases, IP3R, and calcium mobilization from the endoplasmic reticulum (Fig. 1A). However, the precise interactions between these factors and TRIF signaling remain to be explained.
Figure 1.
Receptor proximal activities control TLR4 signaling. (A) Extracellular factors regulate TLR4 trafficking into the endosomal compartment. LBP facilitates CD14-dependent delivery of LPS to TLR4/MD2 complexes. MD2 then promotes dimerization of TLR4/MD2/LPS complexes allowing for signaling from the plasma membrane and selection of TLR4 as a cargo for CD14-dependent endocytosis and subsequent TRIF-dependent signaling. CD13 inhibits TLR4 endocytosis and TRIF signaling but does not inhibit myddosome responses. In DCs, CD11b participates in driving TLR4 endocytosis and works within the endosome to promote TRIF signaling. In macrophages CD11b inhibits TLR4 responses by promoting degradation of MyD88 and TRIF. (B) Receptor-proximal factors modulate PIs to control TLR4 signaling. Sorting adaptors TIRAP and TRAM are prepositioned at membranes by interactions with PIs where they engage receptor ligand complexes for signaling. CD14 can modulate TIRAP association with the membrane. LPS binding promotes CD14 aggregation and PIP5K-dependent conversion of PI(4)P to PI(4,5)P2 resulting in increased TIRAP binding and myddosome signaling. Conversely, CD300b drives PI3K activation and conversion of PI(4,5)P2 to PI(3,4,5)P3 resulting in dissociation of TIRAP and MyD88 from the membrane. By doing so, CD300b promotes receptor trafficking and TRIF signaling.
Although the proteins listed above represent regulators of TLR4 endocytosis, perhaps the most notable feature of this process is what it does not depend on. Genetic analysis demonstrated that LPS retained the ability to promote CD14 endocytosis in the absence of TLR4, whereas TLR4 could not promote endocytosis in the absence of CD14 [13]. This finding was significant as it was previously believed that the sole means by which mammalian cells response to LPS is through signaling activities of TLR4. As such, all other LPS-binding proteins were not expected to induce cellular responses themselves, but rather deliver LPS to TLR4 to induce downstream activities. CD14 endocytosis therefore revealed the first innate immune response to LPS that does not depend on TLR4. This discovery set the stage for the identification of other TLR4-independent responses to LPS, such as the activation of ROS production by BAI1 [14] and the assembly of non-canonical inflammasomes by caspase-11 (caspase4/5 in humans) [15–17].
Recent studies to understand CD14-dependent endocytosis have addressed the question of how CD14 selects TLR4-LPS complexes as cargo for inflammatory endocytosis. Deletion analysis demonstrated that the entire cytosolic tail of TLR4 could be removed without preventing endocytosis, an observation that pointed to an endocytosis-motif being present in the extracellular domain of this receptor [18]. The cargo-selection agent for TLR4 endocytosis was subsequently revealed to be MD-2, which binds to the ecto-domain of TLR4 directly [12, 19]. Functional analysis of TLR4/MD-2 interactions allowed a model to emerge whereby dimerization of TLR4 by MD-2 serves two independent functions: LPS-induced signal transduction and LPS-induced TLR4 endocytosis. The LPS binding and receptor trafficking activities of CD14 and MD-2 therefore distinguish these factors from classically defined chaperone proteins, which only transport ligand to receptors. For these reasons, we have proposed that CD14 and MD-2 represent a novel category of innate immune regulators called Transporters Associated with the eXecution of Inflammation (TAXI) proteins [18]. Future work is needed to determine whether other ligand-binding chaperones also have TAXI activities.
