Abstract
A major challenge for treating cocaine addiction is the propensity for abstinent users to relapse. Two important triggers for relapse are cues associated with prior drug use and stressful life events. To study their interaction in promoting relapse during abstinence, we used the incubation model of craving and relapse in which cue-induced drug seeking progressively intensifies (“incubates”) during withdrawal from extended-access cocaine self-administration. We tested rats for cue-induced cocaine seeking on withdrawal day (WD) 1. Rats were then subjected to repeated restraint stress or control conditions (7 sessions held between WD6-WD14). All rats were tested again for cue-induced cocaine seeking on WD15, one day after the last stress or control session. Although controls showed a time-dependent increase in cue-induced cocaine seeking (incubation), rats exposed to repeated stress in early withdrawal exhibited a more robust increase in seeking behavior between WD1 and WD15. In separate stressed and control rats, equivalent cocaine seeking was observed on WD48. These results indicate that repeated stress in early withdrawal accelerates incubation of cocaine craving, although craving plateaus at the same level observed in controls. However, one month after the WD48 test, rats subjected to repeated stress in early withdrawal showed enhanced cue-induced cocaine seeking following acute (24 h) food deprivation stress. Together, these data indicate that chronic stress exposure enhances the initial rate of incubation of craving during early withdrawal, resulting in increased vulnerability to cue-induced relapse during this period, and may lead to a persistent increase in vulnerability to the relapse-promoting effects of stress.
Keywords: chronic stress, cocaine, incubation of craving, repeated restraint stress, self-administration
INTRODUCTION
One of the greatest challenges to treating cocaine addiction is the high rate of relapse in abstinent users. Stress is one of the most common triggers for relapse, but the underlying neuronal mechanisms are not fully understood. Human studies indicate that exposure to chronic adverse life events is associated with increased relapse vulnerability (i.e., Sinha 2001, 2008), indicating a need for animal models that explore interactions between chronic stress exposure and drug abstinence. However, over the past two decades, most animal work on stress-induced relapse has studied acute stress-induced reinstatement of previously extinguished drug seeking behavior using the reinstatement model of relapse (for review see Shaham et al. 2000; Sinha et al. 2011; Mantsch et al. 2016). While the reinstatement model has greatly advanced our understanding of the neurobiology of drug addiction and relapse vulnerability, relapse in human addicts typically occurs after a drug-free withdrawal period rather than extinction training (Fuchs et al. 2008). Furthermore, studies have shown that extinction training produces distinct neuroadaptations from those observed in rats undergoing abstinence alone (i.e., Sutton et al. 2003; Self et al. 2004; Ghasemzadeh et al. 2009; Knackstedt et al. 2010) and that cocaine seeking in rats that have undergone extinction involves different neural substrates compared to rats studied after abstinence alone (Fuchs et al. 2006). Finally, because seeking behavior must first be extinguished before acute stress-induced reinstatement can be studied, it is difficult to use this model to assess how chronic stress exposure alters relapse vulnerability over time. Together, these findings indicate the need for a rat model that can assess the interplay between cocaine withdrawal and repeated stress exposure on subsequent cue-induced cocaine seeking behavior.
To fill this gap, we assessed the effects of chronic stress exposure on relapse vulnerability using the ‘incubation of craving’ model, in which cue-induced craving in rats progressively intensifies (“incubates”) during withdrawal from extended-access self-administration of cocaine or other addictive drugs (Lu et al. 2004a,b; Pickens et al. 2011; Loweth et al. 2013, 2014a; Wolf, 2016). Incubation provides an animal model for a common human scenario in which withdrawal is imposed by incarceration or hospitalization (Reichel & Bevins 2009) and has recently been demonstrated in humans during abstinence from cocaine (Parvaz et al. 2016) as well as nicotine (Bedi et al. 2011), methamphetamine (Wang et al. 2013) and alcohol (Li et al. 2014). Many studies have investigated the ability of an acute stressor to reinstate previously extinguished cocaine seeking (for review see Shaham et al. 2000 and Mantsch et al. 2016). However, none have investigated the effects of repeated stress exposure on the incubation of cue-induced cocaine seeking, although a few studies have used this strategy to study chronic stress-induced changes in heroin seeking (D’Cunha et al. 2013; Sedki et al. 2013, 2015). The goal of our study was to explore this interaction by assessing the effects of chronic stress exposure during withdrawal on incubated cocaine craving in order to identify time-dependent changes in relapse vulnerability to both cues and stress.
METHODS
Subjects and Surgery
All procedures were approved by the Rosalind Franklin University of Medicine and Science Animal Care and Use Committee in accordance with the USPHS Guide for Care and Use of Laboratory Animals. Male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 250–275g upon arrival were group housed (3/cage) on a reverse light/dark cycle (lights off at 8:00AM, on at 8:00PM) with food and water freely available. After an acclimation period (5–7 days), rats underwent intravenous catheter surgery. As described previously (Loweth et al. 2014b), rats were anesthetized with ketamine-xylazine (80–10 mg/kg, i.p., respectively) and a silastic catheter (Plastics One, Roanoke, VA) was inserted into the right jugular vein and passed subcutaneously to the mid-scapular region. Immediately following surgery and for the remainder of the experiment, rats remained singly-housed in their home cages and were handled 2–3 times per week. Following surgery, rats were allowed a 5–7 day recovery period, during which time their catheters were flushed every 24–48 h with cefazolin (15 mg, i.v.; Webster Veterinary Supply, Devens, MA) dissolved in 0.9% sterile saline.
