Abstract
Hangover refers to the cluster of physiological and behavioral symptoms that occur following the end of a drinking episode. While hangover has been studied after the typical oral consumption of alcohol, the occurrence of hangover following intravenous (IV) alcohol administration in human laboratory studies has not been previously reported. This study characterizes hangover symptoms and post-infusion drinking behavior following acute IV alcohol administration in social drinkers. 21–30 year-old healthy social drinkers (n=24) underwent an alcohol clamp session at breath alcohol concentration of 0.06%. Hangover symptoms as well as any post-infusion drinking that occurred between the end of the session and the following morning were assessed using the Acute Hangover Scale, and examined for influences of recent drinking history, family history of alcoholism and Sex. Results indicated a 79% prevalence of hangover symptoms, with the most common symptoms being “tired”, “thirsty”, and “headache”. Recent drinking measures showed significant effects on Average Hangover Scale scores, with heavier drinkers showing greater hangover symptoms. There was a significant sex difference in average hangover scores, with females reporting higher scores than males. Subjective measures of stimulation and intoxication were also associated with Average Hangover Scale scores. The probability of post-infusion drinking was not predicted by hangover scores, but was related to recent drinking history; subjective response to alcohol was a significant mediator of this relationship. These findings demonstrate that hangover symptoms are experienced following IV alcohol administration, and extend previous studies of influences of risk factors for alcohol use disorders including recent drinking on hangover.
Keywords: Alcohol Hangover Scale (AHS), Drinking History, Family History of Alcoholism, Subjective Responses, Human Laboratory Studies
Introduction
Hangover is commonly referred to as the cluster of symptoms that arise following the end of a drinking episode, and has both physiological and behavioral manifestations. Although hangover is fairly common and highlighted in the popular media, and its occurrence has been reported for several centuries, it has been scarcely investigated. Symptoms of hangover include fatigue, headache, tiredness, and thirst. Other signs include increased sensitivity to light and sound, bloodshot eyes, muscle and stomach aches. Neuropsychological symptoms include dizziness; vertigo; and some possible cognitive and mood disturbances, especially low mood, anxiety, and irritability (Stephens et al., 2008; Swift and Davidson, 1998).
Because hangover is primarily associated with heavy drinking, there is a general perception of hangover as a manifestation of alcohol use disorder. Hangover shares many characteristics with the symptoms of acute alcohol withdrawal, a clinically relevant set of symptoms that manifest when a heavy drinker stops or cuts down their drinking. Indeed, alcohol withdrawal is one of the criteria for diagnosis of alcohol dependence per DSM-IV and alcohol use disorder per DSM-5 (APA). However, most symptoms of hangover can be seen following alcohol consumption in light-to-moderate drinkers as well (Wiese et al., 2000), and hangover has been implicated as a determinant of concurrent and future problem alcohol use (Piasecki et al., 2010; Rohsenow et al., 2012). As recently reviewed by Piasecki et al. (2010), studies have also examined several factors including demographics, family history, genetics, drinking history and subjective response to alcohol, as predictors of hangover symptoms following alcohol administration in human laboratory studies as well as in the natural environment (Howland et al., 2008; Piasecki et al., 2012; Rohsenow et al., 2012; Slutske et al., 2014).
However, there have been no studies reporting on the occurrence of hangover, or characterization of the determinants of hangover following IV alcohol administration. While the IV route is not naturalistic, it is the method of choice for administering alcohol in studies requiring precision in exposure and minimization of variation in pharmacokinetic influences (Ramchandani et al., 2006). With the increase in use of IV alcohol administration methods to study the human pharmacology of alcohol, it is relevant to systematically assess the occurrence of hangover following IV alcohol exposure and examine its determinants, both from a safety viewpoint and for improving our understanding of the etiology of hangover. Additionally, intravenous administration bypasses the gastrointestinal tract thus avoiding the local high concentrations of alcohol and acetaldehyde there and in the liver during hepatic first pass, and may allow the examination of hangover that may be independent of any influences of the gut-liver processes that have been implicated in hangover (Swift and Davidson, 1998). Finally, IV alcohol formulations do not contain congeners that are present in many oral alcohol beverages, which would enable the evaluation of hangover independent from any effect of congeners which have been implicated in the development of hangover (Rohsenow and Howland, 2010)
Thus, the objective of this analysis was to examine the prevalence of acute hangover symptoms and post-infusion drinking following acute IV alcohol administration in male and female social drinkers, and to examine the influence of several factors, including recent drinking history, family history of alcoholism, sex, and subjective response to alcohol as determinants of acute hangover symptoms. Hangover symptoms were assessed using the Acute Hangover Scale, a validated questionnaire that has been used to characterize the determinants and consequences of hangover following oral alcohol consumption (Rohsenow et al., 2007). This study would provide valuable information on a potential safety and monitoring issue in studies using the IV route for alcohol administration as well as help improve our understanding of the determinants of hangover following alcohol, particularly those associated with risk for future alcohol problems.
Material and Methods
Study Design
The specific investigations reported were conducted under a larger protocol approved by the Combined Neuroscience Institutional Review Board at the NIH and participants were enrolled after providing written informed consent. The overall goal of the study was to examine the influence of family history of alcoholism and other factors on acute responses to IV alcohol using the alcohol clamp at 0.06% in healthy male and female social drinkers (results to be reported elsewhere). Participants were contacted 1–2 days following the study visit to obtain information on hangover symptoms and drinking behavior from the time of completion of study session until the next morning.