Cell type specific activities in TLR4 endocytosis and signaling
While we often consider studies of TLR4 signaling in one cell type to be representative of all cells, there are several examples of cell-type specific LPS responses. An example at the receptor proximal level came from recent studies of the integrin CD11b. CD11b can bind LPS and promote TLR4 signaling [20–22] and importantly, may participate in LPS uptake into cells [23]. Recent work has demonstrated an intriguing aspect of CD11b biology, in that it promotes TLR4 endocytosis and TRIF signaling in dendritic cells (DCs) but not macrophages (Fig. 1A) [24]. It was proposed that CD11b plays a role in LPS responses in DCs due to lower CD14 expression in these cells, as compared with macrophages. Indeed, CD11b was required for efficient endocytosis of TLR4 in DCs, but this requirement could be overcome by upregulating the expression of CD14 by stimulating CD11b-deficient cells with CpG-DNA. Under these conditions, CD14 levels at the plasma membrane increase, and this receptor then promotes efficient TLR4 entry into CD11b-deficient cells. Interestingly, despite the ability of CpG-DNA to rescue the TLR4 endocytosis defect of CD11b-deficient DCs, TRIF signaling from endosomes was still defective under these conditions. Thus, CD11b somehow functions within the endosomal compartment to promote TLR4 signaling through TRIF (Fig. 1A). The precise mechanism by which CD11b regulates TRIF signaling remains to be explored.
In addition to its role in promoting TLR4 endocytosis in DCs, CD11b can also inhibit responses to LPS in macrophages by promoting degradation of the myddosome components TIRAP and MyD88 (Fig. 1A) [25]. Thus, the role of CD11b in LPS sensing is complex. Additional future questions regarding this receptor include: what is the mechanism by which CD11b promotes TLR4-LPS binding and how does it facilitate endocytosis? Furthermore, as will be discussed below, CD11b functions to modulate lipid metabolism to regulate TLR4 adaptor protein localization [26]. Whether this activity of CD11b is important to regulate endocytosis or TRIF signaling in DCs is unknown.
Negative regulation of TLR4 endocytosis
An additional potential regulator of TLR4 endocytosis is the metallopeptidase, CD13. CD13 is upregulated during LPS stimulation and functions to restrict TRIF signaling, but not myddosome signaling, in DCs and macrophages (Fig. 1A) [27]. CD13-deficient DCs exhibit enhanced TLR4 endocytosis, suggesting its involvement in regulating the endocytic process. Exactly how CD13, which binds directly to TLR4, inhibits TLR4 endocytosis remains an open question. The mechanism is not likely to be through inhibition of known TLR4 endocytic determinants such as MD-2 or CD14, as myddosome signaling remains intact in CD13-deficient cells and CD13 does not affect CD14 endocytosis or consistently co-localize with CD14 in endosomes [27]. Importantly, the effects of CD13 on myeloid cells may have consequences in vivo as dysregulation of TRIF signaling results in increased iNOS expression and potentially limits tissue repair during ischemic injury in CD13-deficient mice. However, as CD13 appears to have multiple functions [27], future research will be necessary to dissect its important roles in vivo.
Phosphoinositide regulation of TLR4 signaling
The observation that TLR4 signaling and trafficking are interconnected processes places this receptor at the nexus of phosphoinositide (PI) biology, as these lipids control virtually all membrane transport events in eukaryotic cells [28]. Interestingly, several proteins that regulate TLR4 signaling interact with PIs, and these interactions are critical for promoting inflammatory responses to LPS. The TLR4 regulatory proteins that interact with PIs are TIRAP and TRAM, which are collectively called sorting adaptors [29]. Sorting adaptors are unique among TLR4 regulatory factors in that they are the only proteins that are found at the sites of signaling, before signaling has occurred. This feature is provided by their abilities to bind PIs found on organelles that represent eventual site(s) of TLR4 signal transduction. TIRAP localizes to the inner leaflet of the plasma membrane by binding to phosphatidylinositol (4,5) bisphosphate (PI(4,5)2) (Fig. 1B) [30], but also interacts with other acidic PIs (e.g. PI(3)P)) to permit its endosomal localization [4]. TRAM is also found at the plasma membrane and on early endosomes, but its mechanism of localization differs from TIRAP. TRAM contains a bipartite localization domain that contains an N-terminal myristoylation sequence and a neighboring PI binding domain [31]. These sorting adaptors sense dimerized TLR4 at the cell surface or on endosomes, and subsequently induce myddosome formation or TRIF signaling to promote inflammation.