Cocaine self-administration
After the recovery period, rats were trained to self-administer cocaine. As described previously (Loweth et al. 2014b), rats underwent 10 daily sessions, each lasting 6 h, under a fixed-ratio-1 reinforcement schedule. Cocaine was dissolved in saline and self-administered at a dose of 0.5mg/kg/infusion (~0.030 mL/infusion). Training was conducted in self-administration chambers (MED Associates, St. Albans, VT) equipped with two nose-poke holes, an active hole and an inactive hole, located on opposite sides of the chamber (2 cm above the floor). Active hole responses activated the infusion pump and led to the delivery of a 20-sec light cue and a 20-sec timeout period during which nose pokes were recorded, but did not result in an infusion. Nose poking in the inactive hole had no consequences but was recorded as a measure of nonspecific behavioral activation. Self-administration sessions started at the onset of the dark cycle. Any rats that did not learn to self-administer cocaine and/or had faulty catheters were euthanized.
Restraint Stress & Tests for Cue-Induced Cocaine Seeking
Seeking tests were conducted using a within-subjects design in which time-dependent changes in cocaine seeking were assessed by comparing WD1 to a later time-point. A total of 45 rats were tested on WD1 and WD15, while 25 rats were tested on WD1 and WD48. On WD1, all rats received a 30-min test for cue-induced cocaine seeking, during which nose-pokes resulted in presentation of the light cue but not cocaine. The number of responses that rats make under these conditions is the operational measure of cue-induced cocaine craving. Rats were then divided into two equivalent groups destined for either control or repeated restraint stress conditions. Then, from WD6-WD14, rats underwent repeated restraint stress or control conditions as described previously (Rosenkranz 2010; Padival et al. 2013; Zhang & Rosenkranz 2012) and below in more detail.
Rats in the repeated restraint stress group were placed in a restraint hemicylinder for 20 min once a day for 5 days, followed by 2 days off and then an additional 2 days of restraint (for a total of 7 sessions over a 9 day period). Controls were placed in a transparent cage with bedding on the same schedule. This pattern of stress exposure was selected because it minimizes inter-session habituation to the stressor and has been shown to increase adrenal gland weight and anxiety levels as measured by a decrease in elevated plus maze exploration (Rosenkranz et al. 2010; Zhang & Rosenkranz 2012). All stress exposure or control manipulations took place during the dark cycle and were conducted in the afternoon (2:00pm–4:00pm) so that their timing was distinct from the start time of self-administration sessions or seeking tests (both of which began between 8:00am–9:00am, just after the start of the dark cycle). On WD15 or WD48, rats underwent a second cue-induced seeking test (30 min). The rats that were tested on WD48 underwent additional withdrawal in their home cages and, on WD73, food was removed from all rats for 24 hours (at 9:00am). Water was freely available during this period. Exactly 24 hours later (WD74 at 9:00am), all rats were tested for cue-induced cocaine seeking. One outlier was excluded from this study due to active hole counts that were > 2 SDEV from the mean. In a control study designed to determine if acute restraint stress would mimic the effects of repeated stress, rats underwent self-administration and a seeking test on WD1, as described above, and then received a single restraint stress or control session on WD14. On WD15, these animals underwent a second cue-induced seeking test (30 min).
Statistical Analyses
For analyses of self-administration training data, the number of daily infusions, active hole responses and inactive hole responses were averaged over days 1 through 10 of cocaine self-administration and groups were compared using Student’s t-tests. All training data are expressed as raw values (mean ± SEM). For analyses of changes in cocaine seeking over time (incubation experiments), nose-poke responses were analyzed using between-within ANOVA with treatment group (control vs stress) as the between factor and test day (WD1 vs WD15 or WD1 vs WD48) as the within factor. Bonferroni post-hoc tests were used for group comparisons with the exception of the acute food deprivation study, in which just one time point was analyzed and groups were therefore compared using a Student’s t-test. All seeking test data are expressed as raw values (mean ± SEM). Differences between experimental conditions were considered statistically significant when p<0.05.
RESULTS
Repeated restraint stress exposure accelerates incubation of cue-induced cocaine seeking
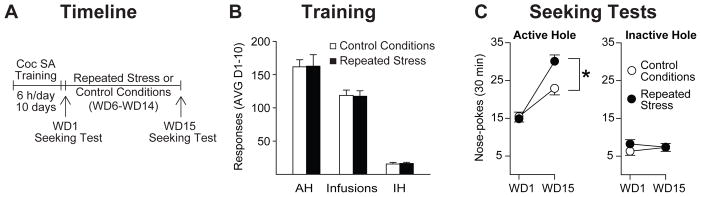
Rats were trained to self-administer cocaine under extended-access conditions (6 h/day for 10 days) and were divided into two groups destined for control conditions (n=13) or repeated stress exposure (n=13) (Fig. 1A). Rats assigned to these groups did not differ in the average number of infusions obtained during self-administration training (t24=0.05, p=0.96), nor were there differences in average active hole (t24=0.05, p=0.96) or inactive hole (t14=0.32, p=0.75) nose-poke responding over the 10 days of self-administration training (Fig. 1B). Rats were then tested for cue-induced cocaine seeking on WD1, followed by a withdrawal period during which rats were exposed to repeated restraint stress sessions or control conditions (7 sessions over a 9 day period from WD6-WD14, 20 min per session). This particular epoch of withdrawal was chosen for the stress exposure based on previous studies showing that, when compared to WD1, cue-induced cocaine craving has incubated to maximal levels after one month of withdrawal and remains at similar levels after 3 months of withdrawal (Lu et al. 2004a,b). Since the first few weeks of withdrawal are when the most rapid change in cue-induced cocaine seeking behavior is occurring, we wanted to assess how stress exposure during this early period affects the time-course of incubation of cocaine craving. All rats were tested again for cue-induced cocaine seeking on WD15, one day after the last restraint stress or control session (see timeline in Fig. 1A).