Participants
The study sample consisted of 24 healthy male and female participants in the age group of 21 to 30 years (Table 1). Participants were recruited as family history positive (FHP) if they had at least 1 first-degree biological relative with alcohol abuse or dependence, as assessed using the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) Family History Assessment Module (described below), or family history negative (FHN) if they had no first or second degree biological relatives with alcohol abuse or dependence. The eligibility of participants was determined following a screening evaluation consisting of medical history, physical exam, and ECG (electrocardiogram), as well as laboratory tests. Participants were excluded if they had a current or past history of disease including cardiovascular, respiratory, gastrointestinal, hepatic, renal, endocrine, or reproductive disorders; current history of axis-I psychiatric disorders or lifetime history of any alcohol or drug dependence or abuse, as assessed using the Structured Clinical Interview for DSM-IV disorders (First et al., 2002), positive urine drug screen for drugs of abuse (amphetamines, benzodiazepine, cocaine, opiates, and cannabinoids); self-reported abstention from alcohol; pregnancy or intention to become pregnant; menstrual cycle irregularities; or use of prescription or over-the-counter medications known to interact with alcohol within two weeks of the study.
Table 1.
Participant Demographics and recent drinking history measures (Mean ± SD).
| Family History Positive | Family History Negative | |||
|---|---|---|---|---|
| Males (n=6) | Females (n=6) | Males (n=4) | Females (n=8) | |
| Age (years) | 25.0 ± 1.3 | 26.2 ± 1.3 | 23.3 ± 1.6 | 24.9 ± 1.1 |
| Height (cm) a | 173.3 ± 3.2 | 168.3 ± 3.2 | 180.0 ± 4.0 | 164.5 ± 2.8 |
| Weight (kg) | 79.8 ± 5.7 | 71.9 ± 5.7 | 76.6 ± 6.9 | 65.7 ± 4.9 |
| BMI (kg/m2) | 26.48 ± 3.09 | 25.31 ± 4.28 | 23.19 ± 2.77 | 24.34 ± 5.86 |
| Recent Drinking History | ||||
| Total Drinks | 44.3 ± 13.8 | 64.2 ± 13.8 | 62.8 ± 16.9 | 61.6 ± 12.0 |
| Drinking Days b | 13.7 ± 5.9 | 33.8 ± 5.9 | 19.5 ± 7.1 | 25.9 ± 5.1 |
| Average Drinks/Day c | 3.0 ± 0.3 | 2.0 ± 0.3 | 3.3 ± 0.4 | 2.5 ± 0.3 |
| Heavy Drinking Days | 2.1 ± 2.0 | 1.8± 2.1 | 4.5 ± 2.5 | 5.4 ± 1.7 |
Significant Sex difference (M > F), p = 0.006
Significant Sex difference (F > M), p = 0.040
Significant Sex difference (M > F), p = 0.017
Procedures
Participants arrived at the NIH Clinical Center (Bethesda, MD) at approximately 7:30AM, having fasted since midnight (approximately 7 hours) prior to the study session and received a metabolic diet (300 Kcal) approximately 1 hour prior to the infusion to standardize the effects of food on alcohol metabolism during the study (Ramchandani et al., 2001). A breath alcohol test was performed to ensure zero alcohol concentrations using the handheld breathalyzer Alcotest 6510 plus (Drager Safety Diagnostics, Inc., Irving, TX), a urine drug screen was performed to ensure absence of recent use of drugs of abuse, and a urine beta-hCG test was performed on all female subjects to ensure that they were not pregnant at the start of the study session. An indwelling intravenous catheter was placed into the antecubital vein of both arms, one for alcohol infusion (non-dominant, preferably), and one for blood sampling (dominant preferably). Following baseline measurements, participants received an infusion of alcohol (6% v/v in saline) starting at approximately 11:30 am) to achieve a target breath alcohol concentration (BrAC) of 0.06 % at 20 min and maintain or clamp the BrAC at the target level for a predetermined duration of 140 min. This IV alcohol solution did not contain congeners that are found in oral alcoholic beverages. Infusion profiles are individualized for each subject using a physiologically-based pharmacokinetic model for alcohol using an algorithm based on age, height, weight and gender (Ramchandani et al., 1999a), and implemented using the computerized alcohol infusion system (CAIS) (available from Sean O’Connor, Indiana Alcohol Research Center, Indiana University School of Medicine, Indianapolis, IN). Serial BrACs obtained during the infusion were input into the CAIS system to enable real-time adjustments to the infusion rates with the goal of maintaining BrAC exposures within 10% of the target level.
Following the completion of the infusion, the IV catheters were removed, and participants were provided with a meal. BrACs were monitored until the level fell below 0.02% at which time, participants were sent home in a taxi, typically around 5:00PM. Participants were contacted by phone 1 to 2 days following the study session to complete an interview on hangover symptoms and post-session drinking, as described below.
Measures
Recent drinking history was obtained during the screening visit using the timeline follow-back, TLFB (Sobell et al., 2003) for the 90-day interval prior to enrollment into the study. Recent drinking history measures examined included total drinks, number of drinking days, average drinks per drinking day, and heavy drinking days. Family history of alcoholism was assessed using the Family Tree Questionnaire (Mann et al., 1985) to identify first- and second- degree relatives that may have had alcohol-related problems, and this was followed-up with the Family History Assessment plus Individual Assessment Modules of the Semi-Structured Assessment for Genetics of Alcoholism or SSAGA (Bucholz et al., 1994). This assessment is widely used in family history based studies, including large genetic studies such as COGA (Collaboration on the Genetics of Alcoholism) for over 20 years, and had been shown to be valid in assessing family history of alcoholism of individuals (Rice et al., 1995).