Not only can PIs control where TLR4 signaling happens, but their modulation by kinases and phosphatases can also serve to control when TLR4 signaling occurs. As a key example, PI(4,5)P2 synthesis is controlled at the plasma membrane of macrophages by signaling via the aforementioned integrin CD11b and the small GTPase ARF6. These proteins regulate type-I PI4-phosphate 5-kinases (PIP5Ks), which convert PI(4)P to PI(4,5)P2 [26]. PIP5K activity is necessary for steady state TIRAP localization and myddosome signaling [26]. Interestingly, recent studies in microglia demonstrate that LPS stimulation activates PIP5K isoform α to promote increased PI(4,5)P2 synthesis at the plasma membrane [32]. PIP5Kα-dependent PI(4,5)P2 production resulted in TIRAP enrichment at the cell surface and is required for normal levels of inflammatory signaling. Furthermore, PIP5Kα co-localized with and bound to TIRAP. As TIRAP’s lipid-binding domain is sufficient to bind PI(4,5)P2 [26], its association with PIP5Kα does not likely determine TIRAP’s localization, but may participate in stabilizing TIRAP at the membrane.
Following TLR4 signaling, PI(4,5)P2 and TIRAP levels return to pre-LPS stimulation levels [32]. Thus, PI(4,5)P2 is required for initiation of TLR4 signaling at the plasma membrane and its modulation provides a mechanism to rapidly increase (and subsequently decrease) the signaling potential of the TLR4 pathway. However, the mechanism by which LPS signaling promotes PIP5Kα activation and PI(4,5)P2 synthesis have been unclear. An important recent finding suggests that CD14 regulates this process. In macrophages, LPS induces aggregation of CD14 in plasma membrane lipid rafts (Fig. 1B) [33]. This CD14 aggregation promotes increased PI(4,5)P2 synthesis and drives increased inflammatory signaling. Notably, this process proceeds in cells that express CD14 and not TLR4, highlighting LPS-induced PI(4,5)P2 synthesis as another process that is independent of TLR4. As with microglial cells, PI(4,5)P2 enrichment after LPS stimulation was dependent on PIP5K isoforms, however both PIP5Kα and γ were shown to contribute to this process (Fig. 1B) [33]. Since CD14 does not have a cytosolic tail for direct signaling to intracellular regulators, the mechanism by which CD14 signals to promote PIP5K α and γ activation is not immediately clear. It was suggested that the mechanism may be analogous to the process by which CD14 utilizes its lipid raft localization to induce Src-PLCγ2-calcineurin-dependent nuclear NFAT translocation [33, 34]. Alternatively, as CD14 can also activate other kinases such as Syk in response to LPS [13], additional pathways may be responsible for activation of PIP5K. Ultimately, understanding the activities of CD14 will be necessary to understand phosphoinositide regulation of TLR4 signaling.