Figure 1. Repeated restraint stress exposure during early withdrawal accelerates incubation of cue-induced cocaine craving.
A. Timeline: Rats were trained to self-administer cocaine for 6 h/day for a total of 10 days, and tested for cue-induced cocaine seeking on either WD1 or WD15. During this two week withdrawal period, rats were exposed to repeated restraint stress sessions or control conditions (7 sessions over a 9 day period, from WD6-WD14). B. Self-Administration Training: Data are presented as mean ± SEM number of cocaine infusions and active hole (AH) and inactive hole (IH) nose-pokes averaged over the 10 days of self-administration training. C. Seeking Tests: Nose-pokes in the previously active hole (left) and the inactive hole (right) during the seeking tests on WD1 and WD15. During the seeking tests, active hole nose-pokes led to contingent presentation of a 20-sec light cue previously paired with each cocaine injection. The number of responses made under these conditions is the operational measure of cue-induced cocaine craving. Nose-pokes in the inactive hole had no consequences. Data are expressed as mean ± SEM number of active and inactive hole nose-pokes. While controls demonstrated a time-dependent increase in cue-induced cocaine seeking behavior on WD15 compared to WD1 (i.e., incubation), animals that were exposed to repeated restraint stress showed significantly greater seeking compared to controls on WD15 (*p<0.05). AVG, average; SA, self-administration; WD, withdrawal day.
As expected, animals that underwent extended-access cocaine self-administration and control conditions during early withdrawal showed greater cue-induced cocaine seeking on WD15 compared to WD1 (i.e., incubation of craving; Fig. 1C). Animals that were exposed to repeated restraint stress during the early withdrawal period showed a more robust increase in seeking behavior on WD15 (Fig. 1C). The ANOVA conducted on these data revealed no significant effect of treatment group (F1,24=2.95, p=0.099) but a significant effect of test day (F1,24=67.87, p<0.001) and a significant treatment group x test day interaction (F1,24=7.96, p=0.01). As expected, Bonferroni post-hoc tests revealed a significant increase in cue-induced cocaine seeking on WD15 compared to WD1 in both the controls (p=0.005) and the repeated restraint group (p<0.001) (i.e., incubation of craving). However, while there was no significant difference in seeking on WD1 between groups, animals that were exposed to repeated restraint stress showed significantly greater seeking on WD15 compared to the control group on WD15 (p=0.02, Fig. 1C) and a greater incubation effect (103% vs 48%, repeated restraint vs controls; percent increase in seeking on WD15 compared to WD1). No group differences in inactive hole responding were observed (treatment group: F1,24=0.64, p=0.43; test day: F1,24=0.01, p=0.93; treatment group x test day interaction: F1,24=1.3, p=0.27; Fig. 1C). Thus, exposure to repeated restraint stress during the first two weeks of withdrawal enhances cue-induced cocaine craving on WD15, one day after the last restraint stress session.
Acute restraint stress exposure does not accelerate incubation of cue-induced cocaine seeking
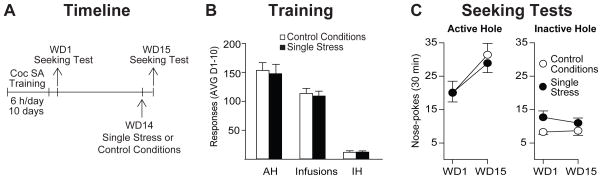
Next, we assessed whether a single restraint stress session is sufficient to enhance cue-induced cocaine seeking or whether it is the repeated stress exposure that is driving this effect. As in the first experiment, rats were trained to self-administer cocaine under extended-access conditions and were divided into two groups destined for control conditions (n=9) or a single stress exposure (n=10) (Fig. 2A). Rats assigned to these groups did not differ in the average number of infusions obtained during self-administration training (t17=0.37, p>0.05), nor were there differences in average active hole (t17=0.27, p>0.05) or inactive hole (t17=0.11, p>0.05) nose-poke responding over the 10 days of self-administration training (Fig. 2B). Rats were then tested for cue-induced cocaine seeking on WD1. On WD14, rats were exposed to a single restraint stress session (20 min) or control conditions. One day later (WD15), all rats received a second cue-induced seeking test (see timeline in Fig. 2A).
Figure 2. A single restraint stress exposure is not sufficient to accelerate incubation of cue-induced cocaine craving.
A. Timeline: As described in Fig. 1A with the exception that, in this experiment, rats received a single restraint stress exposure or control session on WD14. B. Self-Administration Training: Data are presented as mean ± SEM number of cocaine infusions and active hole (AH) and inactive hole (IH) nose-pokes averaged over the 10 days of self-administration training. C. Seeking Tests: Nose-pokes in the previously active hole (left) and the inactive hole (right) during seeking tests on WD1 and WD15 as described in detail in the legend for Fig. 1C. Data are expressed as mean ± SEM number of active and inactive hole nose-pokes. Both groups showed a similar increase in cue-induced cocaine seeking over time, indicating that a single stress exposure is not sufficient to accelerate incubation of craving. AVG, average; SA, self-administration; WD, withdrawal day.
