Abstract
Vitamin D deficiency is highly prevalent in children and adults with cystic fibrosis (CF). Recent studies have found an association between vitamin D status and risk of pulmonary exacerbations in children and adults with CF. The ongoing Vitamin D for enhancing the Immune System in Cystic fibrosis (DISC) study, a multi-center, double-blind, randomized, placebo-controlled trial, will test the hypothesis of whether high dose vitamin D given as a single oral bolus of 250,000 IU to adults with CF during a pulmonary exacerbation followed by a maintenance dose of vitamin D will improve time to next pulmonary exacerbation and re-hospitalization, improve survival and lung function compared to placebo and reduce the rates of pulmonary exacerbation. Subjects will be randomized 1:1 at each clinical site to vitamin D or placebo within 72 h of hospital admission for pulmonary exacerbation. Clinical follow-up visits will occur at 1, 2, 3, and 7 days, and 1, 3, 6 and 12 months after randomization. Blood and sputum will be collected and determination of clinical outcomes will be assessed at each visit. The primary endpoint will be the time to next pulmonary exacerbation requiring antibiotics, re-hospitalization or death. The secondary endpoints will include lung function assessed by forced expiratory volume in 1 s (FEV1), blood markers of inflammatory cytokines, anti-microbial peptide expression by peripheral blood mononuclear cells and circulating concentrations in blood. Other exploratory endpoints will examine the phenotype of neutrophils and monocyte/macrophages in sputum. Nutritional status will be assessed by 3 day food records and food frequency questionnaire.
Keywords: Vitamin D, Cystic fibrosis, Pulmonary exacerbation, Diet, Nutrition, Randomized controlled trial
1. Introduction
Cystic fibrosis (CF) is a chronic lethal genetic condition that results from a mutation of the cystic fibrosis transmembrane conductance regulator (CFTR) gene [1]. CF occurs with a frequency of 1 out of every 3200 Caucasian newborns in the United States [2]. The median age of survival among individuals with CF has now climbed above the age of 40; however, patients still have shortened life expectancy due to progressive decline of lung function and chronic respiratory infections [3]. Other common morbidities that may shorten lifespan in CF include CF related diabetes [4], [5], pancreatic insufficiency and malnutrition [6], CF related bone disease [7], and depression [8].
Vitamin D deficiency is highly prevalent in children and adults with cystic fibrosis [9], [10]. Causes of vitamin D deficiency in cystic fibrosis include fat malabsorption due to pancreatic insufficiency, inadequate dietary intake, variability in the formulation of vitamin D, decreased sunlight exposure and decreased fat storage [11], [12]. Studies suggest a high initial dose of cholecalciferol in powder to be most effective in raising vitamin D status [29]. Higher vitamin D status in the general population has been associated with better lung function [13] and lowered risk of infections [14], both of concern in patients with CF [15]. Higher vitamin D status in children and adults with CF has been associated with better lung function assessed by forced expiratory volume in 1 s (FEV1) [9], [16], [17]. Furthermore, higher vitamin D status in children with CF is associated with fewer pulmonary exacerbations [18], [19]. The mechanisms by which vitamin D may confer beneficial effects on lung function and risk of infections are unclear, but may be through induction of anti-microbial peptides such as human cathelicidin (LL-37) and beta-defensins [20], improved anti-oxidant status [21] and improved lung remodeling [22], [23].
Given the associations between vitamin D status and lung function and infections, we previously conducted a pilot, randomized, double-blinded, placebo-controlled study of high dose vitamin D therapy in 30 adult subjects with CF admitted during a pulmonary exacerbation. We found that a single oral dose of 250,000 IU of vitamin D3 rapidly improved vitamin D status and was associated with improved 1-year survival, decreased plasma tumor necrosis factor- α (TNFα), and a trend towards improved lung function and re-hospitalization rates [24], [25]. Large, prospective, randomized, placebo-controlled, multi-center studies have yet to be conducted to confirm a benefit of vitamin D in patients with CF on survival, lung function, and pulmonary exacerbation rates. The purpose of this study is to confirm the beneficial responses of vitamin D on clinical outcomes, survival, inflammatory cytokine production, and circulating antimicrobial peptides in adults with CF admitted with a pulmonary exacerbation of CF in a randomized, double-blinded, placebo-controlled, multi-center trial.