Recent findings suggest that additional receptor-proximal factors can regulate sorting adaptor localization and modulate the inflammatory response. The type-I transmembrane receptor, CD300b was first implicated in responses to LPS through the ability of its cleaved soluble form to amplify LPS-induced sepsis [35]. Subsequently, a role for CD300b in binding LPS and associating with TLR4 and CD14 was suggested (Fig. 1B) [36]. CD300b promoted activation of the TRIF pathway and IFNβ production, and suppressed MyD88-dependent activation of ERK1/2, collectively leading to reduced production of anti-inflammatory IL-10 and increased inflammatory cytokines. Furthermore, much like CD14, CD300b activated Syk via the ITAM-containing adaptor DAP12. However, if CD300b-dependent activation of Syk plays a role in inflammatory endocytosis remains unclear. Syk activation induces the phosphorylation of Akt, and the activation of phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) (Fig. 1B) [36]. Interestingly, after LPS-stimulation, TIRAP-MyD88 interaction with TLR4 was decreased in a CD300b-dependent manner, suggesting a deficiency in myddosome formation. As PI3K is an important enzyme for converting PI(4,5)P2 to phosphatidylinositol-3,4,5 triphosphate (PI(3,4,5)P3) and TIRAP binds PI(4,5)P2 to remain at the plasma membrane, potentially, CD300b-dependent PI3K activation is responsible for dissociation of TIRAP and MyD88 from TLR4 (Fig. 1B). CD300b-dependent dissociation of TIRAP and MyD88 is also likely responsible for reduction in ERK signaling and may facilitate re-directing TLR4 signaling to the endosomal TRAM-TRIF pathway, ultimately leading to lethal sepsis [36]. However, it remains unclear if the activation of PI3K or alternative mechanisms such as increased endocytosis of TLR4 are responsible for the effects of CD300b on the TLR4 pathway. Overall, these examples highlight the ability of extracellular receptor-proximal activities to control phospholipid synthesis and dictate the location and type of response elicited by LPS stimulation.
Bacterial inhibition of receptor-proximal activities
The importance of receptor-proximal activities is exemplified by the discovery of bacterial strategies to evade them. Bacteria use direct and indirect mechanisms to evade TLR4 signaling (See reviews [37, 38]). Notably, producing TLR4 evasive LPS is effective against CD14 and MD-2 functions. The Lipid A component of LPS, which is responsible for most of its stimulatory capability [39], can contain varying numbers of acyl chains and phosphorylation states, depending on the organism. Escherichia coli LPS, which possesses six acyl chains and is di-phosphorylated, represents the benchmark for TLR4 stimulation. Comparatively, organisms with less than six acyl chains, such as the Yersinia spp., or the environmental bacterium, Rhodobacter sphaeroides, produce less stimulatory LPS [40, 41]. Interestingly, penta-acylated Rhodobacter-LPS is unable to promote TLR4-dimerization and endocytosis, but it efficiently promotes endocytosis of CD14 [18]. This ability to create an inducible CD14 deficiency at the plasma membrane likely explains its ability to serve as a TLR4 signaling inhibitor.
In contrast to Rhodobacter-LPS, hypo-acylated, mono-phosphorylated LPS of the human pathogen, Francisella tularensis, has no ability to engage any LPS receptor [18]. Francisella LPS cannot promote CD14 endocytosis, TLR4 dimerization, endocytosis or signaling. Thus, in the case of this important human pathogen, Francisella has altered its LPS to be immunologically silent. Commensal bacteria of the human intestine also manipulate their LPS structure, with immunological consequences. For example, LPS from the commensal bacterium Bacteriodes thetaiotaomicron, which possesses a penta-acylated, mono-phosphorylated LPS, weakly activated TLR4/MD-2 and CD14 functions [18]. When this organism was rendered incapable of de-phosphorylating its LPS, it could no longer evade CD14, and TLR4 signaling was more efficient. Taken together, pathogenic and commensal bacteria employ similar strategies for survival in the face of TLR4-dependent innate immunity and can use de-phosphorylation of their LPS to evade CD14 and subsequent TLR4/MD-2 related processes. These findings illustrate the importance of receptor-proximal activities in defense against bacteria and highlight the potential for these strategies to reveal important aspects of TLR4 biology. Identification of additional bacterial strategies to evade receptor-proximal components will likely uncover novel aspects of TLR4 regulation.
Perspective
Despite advances in understanding of innate immunity, no effective therapies are currently available for the treatment of sepsis [42]. Therefore, the need remains to fully understand all aspects of the immune response to endotoxin. We suggest that the future of research into TLR4 signaling lies with understanding the synergy between TLR4 pathway components and cell biological activities. We highlight the fact that receptor-proximal components direct all of the underlying cell biological functions that define where and when TLR4 signaling can occur. Future research will likely aid in our understanding of how receptor-proximal activities promote appropriate responses during infection.