As expected and at a magnitude similar to controls in the first experiment (Fig. 1), rats in the control group showed a time-dependent increase in cue-induced cocaine seeking on WD15 compared to WD1 (Fig. 2C). Unlike animals exposed to repeated restraint stress, animals that received a single restraint stress session exhibited a change in seeking behavior similar to that observed in controls (Fig. 2C). The ANOVA conducted on these data revealed a significant effect of test day (F1,17=16.55, p<0.001) but no significant effect of treatment group (F1,17=0.11, p=0.74) and no significant treatment group x test day interaction (F1,17=0.28, p=0.60). Indeed, both groups showed a similar increase in cue-induced cocaine seeking over time (57% vs 44%, controls vs single restraint stress; percent increase in seeking on WD15 compared to WD1). No group differences in inactive hole responding were observed (treatment group: F1,17=3.94, p=0.06; test day: F1,17=0.32, p=0.58; treatment group x test day interaction: F1,17=0.71, p=0.41; Fig. 2C). These data indicate that the facilitation of incubated cocaine craving observed following exposure to repeated restraint stress is due to chronic rather than acute stress exposure.
Repeated restraint stress exposure does not affect the long-term expression of incubation, but enhances cocaine seeking in response to acute food deprivation stress
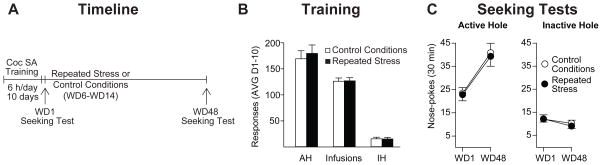
To assess the duration of stress effects on cocaine seeking, animals were generated as in Fig. 1 and seeking was assessed on WD48, weeks after the last stress exposure or control session (Fig. 3A). Rats assigned to the control group (n=13) and the repeated stress exposure group (n=12) did not differ in the average number of infusions obtained during self-administration training (t23=0.095, p>0.05), nor were there differences in average active hole (t23=0.46, p>0.05) or inactive hole (t23=0.055, p>0.05) nose-poke responding over the 10 days of self-administration training (Fig. 3B). As in the first experiment, rats were tested for cue-induced cocaine seeking on WD1 and exposed to repeated restraint stress or control conditions (7 daily sessions from WD6-WD14). After the last session on WD14, rats underwent withdrawal in their home cages for several weeks. On WD48, all rats received a second cue-induced seeking test (see timeline in Fig. 3A).
Figure 3. Repeated restraint stress exposure does not affect the long-term expression of incubated craving.
A. Timeline: As described in Fig. 1A with the exception that, in this experiment, rats received their second cue-induced seeking test on WD48, weeks after the last stress or control session. B. Self-Administration Training: Data are presented as mean ± SEM number of cocaine infusions and active hole (AH) and inactive hole (IH) nose-pokes averaged over the 10 days of self-administration training. C. Seeking Tests: Nose-pokes in the previously active hole (left) and the inactive hole (right) during seeking tests on WD1 and WD48 as described in detail in the legend for Fig. 1C. Data are expressed as mean ± SEM number of active and inactive hole nose-pokes. Both groups showed a similar increase in cue-induced cocaine seeking over time (i.e., incubation), indicating that chronic stress exposure during early withdrawal does not affect the level at which cocaine seeking ultimately plateaus. AVG, average; SA, self-administration; WD, withdrawal day.
As expected, rats in the control group showed a time-dependent increase in cue-induced cocaine seeking on WD48 compared to WD1 (Fig. 3C). The magnitude of incubation detected on WD48 (a 75% increase from WD1) was somewhat greater than that observed when control rats were tested on WD15 (a 45–50% increase from WD1), indicating that seeking has not reached maximal levels on WD15; this is expected based on prior studies showing that WD15 is on the rising phase of incubation (i.e., Grimm et al. 2001; Lu et al. 2004a,b; Loweth et al. 2014a). Interestingly, in animals exposed to repeated restraint stress during early withdrawal, the level of cue-induced cocaine seeking observed on WD48 was similar to that observed in controls, as was the change in seeking between WD1 and WD48 (a 75% increase in controls, and a 73% increase in the repeated stress group) (Fig. 3C). The ANOVA conducted on these data revealed a significant effect of test day (F1,23=54.59, p<0.001) but no significant effect of treatment group (F1,23=0.066, p=0.80) and no significant treatment group x test day interaction (F1,23=0.04, p=0.84). No group differences in inactive hole responding were observed (treatment group: F1,23=0.15, p=0.70; test day: F1,23=2.93, p=0.10; treatment group x test day interaction: F1,23=0.22, p=0.65; Fig. 3C). Together with the findings from the first experiment (Fig. 1), these data indicate that chronic stress exposure enhances cocaine craving during early withdrawal but does not affect the level at which incubation of craving ultimately plateaus.