On the day of the study session, at baseline and during the infusion, two self-report questionnaires were obtained: Biphasic Alcohol Effects Scale (BAES) (Martin et al., 1993) and the Drug Effects Questionnaire (DEQ) (Holdstock et al., 2000; Morean et al., 2013). The Biphasic Alcohol Effects Scale provides two sub-scores: “stimulation” and “sedation”. The DEQ included the following five items; “do you feel drug effects?”; “do you like drug effect?”; “do you feel high?”; “do you feel intoxicated?” and “would you like more” . Two indices of subjective response were derived for each measure: an initial response to alcohol index (IRA) computed as the difference between responses at the start of the clamp interval (20 min) and baseline (IRA = time20 – time0); and an adaptation response index (ADA) computed as the difference between responses at the end of the clamp interval (110 min) and the start of the clamp interval (20 min) (ADA = time110 – time20) (Ramchandani et al., 1999b).
Following the study session, the Alcohol Hangover Scale (AHS) (Rohsenow et al., 2007) and questions related to post-infusion drinking behavior were obtained during a follow-up phone call with the participant. The questionnaire focused on the interval between the participant’s release from the Clinical Center and 10AM of the following day. AHS included measures of the severity of symptoms including hangover, headache, thirsty, tired, nausea, stomach-ache, heart-racing, dizziness, and loss of appetite. All symptoms were rated on a scale of 0 (none) to 7 (incapacitating). Participants were also asked to report on any craving for alcohol (also rated on a scale from 0 to 7) and any use of alcohol (post-infusion drinking behavior) during the evaluation interval.
Data Analysis
The primary dependent measure for the analysis was the Average Hangover Scale score (average of all 9 items of the AHS). The individual scores for items that were most consistently endorsed by the participants, i.e., thirsty, tired and headache, as well as craving for alcohol were examined as secondary measures. Analysis of covariance was used to examine the main effects of sex and FHA on the primary and secondary hangover measures. Recent drinking history measures (total drinks, number of drinking days, drinks per drinking day) were included as covariates to examine their effect on hangover measures. Linear regression analysis was used to examine the relationship between subjective response measures (IRA and ADA, as defined above) and average hangover score. In these analyses, recent drinking history measures (total drinks, number of drinking days, drinks per drinking day), sex and FHA were included as covariates. Logistic regression analysis was used to examine the prevalence of post-infusion drinking as a function of FHA, sex and drinking history measures. Separate analyses were conducted for each of the recent drinking history measures, total drinks, number of drinking days, drinks per drinking day, and heavy drinking days.
Multiple mediation analyses were conducted to better understand the relationships among hangover scores, recent drinking, subjective response and post-infusion drinking. Post-infusion drinking was the dependent variable and total drinks measure was used as the independent variable, primarily because it showed the strongest relationship with post-infusion drinking in the logistic regression analysis. Potential mediators examined were average hangover score and subjective response indices (IRA and ADA) for the BAES-Stimulation and DEQ-intoxication measures. Separate mediation models were fit for the average hangover score (model 1) and for the subjective response indices (model 2). Sex and FHA were included as covariates in both models. Analyses were conducted using the INDIRECT macro developed for SAS (available at http://www.afhayes.com), which uses a bootstrapping method for the analyses (Preacher and Hayes, 2008). Indirect effects were estimated via bootstrap analysis for 5000 randomly generated samples with mediation established if the 95% bias-corrected confidence interval for the indirect parameter estimate did not contain zero.
Statistical analysis was conducted using SPSS 20.0 (IBM, Chicago IL), with the level of statistical significance set at p≤0.05, uncorrected for multiple testing. Mediation analyses were conducted using SAS version 9.3 (SAS Institute, Cary NC).
Results
Participant demographics and characteristics, including drinking history measures are listed in Table 1. Expected differences in morphometric measures were seen between males and females, however there were no main or interaction effects with FHA. Males reported significantly lower number of drinking days but higher average drinks per drinking day than females. There were no differences in recent drinking history measures between family history positive, FHP and family history negative, FHN groups.
Characterization of Hangover: Influence of FHA and Sex, and Recent Drinking History
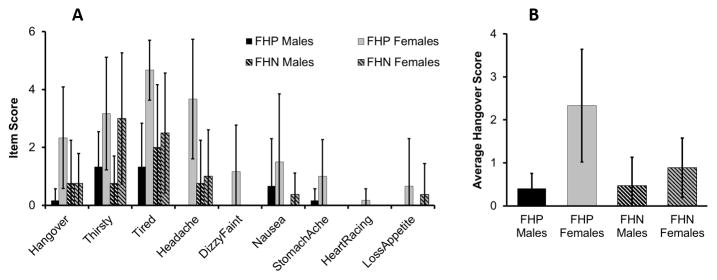
Figure 1a shows the mean (and SD) scores on the individual items of the Alcohol Hangover Scale by sex for the FHP and FHN groups. In general, hangover symptoms were reported in 79% (19 of 24) of participants in the study, with the most common symptoms being “Tired”, “Hangover”, “Thirsty” and “Headache”, reported at moderate severity levels (scores from 2 – 4 on a 7-point scale). The remaining symptoms, when reported, were present at mild levels of severity (score of 1 on the 7-point scale). The average hangover scale score showed a significant main effect of sex (F(1, 20) = 5.423, p = 0.034), with females showing higher scores than males. There was no significant effect of family history (FH) on average hangover score (figure 1b). None of the recent drinking history covariates, total drinks, number of drinking days, or drinks per drinking day had a significant effect on the average hangover score.