Highlights.
MD-2 and CD14 regulate the transport and signaling functions of TLR4.
LPS-induced CD14 aggregation modulates PIP5K activity to promote myddosome signaling downstream of TLR4.
Pathogenic and commensal bacteria modulate LPS structures to evade CD14 and MD-2 functions.
Acknowledgments
We would like to thank the members of the Kagan lab for helpful discussions. JCK is supported by NIH grants AI093589 and P30 DK34854, and an unrestricted gift from Mead Johnson & Company. Dr. Kagan holds an Investigators in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund. CVR is supported by Crohn’s & Colitis Foundation of America CDA 410198.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 2.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 3.Tan Y, Kagan JC. A cross-disciplinary perspective on the innate immune responses to bacterial lipopolysaccharide. Mol Cell. 2014;54:212–223. doi: 10.1016/j.molcel.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonham KS, Orzalli MH, Hayashi K, Wolf AI, Glanemann C, Weninger W, Iwasaki A, Knipe DM, Kagan JC. A promiscuous lipid-binding protein diversifies the subcellular sites of toll-like receptor signal transduction. Cell. 2014;156:705–716. doi: 10.1016/j.cell.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Motshwene PG, Moncrieffe MC, Grossmann JG, Kao C, Ayaluru M, Sandercock AM, Robinson CV, Latz E, Gay NJ. An oligomeric signaling platform formed by the Toll-like receptor signal transducers MyD88 and IRAK-4. The Journal of biological chemistry. 2009;284:25404–25411. doi: 10.1074/jbc.M109.022392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin SC, Lo YC, Wu H. Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature. 2010;465:885–890. doi: 10.1038/nature09121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kagan JC, Magupalli VG, Wu H. SMOCs: supramolecular organizing centres that control innate immunity. Nat Rev Immunol. 2014;14:821–826. doi: 10.1038/nri3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitzgerald KA, Rowe DC, Barnes BJ, Caffrey DR, Visintin A, Latz E, Monks B, Pitha PM, Golenbock DT. LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF. The Journal of experimental medicine. 2003;198:1043–1055. doi: 10.1084/jem.20031023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 10.Schumann RR. Old and new findings on lipopolysaccharide-binding protein: a soluble pattern-recognition molecule. Biochemical Society transactions. 2011;39:989–993. doi: 10.1042/BST0390989. [DOI] [PubMed] [Google Scholar]
- 11.Gioannini TL, Teghanemt A, Zhang D, Coussens NP, Dockstader W, Ramaswamy S, Weiss JP. Isolation of an endotoxin-MD-2 complex that produces Toll-like receptor 4-dependent cell activation at picomolar concentrations. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4186–4191. doi: 10.1073/pnas.0306906101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 13.Zanoni I, Ostuni R, Marek LR, Barresi S, Barbalat R, Barton GM, Granucci F, Kagan JC. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell. 2011;147:868–880. doi: 10.1016/j.cell.2011.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Billings EA, Lee CS, Owen KA, D’Souza RS, Ravichandran KS, Casanova JE. The adhesion GPCR BAI1 mediates macrophage ROS production and microbicidal activity against Gram-negative bacteria. Sci Signal. 2016;9:ra14. doi: 10.1126/scisignal.aac6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science. 2013;341:1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszynski A, et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 17.Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- ••18.Tan Y, Zanoni I, Cullen TW, Goodman AL, Kagan JC. Mechanisms of Toll-like Receptor 4 Endocytosis Reveal a Common Immune-Evasion Strategy Used by Pathogenic and Commensal Bacteria. Immunity. 2015;43:909–922. doi: 10.1016/j.immuni.2015.10.008. Demonstrates that TLR4 dimerization by MD2 selects TLR4 as cargo for CD14-dependent inflammatory endocytosis. Reveals that both pathogenic and commensal bacteria produce LPS variants that are capable of inhibiting both CD14 and MD2 function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim HM, Park BS, Kim JI, Kim SE, Lee J, Oh SC, Enkhbayar P, Matsushima N, Lee H, Yoo OJ, et al. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell. 2007;130:906–917. doi: 10.1016/j.cell.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Ingalls RR, Arnaout MA, Golenbock DT. Outside-in signaling by lipopolysaccharide through a tailless integrin. Journal of immunology. 1997;159:433–438. [PubMed] [Google Scholar]
- 21.Flo TH, Ryan L, Kilaas L, Skjak-Braek G, Ingalls RR, Sundan A, Golenbock DT, Espevik T. Involvement of CD14 and beta2-integrins in activating cells with soluble and particulate lipopolysaccharides and mannuronic acid polymers. Infection and immunity. 2000;68:6770–6776. doi: 10.1128/iai.68.12.6770-6776.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perera PY, Mayadas TN, Takeuchi O, Akira S, Zaks-Zilberman M, Goyert SM, Vogel SN. CD11b/CD18 acts in concert with CD14 and Toll-like receptor (TLR) 4 to elicit full lipopolysaccharide and taxol-inducible gene expression. Journal of immunology. 2001;166:574–581. doi: 10.4049/jimmunol.166.1.574. [DOI] [PubMed] [Google Scholar]
- 23.Scott MJ, Billiar TR. Beta2-integrin-induced p38 MAPK activation is a key mediator in the CD14/TLR4/MD2-dependent uptake of lipopolysaccharide by hepatocytes. The Journal of biological chemistry. 2008;283:29433–29446. doi: 10.1074/jbc.M803905200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •24.Ling GS, Bennett J, Woollard KJ, Szajna M, Fossati-Jimack L, Taylor PR, Scott D, Franzoso G, Cook HT, Botto M. Integrin CD11b positively regulates TLR4-induced signalling pathways in dendritic cells but not in macrophages. Nat Commun. 2014;5:3039. doi: 10.1038/ncomms4039. Describes the cell-type specific importance of CD11b in regulating TLR4 signaling. CD11b binds to TLR4 and promotes TLR4 endocytosis and TRIF signaling independently of CD14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han C, Jin J, Xu S, Liu H, Li N, Cao X. Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat Immunol. 2010;11:734–742. doi: 10.1038/ni.1908. [DOI] [PubMed] [Google Scholar]
- 26.Kagan JC, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell. 2006;125:943–955. doi: 10.1016/j.cell.2006.03.047. [DOI] [PubMed] [Google Scholar]
- •27.Ghosh M, Subramani J, Rahman MM, Shapiro LH. CD13 restricts TLR4 endocytic signal transduction in inflammation. Journal of immunology. 2015;194:4466–4476. doi: 10.4049/jimmunol.1403133. Demonstrates that CD13 inhbits TRIF signaling, but not myddosome signaling, by supressing endocytosis of TLR4. CD13 may participate in tissue repair during ischemic injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schink KO, Tan KW, Stenmark H. Phosphoinositides in Control of Membrane Dynamics. Annu Rev Cell Dev Biol. 2016 doi: 10.1146/annurev-cellbio-111315-125349. [DOI] [PubMed] [Google Scholar]