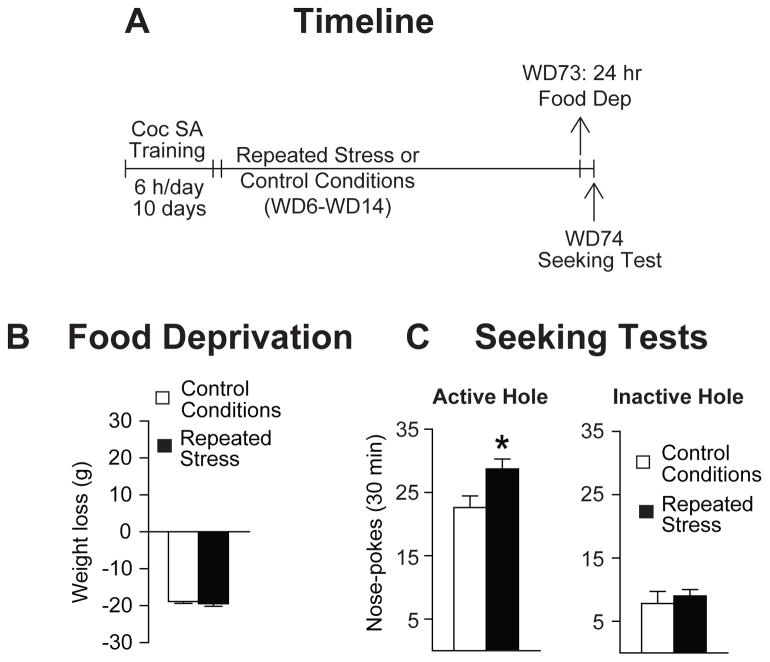
Despite this equivalence after prolonged withdrawal, we wondered whether an acute stressor might be more powerful in enhancing relapse vulnerability in rats previously exposed to repeated stress. To assess this, the same rats tested on WD48 were returned to their home cages for an additional month of withdrawal and, on WD73, underwent acute (24 h) food deprivation, immediately followed by a cue-induced seeking test on WD74 (see timeline in Fig. 4A). We chose this manipulation because our unpublished studies have shown that acute (24 h) food deprivation does not alter incubated cocaine seeking in non-stressed control rats (data not shown) and because short periods of food deprivation (3–5 days) do not alter cue-induced heroin seeking in animals undergoing withdrawal from extended-access heroin self-administration (D’Cunha et al., 2013). Following acute food deprivation, animals in both groups lost a similar amount of weight (controls: −18.9 ± 0.5 grams, −4% of initial body weight; repeated restraint stress: −19.5 ± 0.7 grams, −4% of initial body weight; Fig. 4B). Interestingly, animals that were previously exposed to repeated restraint stress showed an increase in cue-induced cocaine seeking compared to controls (t22=2.5, p=0.02; Fig. 4C). No group differences in inactive hole responding were observed (t22=0.54, p=0.59; Fig. 4C). Together, these data indicate that, while exposure to repeated restraint stress does not affect the level at which incubated craving ultimately stabilizes, it may make animals more susceptible to the relapse-promoting effects of a subsequent stressful episode (Figs. 3C & 4C). We cannot, however, rule out an alternative explanation, namely that the additional withdrawal period between the WD48 and WD74 seeking tests enabled the emergence of enhanced cue-reactivity in the previously restraint-stressed rats.
Figure 4. Rats previously exposed to repeated restraint stress show increased cocaine seeking in response to acute food deprivation stress.
A. Timeline: Rats used in Fig. 3 (see Fig. 3A legend for details) underwent additional withdrawal and, on WD73, all rats underwent acute food deprivation for 24 hours, followed by a cue-induced seeking test. B. Acute Food Deprivation: Rats previously exposed to control or repeated stress conditions lost a similar amount of weight following 24 h of food deprivation. C. Seeking Tests: Nose-pokes in the previously active hole (left) and the inactive hole (right) during a seeking test on WD74, immediately following 24 h food deprivation, as described in detail in the legend for Fig. 1C. Data are expressed as mean ± SEM number of active and inactive hole nose-pokes. Rats that received repeated restraint stress sessions showed increased cocaine seeking behavior in response to acute (24 h) food deprivation stress. *p<0.05. Note that these rats underwent 3 extinction tests including the WD74 test, which may account for somewhat lower seeking on WD74 compared to WD48 (Fig. 3). SA, self-administration; WD, withdrawal day.
DISCUSSION
We assessed the effect of chronic stress exposure in early withdrawal on the subsequent incubation of cue-induced cocaine seeking. The goal was to understand interactions between the relapse-promoting effects of cues and stress. This will help identify time periods when animals are more vulnerable to such effects and thereby inform the development of effective strategies to reduce craving and prevent relapse in abstinent cocaine addicts.
Stress exposure & incubation of craving
To study how chronic stress and cues interact to promote relapse during withdrawal, we used the incubation of craving model in which cue-induced cocaine craving intensifies during the first month of withdrawal from extended-access drug self-administration (i.e., Lu et al. 2004a,b; Pickens et al. 2011, Loweth et al. 2013, 2014a; Wolf, 2016). Our results show that chronic but not acute stress exposure during early withdrawal accelerates the incubation of cocaine craving (Figs. 1C & 2C), but does not affect the level at which craving ultimately plateaus (Fig. 3C). Furthermore, we found that animals previously exposed to chronic stress during the first two weeks of withdrawal showed enhanced cue-induced cocaine seeking on WD74, following acute (24 h) food deprivation stress (Fig. 4C), indicating that stress exposure during early withdrawal may lead to a long-lasting enhancement in the ability of a stressful episode to promote cue-induced relapse.