2. Materials and methods
2.1. Overview of study design
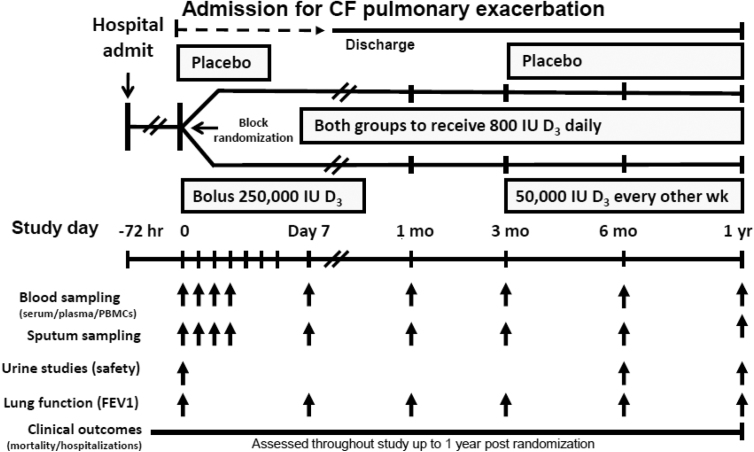
This is a double-blind, randomized, placebo-controlled, multi-center trial to define a role for high dose vitamin D given at the time of admission for a pulmonary exacerbation of CF on mortality and recurrent pulmonary exacerbations. Adults with CF are randomized to 250,000 IU vitamin D3 or placebo given orally as a single bolus within 72 h of admission for an acute pulmonary exacerbation of CF. If subjects are already on maintenance vitamin D therapy at the time of enrollment, they will be allowed to continue taking vitamin D as long as the vitamin D dose remains under 2000 IU daily. If subjects are not taking any vitamin D at enrollment, they will be provided 800 IU daily to prevent severe vitamin D deficiency. Subjects are re-dosed with 50,000 IU of vitamin D3 or placebo every other week starting at 3 months after randomization and in accordance to their initial randomization group. Subjects are followed for 1 year for clinical trial endpoints. Blood sampling for secondary endpoints and safety monitoring occurs at baseline, 1, 2, 3, and 7 days during the initial hospitalization and at 1, 3, 6 and 12 months after randomization. Questionnaires on vitamin D intake and habitual diet are being administered at baseline and at the final visit. Potential adverse events are assessed at each follow-up study visit in person. Additionally, clinical outcome study endpoints e.g. rehospitalization for pulmonary exacerbation and/or adverse events that occur between study visits are documented from the electronic medical record. A summary of the overall study design is depicted in Fig. 1.
Fig. 1.
Overview study design of the Vitamin D for Enhancing the Immune System in Cystic fibrosis (DISC) trial.
2.2. Hypothesis and aims
We hypothesize that a single high dose bolus of vitamin D3 given during a pulmonary exacerbation of CF followed by a bi-weekly maintenance dose of vitamin D3 will improve 1- year survival, time to next pulmonary exacerbation or re-hospitalization due to pulmonary exacerbation, and markers of immunity and inflammation. The primary aim is to test whether 250,000 IU of vitamin D3 given within 72 h of hospitalization for a pulmonary exacerbation followed by maintenance vitamin D3 dosing will improve freedom from pulmonary exacerbation or re-hospitalization over 1 year compared to placebo. The secondary aims are to test whether vitamin D3 improves serial lung function measured by FEV1, increases production of the anti-microbial peptide human cathelicidin (LL-37) in the lung and blood, enhances expression of cathelicidin in peripheral blood mononuclear cells (PBMC) and lung macrophages and neutrophils, and decreases concentrations of circulating pro-inflammatory cytokines.