- 29.Kagan JC. Defining the subcellular sites of innate immune signal transduction. Trends Immunol. 2012;33:442–448. doi: 10.1016/j.it.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marek LR, Kagan JC. Phosphoinositide binding by the Toll adaptor dMyD88 controls antibacterial responses in Drosophila. Immunity. 2012;36:612–622. doi: 10.1016/j.immuni.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen TTN, Kim YM, Kim TD, Le OTT, Kim JJ, Kang HC, Hasegawa H, Kanaho Y, Jou I, Lee SY. Phosphatidylinositol 4-phosphate 5-kinase α facilitates Toll-like receptor 4-mediated microglial inflammation through regulation of the Toll/interleukin-1 receptor domain-containing adaptor protein (TIRAP) location. Journal of Biological Chemistry. 2013;288:5645–5659. doi: 10.1074/jbc.M112.410126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••33.Płóciennikowska A, Zdioruk MI, Traczyk G, Świątkowska A, Kwiatkowska K. LPS-induced clustering of CD14 triggers generation of PI (4, 5) P2. J Cell Sci. 2015;128:4096–4111. doi: 10.1242/jcs.173104. Demonstrates that LPS-induced CD14 aggregation in lipid rafts promotes PIP5K activity and increased PI(4,5)P2 at the membrane. Increased PI(4,5)P2 production drives TLR4 signlaing from the plasma membrane. [DOI] [PubMed] [Google Scholar]
- 34.Zanoni I, Ostuni R, Capuano G, Collini M, Caccia M, Ronchi AE, Rocchetti M, Mingozzi F, Foti M, Chirico G, et al. CD14 regulates the dendritic cell life cycle after LPS exposure through NFAT activation. Nature. 2009;460:264–268. doi: 10.1038/nature08118. [DOI] [PubMed] [Google Scholar]
- 35.Yamanishi Y, Takahashi M, Izawa K, Isobe M, Ito S, Tsuchiya A, Maehara A, Kaitani A, Uchida T, Togami K, et al. A soluble form of LMIR5/CD300b amplifies lipopolysaccharide-induced lethal inflammation in sepsis. Journal of immunology. 2012;189:1773–1779. doi: 10.4049/jimmunol.1201139. [DOI] [PubMed] [Google Scholar]
- ••36.Voss OH, Murakami Y, Pena MY, Lee HN, Tian L, Margulies DH, Street JM, Yuen PS, Qi CF, Krzewski K, et al. Lipopolysaccharide-Induced CD300b Receptor Binding to Toll-like Receptor 4 Alters Signaling to Drive Cytokine Responses that Enhance Septic Shock. Immunity. 2016;44:1365–1378. doi: 10.1016/j.immuni.2016.05.005. Demonstrates that CD300b activates PI3K to inhibit myddosome signaling and promote TLR4 endocytosis and TRIF signaling. In doing so, CD300b promotes loss of ERK signaling and increased TRIF signaling leading to enhanced cytokine responses and lethal sepsis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosadini CV, Kagan JC. Microbial strategies for antagonizing Toll-like-receptor signal transduction. Curr Opin Immunol. 2015;32:61–70. doi: 10.1016/j.coi.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reddick LE, Alto NM. Bacteria fighting back: how pathogens target and subvert the host innate immune system. Mol Cell. 2014;54:321–328. doi: 10.1016/j.molcel.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rietschel ET, Kirikae T, Schade FU, Mamat U, Schmidt G, Loppnow H, Ulmer AJ, Zahringer U, Seydel U, Di Padova F, et al. Bacterial endotoxin: molecular relationships of structure to activity and function. Faseb J. 1994;8:217–225. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- 40.Montminy SW, Khan N, McGrath S, Walkowicz MJ, Sharp F, Conlon JE, Fukase K, Kusumoto S, Sweet C, Miyake K. Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat Immunol. 2006;7:1066–1073. doi: 10.1038/ni1386. [DOI] [PubMed] [Google Scholar]
- 41.Golenbock DT, Hampton RY, Qureshi N, Takayama K, Raetz CR. Lipid A-like molecules that antagonize the effects of endotoxins on human monocytes. The Journal of biological chemistry. 1991;266:19490–19498. [PubMed] [Google Scholar]
- 42.Marshall JC. Why have clinical trials in sepsis failed? Trends in molecular medicine. 2014;20:195–203. doi: 10.1016/j.molmed.2014.01.007. [DOI] [PubMed] [Google Scholar]