Figure 1.
Panel A: Alcohol Hangover Scale individual item scores by FHA and Sex. Bars indicate standard deviations. Significant Sex effects seen for the items “hangover”, “tired”, and “headache” (females showing higher scores than males. Significant Sex effects seen for item “thirsty” (F > M). Panel B: Average Alcohol Hangover Scale Score by FHA and Sex. Bars indicate standard deviations. Females showed significantly higher average hangover scores than males. No significant FHA effects were seen.
Analysis of individual AHS items indicated a pattern of Sex effects that was consistent with that seen for the average hangover score. Sex differences were seen for the items “Headache” (F(1, 23) = 8.062, p = 0.01), “Tired” (F(1, 23) = 6.611, p = 0.018), and “Thirsty” (F(1, 23) = 7.521, p = 0.012). For all items, females showed higher scores than males. FHA did not have a significant effect on the individual items. No significant effects were seen for the measure of craving obtained on the questionnaire.
Relationship between Average Hangover Score with Initial and Adaptive Subjective Response
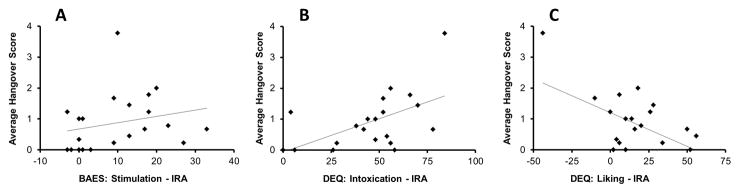
Multiple regression analyses indicated significant associations between the initial response index for some subjective measures and the average hangover score, after controlling for recent drinking history (total drinks), sex and FHA. Figure 2 shows the initial response measures that showed significant effects on hangover. Specifically, significant positive associations were seen for DEQ-Intoxication (partial R2 = 0.355, p = 0.009) and BAES-Stimulation (partial R2 = 0.257, p = 0.032) initial response indices. On the other hand, a significant negative association was seen for DEQ-Liking (partial R2 = 0.245, p = 0.037) initial response index. In all analyses, sex was a significant predictor of average hangover score. Regression diagnostics, including histograms and P-P plots as well as normality tests on the residuals, did not indicate any influential observations affecting the observed relationships. There were no significant associations between the average hangover score and the other initial response index measures or any of the adaptation index measures.
Figure 2.
Regression analysis of average hangover score with subjective response measures. Panel A: IRA for BAES stimulation (R2=0.257), Panel B: IRA for DEQ Intoxication (R2=0.355), Panel C: IRA for DEQ Liking (R2=0.245). All analyses controlled for recent drinking history (total drinks), sex and family history of alcoholism. Statistical significance was set at p≤0.05.
Post-infusion Drinking Behavior: Association with Hangover and Drinking History
Results of logistic regression analysis indicated that the probability of post-infusion drinking was significantly predicted by recent drinking history measures. Table 2 shows the results of the analysis conducted separately for each drinking measure. Total drinks showed the most significant effect on the probability of post-infusion drinking (Wald Chi = 5.445, p = 0.02), with a 6% increase in odds of post-infusion drinking with each unit increase in total drinks (in the past 90 days). The AvgHS score was not a significant predictor of post-infusion drinking (Wald Chi = 1.655, p = 0.198), even in the absence of drinking history measures in the regression models analyzed.
Table 2.
Effects of recent drinking history on Post-infusion Drinking Behavior. Note that each recent drinking history measure was analyzed separately as described in the text.
| Predictor | Estimate | Standard Error | Wald's Chi- square | p-value | Odds ratio (95% CI) |
|---|---|---|---|---|---|
| Total Drinks | 0.059 | 0.026 | 5.224 | 0.022 | 1.060 (1.008, 1.115) |
| No of Drinking Days | 0.066 | 0.047 | 2.000 | 0.157 | 1.068 (0.975, 1.170) |
| Average Drinks per Day | 1.515 | 0.692 | 4.791 | 0.029 | 4.549 (1.172, 17.660) |
| Heavy Drinking Days | 0.246 | 0.128 | 3.702 | 0.054 | 1.278 (0.995, 1.642) |
Mediation analyses of post-infusion drinking (PIDB)
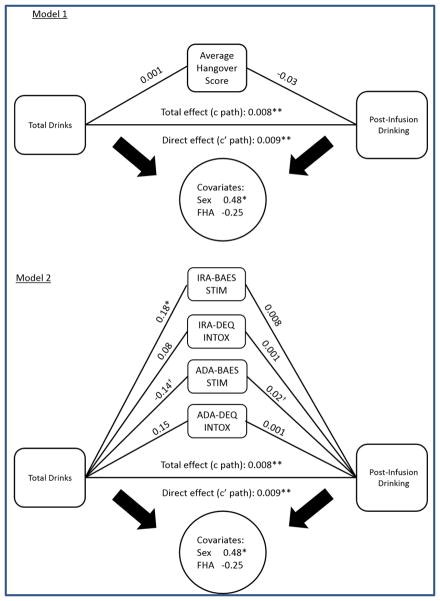
Given the significant effect of recent drinking history, particularly total drinks on post-infusion drinking behavior, mediation analysis was done to examine if this effect was mediated by average hangover scale scores or subjective response measures. Table 3 shows the results of mediation models using total drinks as a predictor and post-infusion drinking as the outcome, with separately conducted tests for the average hangover scale score and the subjective response indices. Model 1 (figure 3A) with average hangover scale score showed a significant direct effect of total drinks and post-infusion drinking (adjusted R2 = 0.43, p = 0.01), however the average hangover score did not have a significant mediation effect on the relationship. Model 2 (figure 3b) with subjective measures was also significant (adjusted R2 = 0.48, p = 0.01), with the initial response index to alcohol, IRA for BAES-Stimulation showing significant relationship with total drinks (b=0.176, p=0.006) although the direct effect of the mediator on post-infusion drinking was not significant. The adaptation response index to alcohol, ADA for BAES-Stimulation showed trend-level significance (p=0.06) for mediation of the relationship between total drinks and post-infusion drinking.