2.3. Sponsors
The primary sponsor of the “Vitamin D for enhancing the Immune System in Cystic fibrosis (DISC)” trial is the Cystic Fibrosis Foundation and the Cystic Fibrosis Foundation Therapeutic Drug Network and the secondary sponsor is the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454 for the Atlanta Clinical and Translational Science Institute.
2.4. Setting and clinical sites
This multi-center study is being conducted at five United States based CF Foundation Care Centers and members of the CF Therapeutics Development Network (TDN). The sites include Emory University and Emory University Hospital, Atlanta, GA, The University of Alabama Hospital at Birmingham, Birmingham, AL, Case Western Reserve University and Rainbow Babies and Children's Hospital, Cleveland, OH, University of Iowa and University of Iowa Hospitals and Clinic, Iowa City, IA, and the University of Cincinnati, Cincinnati, OH. All of the subjects who are enrolled are patients receiving clinical care for CF at these centers.
2.5. Intervention arms: Vitamin D supplement and placebo
The vitamin D3 50,000 IU capsule and matching placebo has been obtained from BioTech Pharmacal, Inc (Fayetteville, AR). The vitamin D and placebo are identical in color, shape and size. The vitamin D3 capsules have been analyzed by an independent laboratory (Analytical Research Laboratories, Oklahoma City, OK) to confirm that they contained the stated amount of vitamin D. The investigational drug service at each site stores and dispenses the study medication at baseline as 5 pills for the initial dose (to equal 250,000 IU of vitamin D3 or placebo) and at 3 months post randomization to be taken as 1 pill (50,000 IU of vitamin D3 or placebo) every other week for 40 weeks.
2.6. Trial eligibility
The following criteria are used to determine eligibility for participation in the DISC study.
Inclusion Criteria: 1) Adult and adolescent CF patients (age ≥ 16 years), 2) admitted to the inpatient hospital setting for a pulmonary exacerbation of CF, 3) enrolled within 72 h of admission, 4) able to tolerate oral medications, 5) expected to survive hospitalization.
Exclusion Criteria: 1) Inability to obtain consent from the subject and/or legally authorized representative, 2) Most recent serum total 25-hydroxyvitamin D (25(OH)D) > 55 ng/mL within the past 12 months, 3) Most recent serum 25(OH)D < 10 ng/mL within the past 12 months, 4) Current intake of more than 2000 IU of vitamin D above what is taken from multivitamins, 5) Intake of >2000 IU of vitamin D or its equivalent weekly dose (14,000 IU) for more than 1 week at any time within the past 60 days or intake of greater than vitamin D 10,000 IU once at anytime in the past 60 days (above what is taken from multivitamins), 6) Current pregnancy or plans to become pregnant in the next year, 7) History of disorders associated with hypercalcemia including parathyroid disease, 8) Current hypercalcemia (albumin-corrected serum calcium >10.8 mg/dL or ionized calcium >5.2 mg/dL) 9) History of nephrolithiasis with active symptoms within the past two years, 10) Chronic kidney disease worse than stage 3 (estimated glomerular filtration rate (GFR) < 60 mL/min), 11) Oral or intravenous glucocorticoid use currently or in the past month, 12) History of lung transplantation or awaiting lung transplant, or patient in hospice care, 13) FEV1% predicted <20%, 14) Current significant hepatic dysfunction total bilirubin >2.5 mg/dL with direct bilirubin >1.0 mg/dL, 15) Current use of cytotoxic or immunosuppressive drugs; 16) History of AIDS; 17) History of illicit drug abuse (defined as history of enrollment into a drug rehabilitation program or hospital visits due to drug use within the past 3 years or any use of the following drugs in the past 6 months (cocaine, opiates, amphetamines, marijuana) or any positive toxicology screen for (cocaine, opiates, amphetamines, marijuana); 18) Previous enrollment in the study; 19) Too ill to participate in study based on investigator's or study team's opinion; or 20) Current enrollment in another intervention trial.