This is the first study to assess the effects of repeated stress exposure during withdrawal (with no extinction training) on time-dependent changes in cue-induced cocaine seeking. In contrast, much more is known about stress-induced reinstatement of cocaine seeking, in which an acute stressor administered prior to the seeking test reinstates previously extinguished drug seeking (see below). While studies using the reinstatement model have greatly advanced our understanding of relapse vulnerability, this model is not well suited to understanding how stress during abstinence may predispose towards relapse (see Introduction). In contrast, the incubation model enables us to examine how stress during forced abstinence (e.g., during an interruption of drug-taking due to hospitalization or incarceration; Reichel & Bevins, 2009) may influence the likelihood of relapse. Our finding that exposure to repeated restraint stress during the first two weeks of withdrawal accelerates incubation of cue-induced cocaine craving (Fig. 1C) indicates that the first two weeks of withdrawal may be a time period when abstinent addicts are vulnerable to the effects of stress exposure, and a time period that stress-reducing interventions could productively target. Furthermore, based on our observation that early stress enhanced the relapse promoting effect of a subsequent acute stressor presented ~2 months later (Fig. 4C), it is likely that stress reduction in early withdrawal could have lasting beneficial effects. However, additional studies are necessary to assess how stress exposure during other withdrawal periods (e.g., after one month) affects incubation of cocaine craving over time.
One question of interest is to what extent this chronic stress-induced enhancement of cocaine seeking generalizes to other stressors and other drugs of abuse. The Shalev laboratory has studied the effects of chronic stress in rats that undergo extended-access heroin self-administration followed by forced abstinence (without extinction training). Their results show that chronic mild food restriction stress (weight maintained at 90% of baseline weight) in male rats during the first two weeks of withdrawal (WD2-WD15) from heroin self-administration produces a robust increase in cue-induced heroin seeking on WD15 compared to non-food restricted controls (D’Cunha et al. 2013; Sedki et al. 2013). This is due to chronic food restriction, as a shorter (3–5 day) food restriction period did not enhance heroin seeking (D’Cunha et al., 2013). However, blocking corticotropin-releasing factor (CRF) receptors and lowering corticosterone levels via adrenalectomy did not prevent the enhanced heroin seeking observed in the food restricted rats (Sedki et al. 2013). While these findings do not rule out a role for stress hormones in the enhanced heroin seeking observed following chronic food restriction (i.e., via their downstream signaling cascades), they do suggest that, after two weeks of food restriction, stress hormones no longer directly mediate this effect. Interestingly, unpublished preliminary findings from our laboratory show that an identical period and degree of chronic mild food restriction stress in male rats (WD2-WD15) also enhances incubation of cue-induced cocaine seeking on WD15 (Loweth et al. 2015). While the role that corticosterone and CRF receptors play in mediating this effect is unknown, together these results indicate that chronic mild food restriction during the first two weeks of withdrawal enhances cue-induced craving for both cocaine and heroin. Furthermore, together with the present findings, it can be concluded that either chronic mild food restriction stress or repeated restraint stress during early withdrawal enhance subsequent cue-induced cocaine seeking (Loweth et al. 2015 & Fig. 1). Future studies will be needed to determine if similar findings are observed following repeated restraint stress in the incubation model with heroin and other drugs.
Stress exposure and drug seeking in the reinstatement model compared to the incubation model
In seeking to understand effects of stress on cue-induced relapse vulnerability, much can be learned from studies conducted over the past twenty years on stress-induced reinstatement of drug seeking after extinction training. Detailed work has been done to identify which types of stressors produce reinstatement and how the environment in which the stressor is administered can affect reinstatement, as well as how these effects vary across different drugs of abuse (Shaham et al. 2000; Mantsch et al. 2016). The main stressor studied has been acute intermittent footshock stress, administered immediately before the reinstatement session. Acute footshock stress has been shown to reinstate responding for heroin, cocaine, alcohol, nicotine and methamphetamine (see Mantsch et al. 2016 for review). In addition, one study assessed time-dependent changes in footshock-induced reinstatement of heroin seeking by conducting the extinction training and reinstatement testing on the same day at different time points (WD1, 6, 12, 25 & 66). These studies showed that lever pressing during both the extinction and the reinstatement session followed an inverted U-shape curve and that footshock reinstated seeking at all time points except WD1, with maximal reinstatement on WD6 and WD12 (Shalev et al. 2001). Additional studies with the incubation model are needed to assess whether the effects of chronic stress exposure on incubation of cocaine craving vary depending on when the stress is administered during withdrawal.
In addition to footshock, several other acute stressors have also been shown to reinstate drug seeking following extinction training, including acute 1-day food deprivation in both rats (heroin, Shalev et al. 2000; cocaine, Shalev et al. 2003) and mice (cocaine, Highfield et al. 2002), as well as acute exposure to cold swim stress administered 24 hours prior to the reinstatement session (cocaine, Conrad et al. 2010). While the majority of studies using the reinstatement model to study the effects of stress on cue-induced relapse vulnerability have studied acute stress-induced reinstatement, one recent study has used this model to assess how chronic variable stress exposure prior to drug self-administration affects methamphetamine intake, extinction training and cue-induced reinstatement. Interestingly, no effect of chronic variable stress was observed for any of these measures (Lewis et al. 2016), but the effects of this treatment during withdrawal on subsequent extinction and reinstatement have yet to be assessed.
Aside from chronic food restriction stress (described in the previous section of the Discussion) and repeated restraint stress (current results), it is unknown whether exposure to other types of stress during withdrawal will also enhance cue-induced cocaine seeking in the incubation model and whether the duration of these stressors (i.e., acute vs chronic) impacts craving. In addition, it is unknown whether the facilitating effects of chronic stress exposure on incubated craving are observed for drugs other than heroin (D’Cunha et al. 2013; Sedki et al. 2013, 2015) and cocaine (current results). Since the incubation model is relevant to understanding persistent vulnerability to relapse (i.e., Reichel & Bevins, 2009; Pickens et al. 2011) and incubation has been shown to occur in human users of several drugs including cocaine (Bedi et al. 2011; Wang et al. 2013; Li et al. 2014; Parvaz et al. 2016), further characterizing these effects will help to identify more effective strategies with which to treat craving and relapse in recovering addicts.