Table 3.
Results of mediation analyses for total drinks (TD90) with post-infusion drinking behavior as the outcome. Model 1 included the average hangover score as mediator. Model 2 included the subjective response indices in a multiple mediation model.
| Independent Variable | Mediator | Effect of Independent Variable on Mediator1 | Effect of Mediator on Outcome | Indirect Effect Estimate2 | 95% Bias- corrected Confidence Interval | Total Effect of Independent Variable | Direct Effect of Independent Variable |
|---|---|---|---|---|---|---|---|
| Model 1 | |||||||
| Total Drinks (TD90) | Average Hangover Score | 0.001 | −0.034 | −0.000 | −0.004, 0.000 | 0.008* | 0.008* |
| Model 2 | |||||||
| Total Drinks (TD90) | IRA-BAES STIM | 0.176* | 0.008 | −0.002 | −0.003, 0.007 | 0.009** | 0.010** |
| IRA-DEQ-Intox | 0.084 | 0.001 | 0.000 | −0.002, 0.002 | |||
| ADA-BAES STIM | −0.142† | 0.020† | −0.003 | −0.011, 0.001 | |||
| ADA-DEQ-Intox | 0.159 | 0.001 | 0.0001 | −0.002, 0.003 |
Values are un-standardized regression coefficients;
Based on 5000 bootstrapped samples;
p < 0.05;
p < 0.005;
p = 0.06 (trend-level).
Figure 3.
Panel A: Mediation model of the effect of hangover on relationship between drinking history and post-infusion drinking. Panel b: Mediation model of the effect of subjective response on relationship between drinking history and post-infusion drinking, with separately conducted tests for each mediator. Values are unstandardized coefficients. Symbols indicate level of significance (* p < 0.01; ** p < 0.005; † p = 0.06).
Discussion
This investigation is the first to report on hangover consequences following acute intravenous alcohol administration in social drinkers. Mild to moderate hangover symptoms, in particular “tired”, “thirsty”, and “headache”, were observed following IV alcohol exposure to moderate BrACs levels of 0.06% using the alcohol clamp method. Females, particularly those with heavier drinking history showed higher prevalence and distribution of hangover symptoms and post-infusion drinking. Previous studies examining hangover in females have shown that younger women who reported first being high/drunk at a relatively earlier age were more likely to show signs of hangover following drinking (Harburg et al., 1993), and that female social drinkers show hangover symptoms along with greater choice reaction errors on a decision-making task on the morning after consuming low to moderate standard quantities of alcohol (Kruisselbrink et al., 2006). A recent report includes data from a survey suggesting significantly more severe and longer lasting hangover among women when compared to men (Verster et al., 2013).
In addition, as recently reviewed by Piasecki et al. (2010), individuals at elevated risk for alcoholism may experience greater hangover symptoms, and this may initiate further drinking to relieve these aversive symptoms (Earleywine, 1993). Hangover symptoms have also shown close association with familial risk for alcoholism in males (Span and Earleywine, 1999); and hangover may be interpreted as early signs of acute withdrawal syndrome (Newlin and Pretorius, 1990) that might lead individuals to drink alcohol again in order to alleviate withdrawal symptoms. A recent study examining genetic influences on hangover has reported that genetic factors account for 40–45% of the variance in hangover frequency (Slutske et al., 2014). Another recent study in twins reported a heritability of 55.4% in hangover (Wu et al., 2014). However, other recent studies did not show any association between family history of alcoholism and hangover symptoms (Howland et al., 2008; Epler et al., 2014). Taken together, these studies, along with the current findings point to the need to examine genetic, epigenetic as well as non-genetic influences on hangover to get a better understanding of the etiology of and developing interventions for the consequences of drinking.
The current study also demonstrated significant association between subjective response to alcohol, specifically self-reported stimulation and intoxication, and hangover. While, most studies examining the relationship between subjective and impairing effects of alcohol and hangover have focused on residual effects concurrent with hangover symptoms, a recent study by Rohsenow et al. (2012) has demonstrated that ratings of intoxication at peak BAC after oral alcohol challenge were positively correlated with hangover symptoms the next day. These findings are consistent with the results of the current study. The subjective responses examined in this study include measures of stimulation and intoxication, which have been shown to be associated with drinking history (Ramchandani et al., 2002), as well as risk for alcohol problems (King et al., 2011), and the positive association between these perceptions and the hangover scale score, after adjusting for drinking history, suggests that greater alcohol response may be a risk factor for alcohol use problems, and alcohol hangover may be a marker of this risk. On the other hand, there are published reports that a lower sensitivity to alcohol, measured using the retrospective self-rating of the effects of alcohol (SRE) scale, may be associated with greater hangover, although this effect may be influenced by the greater drinking history (more drinks per drinking episode) typically seen in these low-sensitivity individuals (Piasecki et al., 2012). Indeed, after accounting for drinking history it appeared that a lower alcohol sensitivity was associated with lower odds of hangover. Thus, the current study findings, along with recently published reports (Rohsenow, et al., 2012, Piasecki et al., 2012) all seem to converge on the conclusion that an enhanced sensitivity to acute alcohol effects is associated with enhanced sensitivity to hangover symptoms after drinking.