2.7. Recruitment of the study population
The study investigators identify potential subjects for the study by referral from the inpatient hospital team for new admissions for pulmonary exacerbations of CF or by screening hospital admission logs of subjects with pulmonary exacerbation participating in the national cystic fibrosis registry who provided prior consent for screening in future studies. Patients are assessed for eligibility based on inclusion and exclusion criteria by examining the electronic medical record and by interviewing the subject within 72 h of hospital admission. All potential study participants are approached for informed consent. They are either enrolled in the study or listed on a screening log as screen fails.
2.7.1. Enrollment
All subjects provide written informed consent prior to participating in study procedures. Subjects also provide an additional written informed consent, if interested, to allow for long term banking of specimens for future investigation not related to the current study. Women of child bearing age undergo a urine pregnancy test to exclude pregnancy. Subjects already on vitamin D are asked to discontinue the supplemental vitamin D. Subjects on multi-vitamins are allowed to continue on the multivitamin as long as the amount of vitamin D contained in the multi-vitamin did not exceed 2000 IU.
2.7.2. Randomization
Subjects are randomized using a block design using blocks of 10 stratified by site, as sites will be enrolling at varying rates. Randomization has been performed by a biostatistician not involved in the recruitment of subjects. Research pharmacists dispense the study drugs to the investigators with new labels (labeled as “A” or “B”) to keep the study medication blinded to all study investigators, staff and the subjects. Pharmacists are be blinded as to the true allocation (vitamin D or placebo).
2.8. Specimen collection and assays
2.8.1. Blood collection and processing
Subjects provide approximately 35 mL of whole blood from a peripheral vein collected in Benton-Dickinson vacutainer tubes (Franklin Lakes, New Jersey) in the following order: 1) 8.5 mL serum separator tube (SST) for serum specimens, 2) 6 mL trace element free tube for trace element free serum specimens, 3) two 6 mL spray EDTA tubes for plasma specimens, 4) 8 mL cell preparation tube (CPT) for PBMC collection. Time of blood draw and number of hours since last meal is recorded. Specimens will be collected fasting when possible and the time of the last meal will be recorded. Plasma is processed at 4 °C by sequential centrifugation of EDTA tubes at 400G to pellet cells and generate platelet-rich plasma, and 3000G to pellet platelets and generate platelet-free plasma, as detailed before [26]. The cell pellet from the 400G centrifugation is washed once and resuspended at the original volume in PBS-EDTA (2.5 mM final) for use in flow cytometry (see below). Serum is processed in a similar fashion with centrifugation of the SST tubes.
2.8.1.1. Blood assays
2.8.1.1.1. Vitamin D
Vitamin D status will be determined by measurement of serum total 25-hydroxyvitamin D (25(OH)D) at all of the study time points by using a chemiluminescent assay (Catalog Number IS-2700S, IDS-iSYS, Immunodiagnostic Systems, Scottsdale, AZ) on an automated Immunoassay system (IDS-iSYS). The laboratory performing the measurement actively participates in the Vitamin D External Quality Assessment Scheme (DEQAS) and the NIST/NIH Vitamin D Metabolites QA Program (VitDQAP). The IDS-iSYS machine will be calibrated using manufacturer controls of known concentration levels. The study samples will also be run with internal house controls of known concentrations of 25(OH)D.
2.8.1.1.2. Calcium and albumin
A serum aliquot will be delivered to the Emory University Hospital or local site hospital chemistry laboratory for determination of serum calcium and albumin in a CLIA-certified laboratory. A Beckman Coulter DxC chemistry analyzer will be used to measure total calcium concentration by indirect potentiometry utilizing a calcium ion selective electrode in conjunction with a sodium reference electrode. The DxI System will be used to determine albumin concentration by means of a bichromatic digital endpoint methodology using bromocresol purple (BCP) reagent.
2.8.1.1.3. Parathyroid Hormone and cathelicidin
Plasma intact parathyroid hormone (iPTH) will be performed on the Immunodiagnostic iSYS automated chemiluminescent assay (Catalog Number IS-3600 IDS-iSYS, Immunodiagnostic Systems, Scottsdale, AZ). Human cathelicidin (LL-37) will be measured in plasma using enzyme-linked immunosorbent assay (ELISA) (Hycult Biotech Inc., Plymouth Meeting, PA).