Neuroadaptations driving stress-induced changes in incubated craving
Of great interest are the neurobiological mechanisms that are driving the chronic stress- induced acceleration of incubated cocaine craving observed here. Previous studies have shown that repeated restraint stress exposure enhances excitatory drive to the basolateral amygdala (BLA) (Rosenkranz et al. 2010; Padival et al. 2013), a region critical for behavioral responses to stress (Roozendaal et al. 2009). Given that glutamate projections from the BLA to the NAc are critical for incubation of cocaine craving (Lee et al. 2013), one possibility is that cocaine withdrawal and chronic stress exposure produce a synergistic increase in BLA neuronal activity which facilitates incubation of craving, whereas treatments promoting stress resilience or stress reduction will prevent this facilitation by producing opposing adaptations in the BLA. Some insight into the latter possibility can be gained from recent studies examining the effects of environmental enrichment (EE) during withdrawal on the incubation of cocaine craving. Although one study found that EE decreases cocaine seeking during both early and late withdrawal but does not prevent incubation (Thiel et al. 2012), another study using longer cocaine self-administration sessions, a longer withdrawal period and a longer period of enrichment found that EE attenuates incubation but that incubation re-emerges when EE is removed (Chauvet et al. 2012). Interestingly, a very recent study suggested that incubation can be prevented in a more persistent manner when EE is administered in conjunction with intervention in AMPAR plasticity in nucleus accumbens (NAc) neurons postsynaptic to BLA afferents (Ma et al. 2016). Together, these findings raise the intriguing possibility that the behavioral impact of stress exposure on incubation of craving observed here could also be due to alterations in activity within the BLA-NAc pathway.
In conclusion, our results show that exposure to repeated restraint stress during early withdrawal accelerates incubation of cue-induced cocaine craving and may lead to a persistent enhancement in the relapse-promoting effects of stress. By identifying such time-dependent changes in relapse vulnerability during withdrawal, these studies will ultimately bring us closer to developing effective strategies to reduce craving and prevent relapse in abstinent cocaine addicts.
Acknowledgments
This research was supported by NIH grant K99DA038110 to JAL. We thank Dr. Yavin Shaham for his insight and advice in the design and development of these studies.
Footnotes
Supplemental information: None
Conflict of Interest: None declared
AUTHORS CONTRIBUTIONS: JAL, MEW and JAR were responsible for designing the studies. JAL and RMG conducted all behavioral studies, along with help from AC, FS and ABS. JAL conducted all statistical analysis. JAL and MEW wrote the manuscript. RMG and JAR provided critical revision of the manuscript. All authors reviewed and approved the final version for publication.
References
- Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y, de Wit H. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biological Psychiatry. 2011;69:708–11. doi: 10.1016/j.biopsych.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet C, Goldberg SR, Jaber M, Solinas M. Effects of environmental enrichment on the incubation of cocaine craving. Neuropharmacology. 2012;63:635–641. doi: 10.1016/j.neuropharm.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, McCutcheon JE, Cotterly LM, Ford KA, Beales M, Marinelli M. Persistent increases in cocaine-seeking behavior after acute exposure to cold swim stress. Biol Psychiatry. 2010;68:303–305. doi: 10.1016/j.biopsych.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Cunha TM, Sedki F, Macri J, Casola C, Shalev U. The effects of chronic food restriction on cue-induced heroin seeking in abstinent male rats. Psychopharmacology. 2013;225:241–50. doi: 10.1007/s00213-012-2810-1. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26:3584–88. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Lasseter HC, Ramirez DR, Xie X. Relapse to drug seeking following prolonged abstinence: the role of environmental stimuli. Drug Discov Today Dis Models. 2008;5:251–258. doi: 10.1016/j.ddmod.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Vasudevan P, Mueller C, Seubert C, Mantsch JR. Neuroadaptations in the cellular and postsynaptic group 1 metabotropic glutamate receptor mGluR5 and Homer proteins following extinction of cocaine self-administration. Neurosci Lett. 2009;452:167–171. doi: 10.1016/j.neulet.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highfield D, Mead A, Grimm JW, Rocha BA, Shaham Y. Reinstatement of cocaine seeking in mice: effects of cocaine priming, cocaine cues and food deprivation. Psychopharmacology. 2002;161:417–424. doi: 10.1007/s00213-002-1047-9. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Kluggmann M, Kalivas PW. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J Neurosci. 2010;30:7984–7992. doi: 10.1523/JNEUROSCI.1244-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BR, Ma Y-Y, Huang YH, Wang X, Otaka M, Shikawa M, Neurmann PA, Graziane NM, Brown TE, Suska A, Guo C, Lobo MK, Sesack SR, Wolf ME, Nestler EJ, Saham Y, Schluter OM, Dong Y. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat Neurosci. 2013;16:1644–51. doi: 10.1038/nn.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CR, Staudinger K, Tomek SE, Hernandez R, Manning T, Olive MF. Early life stress and chronic variable stress in adulthood interact to influence methamphetamine self-administration in male rats. Behav Pharmacol. 2016;27:182–184. doi: 10.1097/FBP.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Wu P, Xin X, Fan Y-L, Wang G-B, Wang F, Ma M-Y, Xue M-M, Luo Y-X, Yang F-D, Bao Y-P, Shi J, Sun H-Q, Lu L. Incubation of alcohol craving during abstinence in patients with alcohol dependence. Addict Biol. 2014;20:513–522. doi: 10.1111/adb.12140. [DOI] [PubMed] [Google Scholar]