This lower sensitivity is also thought to be a risk factor for alcohol use problems, indicating that regardless of the underlying etiology, high risk for alcohol problems seem to be associated with greater hangover (Piasecki et al., 2005). The results of this study did not demonstrate a relationship between the adaptation response, i.e., acute tolerance to alcohol and hangover, which is inconsistent with previous theories that hangover may be a form of acute withdrawal related to alcohol tolerance. While this study did not examine acute withdrawal as it would not be relevant in our study sample of non-dependent drinkers, results do indicate a significant relationship between drinking history and alcohol hangover. To the extent that heavier drinking reflects the development of chronic tolerance, these data do suggest a relationship between hangover and development of tolerance and escalation in drinking patterns. The other important question that arises following alcohol exposure is post-infusion drinking, which may be employed by the drinking population to reduce the vigorous nature of hangover or acute alcohol withdrawal-like symptoms. This study showed that the risk of post-infusion drinking was associated with recent drinking history, however there was no significant relationship between hangover (either as a predictor or mediator) on post-study drinking, and is consistent with a recent ecological momentary assessment study that also did not find a significant relationship between hangover and subsequent drinking measures (Epler et al., 2014). Despite the practice of using alcohol to ameliorate hangover (“hair of the dog”), the clinical characterization, treatment, and prevention aspects are not well studied and previous studies have not been able to substantially identify any conventional or complementary intervention that could be effective for preventing or treating alcohol hangover, which could be attributed to the gap in understanding the causality. One of the most effective ways that has been supported to avoid the symptoms of alcohol induced hangover has been associated with practicing abstinence or moderation (Pittler et al., 2005).
The results of this study should be considered in light of its limitations, primarily the lack of a placebo condition and the small sample size. The design of the overall study was not amenable to the inclusion of a placebo condition to examine the extent to which the hangover symptoms were attributable to alcohol, per se. Placebo-controlled studies of hangover reveal very minimal reporting of symptoms on the Alcohol Hangover Scale in the placebo condition, for example, the study by Howland et al. (2008) reported that only 3% (5 of 167) of participants receiving placebo reported any hangover symptoms. While some contribution of participating in the study on the symptoms in the hangover scale cannot be ruled out, the consistent endorsement of the item “hangover” with the other items on the AHS suggests that the symptoms reported by the participants are probably a result of the alcohol exposure. There was also no oral alcohol condition for evaluation of the effect of route of administration on the manifestation of hangover symptoms. However, this was not the objective of our study, and the critical issue of route of administration will have to be examined in a future prospective study. Additionally, the sample size of the study is small, particularly for examining sex and FHA-related differences, and therefore these findings should be considered as preliminary until confirmed in larger samples. Finally, the IV alcohol used in this study was free of congeners that have been previously been associated to hangover symptoms following oral consumption of alcoholic beverages (Rohsenow and Howland, 2010).
There has been an increasing use of intravenous alcohol in human laboratory studies examining various genetic and non-genetic determinants of the pharmacokinetics and pharmacodynamics of alcohol. Studies using the alcohol clamp that provides a steady-state BrAC exposure profile following IV alcohol (Ramchandani et al., 1999a) have been used in studies by Gilman et al., 2008; Ramchandani et al. 2011; Wetherill et al., 2012; Kerfoot et al., 2013; Vatsalya et al., 2014; to name a few. Other BrAC exposure profiles utilizing IV alcohol administration have also been developed (Ramchandani et al., 2009) and yet others have been used to examine the influence of medications on alcohol response (Ray and Hutchison, 2007). More recently IV self-administration paradigms have been developed to examine alcohol seeking and consumption behavior and its determinants (Zimmermann et al., 2013; Plawecki et al., 2013; Hendershot et al., 2014). The BrAC exposures in these studies may reach peak levels of 0.08 to 0.12 g%, and may be associated with post-infusion hangover symptoms, as seen in the current study. Thus, the continued assessment of hangover symptoms and its determinants such as drinking history, family history of alcoholism, and demographic measures, may be valuable not only for safety and monitoring purposes, but also to better characterize the biological and behavioral underpinnings of hangover, and its impact and clinical implications in individuals at risk for alcohol use disorder.
Acknowledgments
Funding
Supported by the NIAAA Division of Intramural Clinical and Biological Research (ZIA AA000466). Development and use of the Computerized Alcohol Infusion System (CAIS) software used for the alcohol clamp was supported by Sean O’Connor, M.D. at the Indiana Alcohol Research Center (NIH P60 AA007611). The authors gratefully acknowledge the NIH Clinical Center Alcohol Clinic and 5SWS Day Hospital staff for clinical support, Mary Lee, M.D., and the late Dan Hommer, M.D. and for medical support, additional laboratory research staff for data collection support, and the study participants for their participation in the study.
Footnotes
Authorship: VAR is the principal investigator of the study. VV and VAR were responsible for the study concept and design. VV and VS contributed to the acquisition of clinical data. VV and VS conducted data validation and quality assurance. VV, BLS, VS and VAR performed analysis of the data. VV drafted the manuscript. All authors critically reviewed content and approved final version for publication.