2.8.2. Sputum collection and processing
Subjects are asked to produce non-induced expectorated sputum upon coughing. The sputum sample will be collected in a sterile plastic cup and kept on ice until processing (within 60 min of collection), as detailed by elsewhere [26]. In brief, 6 mL of PBS-EDTA (2.5 mM) will be added and mixed with the sputum by gentle aspiration and expulsion (24 cycles) using a sterile syringe and 18G x 1.5″ sterile needle. The processed sputum will be centrifuged at 800 g for 10 min at 4 °C and the turbid supernatant will be collected without disturbing the cell pellet and further centrifuged at 3000 g for 10 min at 4 °C to generate a clear supernatant. This clear sputum supernatant will be stored at −70 °C until analysis. The cell pellet generated by the first 800 g centrifugation will be washed twice with PBS-EDTA, counted, and resuspended in PBS-EDTA at 2.10ˆ6 cells/ml for downstream assays.
2.8.2.1. Plasma and sputum fluid-based assays
2.8.2.1.1. Inflammatory cytokine assay
Aliquots of platelet-free plasma and clear sputum supernatant will be used for determination of the inflammatory cytokine signature including TNFα using a multi-plex ELISA assay, to be performed when the trial is completed.
2.8.2.1.2. Mass spectrometry
Aliquots of platelet-free plasma and clear sputum supernatant will be used for untargeted and targeted mass spectrometry assays to determine carbohydrate, amino acid, and lipid composition of these biofluids, and assess effects of vitamin D administration on the metabolomic profile.
2.8.2.2. Plasma and sputum cell flow cytometry
Immediately upon processing, washed, resuspended whole blood and sputum cell suspensions are subjected to metabolic probe and antibody staining, followed by flow cytometry analysis (Emory site only). Details of this protocol have been published elsewhere [26]. In brief, these assays are designed to profile the phenotype and function of neutrophil and monocyte/macrophage subsets present in the blood and airway compartments of CF patients and assess their responsiveness to vitamin D treatment. In addition, an aliquot of whole blood and sputum cells is fixed in Lyse/Fix Phosflow buffer (BD Biosciences, San Jose, CA, USA) and frozen at −70 °C for batch analysis of surface and intracellular outcomes (e.g., vitamin D receptor translocation), as described elsewhere [27].
2.8.3. Urine collection
Subjects provide a urine specimen at baseline, 6 and 12 months.
2.8.3.1. Urine assays
2.8.3.1.1. Calcium and albumin ratio in urine
Using standard hospital methods, an aliquot of the collected urine will be analyzed for calcium and creatinine as a safety measure using standard hospital laboratory methods.
2.9. Assessment of diet and vitamin D intake and sun exposure
Subjects are asked to self-report dietary vitamin D intake at months 1 and 12 (visits 6 and 9) and sun exposure at baseline (visit 1) and month 12 (visit 9) using a structured questionnaire. Participants are given two 3-day food record diaries to complete over the course of the study. For each diary, subjects are asked to record all dietary intake for two weekdays and one weekend day. All food records will be analyzed by the same research registered dietitian in the Atlanta Clinical and Translational Science Institute to determine daily habitual vitamin D intake using the Nutrition Data System for Research software (University of Minnesota, Minneapolis, MN). Food frequency questionnaires focused on vitamin D containing foods were also administered at months 1 and 12 (visits 6 and 9).
2.10. Follow-up and study endpoint determination
2.10.1. Determination of primary endpoint (survival, pulmonary exacerbations and re-hospitalizations due to pulmonary exacerbations)
At the conclusion of the study, survival will be assessed by either completion of the 12-month visit or, if lost to follow-up, by verification over phone or medical records of alive status. The number of pulmonary exacerbations will be assessed by a thorough evaluation of all inpatient and outpatient records for the duration of the study for each participant. Possible pulmonary exacerbations will be identified by study investigators based on medical records and on the prescription of antibiotics. A pulmonary exacerbation form developed by the CFF TDN and will be adapted for our study and will be used to capture and verify discrete inpatient and outpatient pulmonary exacerbations. These pulmonary exacerbations will be adjudicated by a pulmonologist blinded to the randomized treatment assignment.