- Loweth JA, Tseng KY, Wolf ME. Using metabotropic glutamate receptors to modulate cocaine’s synaptic and behavioral effects: mGluR1 finds a niche. Curr Opin Neurobiol. 2013;23:500–6. doi: 10.1016/j.conb.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loweth JA, Tseng KY, Wolf ME. Adaptations in AMPA receptor transmission in the nucleus accumbens contributing to incubation of cocaine craving. Neuropharmacology. 2014a;76(Pt B):287–300. doi: 10.1016/j.neuropharm.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loweth JA, Scheyer AF, Milovanovic M, LaCrosse AL, Flores-Barrera E, Werner CT, Li X, Ford KA, Le T, Olive MF, Szumlinski KK, Tseng KY, Wolf ME. Synaptic depression via mGluR1 positive allosteric modulation suppresses cue-induced cocaine craving. Nat Neurosci. 2014b;17:73–80. doi: 10.1038/nn.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loweth JA, Glynn RM, Rosenkranz JA, Wolf ME. Chronic stress exposure during early withdrawal from extended access cocaine self-administration facilitates incubation of cue-induced cocaine craving. Soc Neurosci Abstr. 2015;41 315.20. [Google Scholar]
- Lu L, Grimm JW, Dempsey J, Shaham Y. Cocaine seeking over extended withdrawal period in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology. 2004a;176:101–108. doi: 10.1007/s00213-004-1860-4. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004b;47(Suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Ma Y-Y, Wang X, Huang Y, Marie H, Nestler EJ, Schluter OM, Dong Y. Re-silencing of silent synapses unmasks anti-relapse effects of environmental enrichment. PNAS. 2016;113:5089–5094. doi: 10.1073/pnas.1524739113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Funk D, Lê AD, Shaham Y. Stress-induced reinstatement of drug seeking: 20 years of progress. Neuropsychopharmacology. 2016;41:335–56. doi: 10.1038/npp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padival M, Quinette D, Rosenkranz JA. Effects of repeated stress on excitatory drive of basal amygdala neurons in vivo. Neuropsychopharmacology. 2013;38:1748–1762. doi: 10.1038/npp.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvaz MA, Moeller SJ, Goldstein RZ. Incubation of cue-induced craving in adults addicted to cocaine measured by electroencephalography. JAMA Psychiatry. 2016 doi: 10.1001/jamapsychiatry.2016.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Bevins RA. Forced abstinence model of relapse to study pharmacological treatments of substance use disorder. Curr Drug Abuse Rev. 2009;2:184–194. doi: 10.2174/1874473710902020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Venheim ER, Padival M. Chronic stress causes amygdala hyperexcitability in rodents. Biol Psychiatry. 2010;67:1128–1136. doi: 10.1016/j.biopsych.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedki F, Abbas Z, Angelis S, Martin J, D’Cunha T, Shalev U. Is it stress? The role of stress related systems in chronic food restriction-induced augmentation of heroin seeking in the rat. Front Neurosci. 2013;7:98. doi: 10.3389/fnins.2013.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedki F, Gregory JG, Luminare A, D’Cunha TM, Shalev U. Psychopharmacology. 2015;232:3773–3782. doi: 10.1007/s00213-015-4037-4. [DOI] [PubMed] [Google Scholar]
- Self DW, Choi KH, Simmons D, Walker JR, Smagula CS. Extinction training regulates neuroadaptive responses to withdrawal from chronic cocaine self-administration. Learn Mem. 2004;11:648–57. doi: 10.1101/lm.81404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shalev U, Highfield D, Yap J, Shaham Y. Stress and relapse to drug seeking in rats: studies on the generality of the effect. Psychopharmacology. 2000;150:337–346. doi: 10.1007/s002130000441. [DOI] [PubMed] [Google Scholar]
- Shalev U, Morales M, Hope B, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology. 2001;156:98–107. doi: 10.1007/s002130100748. [DOI] [PubMed] [Google Scholar]
- Shalev U, Marinelli M, Baumann MH, Piazza PV, Shaham Y. The role of corticosterone in food deprivation-induced reinstatement of cocaine seeking in the rat. Psychopharmacology. 2003;168:170–176. doi: 10.1007/s00213-002-1200-5. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse. Psychopharmacology. 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–30. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Shaham Y, Heilig M. Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacology. 2011;218:69–82. doi: 10.1007/s00213-011-2263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Schmidt EF, Choi K-H, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL, Self DW. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behavior. Nature. 2003;421:70–75. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- Thiel KJ, Painter MR, Pentkowksi NS, Mitroi D, Crawford CA, Neisewander JL. Environmental enrichment counters cocaine abstinence-induced stress and brain reactivity to cocaine cues but fails to prevent the incubation effect. Addict Biol. 2012;17:365–377. doi: 10.1111/j.1369-1600.2011.00358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Shi J, Chen N, Xu L, Li J, Li P, Sun Y, Lu L. Effects of length of abstinence on decision-making and craving in methamphetamine users. PLoS One. 2013;8:e68791. doi: 10.1371/journal.pone.0068791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci. 2016 doi: 10.1038/nrn.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Rosenkranz JA. Repeated restraint stress increases basoalteral amygdala neuronal activity in an age-dependent manner. Neuroscience. 2012;226:459–474. doi: 10.1016/j.neuroscience.2012.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]