References
- Earleywine M. Personality risk for alcoholism covaries with hangover symptoms. Addict Behav. 1993;18(4):415–420. doi: 10.1016/0306-4603(93)90058-h. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994 Mar;55(2):149–58. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Epler AJ, Tomko RL, Piasecki TM, Wood PK, Sher KJ, Shiffman S, Heath AC. Does hangover influence the time to next drink? An investigation using ecological momentary assessment. Alcohol Clin Exp Res. 2014;38:1461–1469. doi: 10.1111/acer.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV-TR axis I disorders, Research version, Non-patient edition. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Gilman JM, Ramchandani VA, Davis MB, Bjork JM, Hommer DW. Why we like to drink: a functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. J Neurosci. 2008;28(18):4583–4591. doi: 10.1523/JNEUROSCI.0086-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harburg E, Gunn R, Gleiberman L, DiFranceisco W, Schork A. Psychosocial factors, alcohol use, and hangover signs among social drinkers: a reappraisal. J Clin Epidemiol. 1993;46(5):413–422. doi: 10.1016/0895-4356(93)90017-u. [DOI] [PubMed] [Google Scholar]
- Hendershot CS, Claus ED, Ramchandani VA. Associations of OPRM1 A118G and alcohol sensitivity with intravenous alcohol self-administration in young adults. Addict Biol. 2014 doi: 10.1111/adb.12165. In press. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdstock L, King AC, de Wit H. Subjective and objective responses to ethanol in moderate/heavy and light social drinkers. Alcohol Clin Exp Res. 2000;24(6):789–794. [PubMed] [Google Scholar]
- Howland J, Rohsenow DJ, Allensworth-Davies D, Greece J, Almeida A, Minsky SJ, Arnedt JT, Hermos J. The incidence and severity of hangover the morning after moderate alcohol intoxication. Addiction. 2008;103(5):758–765. doi: 10.1111/j.1360-0443.2008.02181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerfoot K, Pittman B, Ralevski E, Limoncelli D, Koretski J, Newcomb J, Arias AJ, Petrakis IL. Effects of family history of alcohol dependence on the subjective response to alcohol using the intravenous alcohol clamp. Alcohol Clin Exp Res. 2013;37(12):2011–2018. doi: 10.1111/acer.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry. 2011;68(4):389–399. doi: 10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruisselbrink LD, Martin KL, Megeney M, Fowles JR, Murphy RJ. Physical and psychomotor functioning of females the morning after consuming low to moderate quantities of beer. J Stud Alcohol. 2006;67(3):416–420. doi: 10.15288/jsa.2006.67.416. [DOI] [PubMed] [Google Scholar]
- Mann RE, Sobell LC, Sobell MB, Sobell DP. Reliability of a family tree questionnaire for assessing family history of alcohol problems. Drug and Alcohol Dependence. 1985;15:61–67. doi: 10.1016/0376-8716(85)90030-4. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17(1):140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Morean ME, de Wit H, King AC, Sofuoglu M, Rueger SY, O'Malley SS. The drug effects questionnaire: psychometric support across three drug types. Psychopharmacology (Berl) 2013;227(1):177–192. doi: 10.1007/s00213-012-2954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlin DB, Pretorius MB. Sons of alcoholics report greater hangover symptoms than sons of nonalcoholics: a pilot study. Alcohol Clin Exp Res. 1990;14(5):713–716. doi: 10.1111/j.1530-0277.1990.tb01231.x. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Alley KJ, Slutske WS, Wood PK, Sher KJ, Shiffman S, Heath AC. Low sensitivity to alcohol: relations with hangover occurrence and susceptibility in an ecological momentary assessment investigation. J Stud Alcohol Drugs. 2012;73(6):925–932. doi: 10.15288/jsad.2012.73.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Robertson BM, Epler AJ. Hangover and risk for alcohol use disorders: existing evidence and potential mechanisms. Curr Drug Abuse Rev. 2010;3(2):92–102. doi: 10.2174/1874473711003020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Sher KJ, Slutske WS, Jackson KM. Hangover frequency and risk for alcohol use disorders: evidence from a longitudinal high-risk study. Journal of abnormal psychology. 2005;114(2):223. doi: 10.1037/0021-843X.114.2.223. [DOI] [PubMed] [Google Scholar]
- Pittler MH, Verster JC, Ernst E. Interventions for preventing or treating alcohol hangover: systematic review of randomised controlled trials. BMJ. 2005;331(7531):1515–1518. doi: 10.1136/bmj.331.7531.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plawecki MH, Wetherill L, Vitvitskiy V, Kosobud A, Zimmermann US, Edenberg HJ, O'Connor S. Voluntary intravenous self-administration of alcohol detects an interaction between GABAergic manipulation and GABRG1 polymorphism genotype: a pilot study. Alcohol Clin Exp Res. 2013;37(Suppl 1):E152–160. doi: 10.1111/j.1530-0277.2012.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavioral Research Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Bolane J, Li TK, O'Connor S. A physiologically-based pharmacokinetic (PBPK) model for alcohol facilitates rapid BrAC clamping. Alcohol Clin Exp Res. 1999a;23(4):617–623. [PubMed] [Google Scholar]
- Ramchandani VA, Flury L, Morzorati SL, Kareken D, Blekher T, Foroud T, Li TK, O'Connor S. Recent drinking history: association with family history of alcoholism and the acute response to alcohol during a 60 mg% clamp. J Stud Alcohol. 2002;63(6):734–744. doi: 10.15288/jsa.2002.63.734. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Kwo PY, Li TK. Effect of food and food composition on alcohol elimination rates in healthy men and women. J Clin Pharmacol. 2001;41(12):1345–1350. doi: 10.1177/00912700122012814. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, O'Connor S, Blekher T, Kareken D, Morzorati S, Nurnberger J, Jr, Li TK. A preliminary study of acute responses to clamped alcohol concentration and family history of alcoholism. Alcohol Clin Exp Res. 1999b;23(8):1320–1330. [PubMed] [Google Scholar]
- Ramchandani VA, O'Connor S, Neumark Y, Zimmermann US, Morzorati SL, de WH. The alcohol clamp: applications, challenges, and new directions--an RSA 2004 symposium summary. Alcohol Clin Exp Res. 2006;30(1):155–164. doi: 10.1111/j.1530-0277.2006.00017.x. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Plawecki M, Li TK, O'Connor S. Intravenous ethanol infusions can mimic the time course of breath alcohol concentrations following oral alcohol administration in healthy volunteers. Alcohol Clin Exp Res. 2009;33(5):938–944. doi: 10.1111/j.1530-0277.2009.00906.x. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Umhau J, Pavon FJ, Ruiz-Velasco V, Margas W, Sun H, Damadzic R, Eskay R, Schoor M, Thorsell A, Schwandt ML, Sommer WH, George DT, Parsons LH, Herscovitch P, Hommer D, Heilig M. A genetic determinant of the striatal dopamine response to alcohol in men. Mol Psychiatry. 2011;16(8):809–817. doi: 10.1038/mp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: a double-blind placebo-controlled study. Arch Gen Psychiatry. 2007;64(9):1069–1077. doi: 10.1001/archpsyc.64.9.1069. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Howland J. The role of beverage congeners in hangover and other residual effects of alcohol intoxication: a review. Curr Drug Abuse Rev. 2010;3(2):76–79. doi: 10.2174/1874473711003020076. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Howland J, Minsky SJ, Greece J, Almeida A, Roehrs TA. The Acute Hangover Scale: A new measure of immediate hangover symptoms. Addict Behav. 2007;32(6):1314–1320. doi: 10.1016/j.addbeh.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Howland J, Winter M, Bliss CA, Littlefield CA, Heeren TC, Calise TV. Hangover sensitivity after controlled alcohol administration as predictor of post-college drinking. J Abnorm Psychol. 2012;121(1):270–275. doi: 10.1037/a0024706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutske WS, Piasecki TM, Nathanson L, Statham DJ, Martin NG. Genetic influences on alcohol-related hangover. Addiction. 2014;109(12):2027–2034. doi: 10.1111/add.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Agrawal S, Sobell MB, Leo GI, Young LJ, Cunningham JA, Simco ER. Comparison of a quick drinking screen with the timeline followback for individuals with alcohol problems. Journal of Studies on Alcohol and Drugs. 2003;64(6):858. doi: 10.15288/jsa.2003.64.858. [DOI] [PubMed] [Google Scholar]
- Span SA, Earleywine M. Familial risk for alcoholism and hangover symptoms. Addictive behaviors. 1999;24(1):121–125. doi: 10.1016/s0306-4603(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Stephens R, Ling J, Heffernan TM, Heather N, Jones K. A review of the literature on the cognitive effects of alcohol hangover. Alcohol Alcohol. 2008;43(2):163–170. doi: 10.1093/alcalc/agm160. [DOI] [PubMed] [Google Scholar]
- Swift R, Davidson D. Alcohol hangover: mechanisms and mediators. Alcohol Health Res World. 1998;22(1):54–60. [PMC free article] [PubMed] [Google Scholar]
- Vatsalya V, Momenan R, Hommer DW, Ramchandani VA. Cardiac reactivity during the ascending phase of acute intravenous alcohol exposure and association with subjective perceptions of intoxication in social drinkers. Alcohol Clin Exp Res. 2014;38(5):1247–1254. doi: 10.1111/acer.12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verster JC, Alford C, Bervoets AC, de Klerk S, Grange JA, Hogewoning A, Jones K, Kruisselbrink DL, Owen L, Piasecki TM, Raasveld SJ, Royle S, Slutske WS, Smith GS, Stephens R Alcohol Hangover Research Group. Hangover research needs: proceedings of the 5th Alcohol Hangover Research Group meeting. Curr Drug Abuse Rev. 2013;6:245–251. doi: 10.2174/1874473707999140121141538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill L, Morzorati SL, Foroud T, Windisch K, Darlington T, Zimmerman US, Plawecki MH, O'Connor SJ. Subjective perceptions associated with the ascending and descending slopes of breath alcohol exposure vary with recent drinking history. Alcohol Clin Exp Res. 2012;36(6):1050–1057. doi: 10.1111/j.1530-0277.2011.01642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese JG, Shlipak MG, Browner WS. The alcohol hangover. Ann Intern Med. 2000;132(11):897–902. doi: 10.7326/0003-4819-132-11-200006060-00008. [DOI] [PubMed] [Google Scholar]
- Wu SH, Guo Q, Viken RJ, Reed T, Dai J. Heritability of usual alcohol intoxication and hangover in male twins: the NAS-NRC twin registry. Alcohol Clin Exp Res. 2014;38(8):2307–2313. doi: 10.1111/acer.12487. [DOI] [PubMed] [Google Scholar]
- Zimmermann US, O'Connor S, Ramchandani VA. Modeling alcohol self-administration in the human laboratory. Curr Top Behav Neurosci. 2013;13:315–353. doi: 10.1007/7854_2011_149. [DOI] [PubMed] [Google Scholar]