2.10.2. Determination of secondary endpoints
Secondary endpoints will include changes in the concentrations of blood inflammatory cytokines, anti-microbial peptides and markers of vitamin D status as outlined in the blood assays section. Other exploratory endpoints for future investigation will be markers of glucose metabolism.
2.11. Assessment of adherence
Participant adherence with the study drug regimen will be assessed through a count of remaining pills in the returned pill bottle at the final study visit. In the absence of a returned pill the bottle, the participant will be queried on the number of pills remaining at the end of the study. At the 6 month and 12 month/final study visit, participants will be queried in regards to their adherence to the study drug regimen to determine if any pills have been missed. To encourage adherence, participants will be given a schedule outlining the days on which the study drug should be taken. A member of the study team will call the participant on a monthly basis to verify that the pills have been taken as prescribed.
2.12. Data management
All clinical trial data will be entered on paper case report forms and transferred to a secure electronic database, the Research Electronic Data Capture (REDCap) tool. The lead biostatistician of the study will provide reports to the DSMB every 6 months or at more frequent intervals as required by the DSMB. The data from each site will be entered in real time. The study team at the coordinating site at Emory will periodically review the REDCap database for each site to assess data quality. If there are problems with the data, the coordinating site will send a reminder email to fill in missing data or correct data entry errors. Any persistently missing data will be discussed on monthly study conference calls with the study PI and site PIs and study teams to resolve any issues and ensure that the quality of data is maintained. A final reconciliation of the case report forms and REDCap database entry will be conducted by the study PI to ensure that all data entered matches the source paper documentation.
2.13. Sample size determination and analysis plan
The original statistical analysis plan for DISC was to test the hypothesis that vitamin D3 improves outcomes in CF patients using the composite of death or re-hospitalization. About halfway through the study, investigators became concerned about the very limited number of observed events in the study population, namely number of deaths. Concerned about the futility of the study, the DSMB asked the investigators to recalculate the sample size based on a secondary outcome. The DSMB approved the investigators proposed change that the new primary endpoint would be the time from study enrollment to next pulmonary exacerbation requiring any antibiotics, re-hospitalization or death. Data on pulmonary exacerbations were not available to either study investigators or the DSMB at the time of the change in primary study endpoint. With 45 patients in each group (90 total) there is 80% power to detect a hazard ratio of 0.5 between the high-dose oral vitamin D3 group and the standard therapy group, assuming an event rate of 86% in the control group at 1 year using 0.05 significance level. A drop-out rate of 10% was converted into a group loss hazard to allow it to be incorporated in the sample size calculation.
2.13.1. Analysis plan
Analysis will be conducted on an intention-to-treat approach. A Cox-proportional hazards model will be constructed to estimate and compare the hazard rates of pulmonary exacerbation, re-hospitalization or death of each group. Kaplan-Meier curves will also be drawn to describe and compare graphically the two groups' survival experience. One of the benefits of the randomized study design is that the two groups are very likely to be comparable at baseline. In the event that a pre-intervention variable significantly differentiates between groups, this variable will be included a covariate in the Cox model to reduce the impact of the baseline imbalance. The proportional hazards assumption will be assessed using Schoenfeld residuals. Additionally, a generalized linear model, assuming a Poisson distribution for the total number of pulmonary exacerbations, will be fit, with the natural logarithm as the link function. This model will provide an estimate of the incidence rate ratio to compare the rates of pulmonary exacerbations between groups. In the presence of over dispersion, the Poisson distribution will be replaced by the negative binomial distribution. All tests of hypotheses will be two-sided and use a 0.05 level of significance. SAS® software 9.4 (SAS Institute Inc., Cary, NC) was used in the sample size calculation (i.e., proc power) and will be used in the data analyses.
2.14. Ethics and data safety
2.14.1. Human subjects (IRB)- consent and confidentiality
The study was approved by the Emory University Institutional Review Board (IRB) and the local IRBs of each of the participating site. The study was registered at clinicaltrials.gov (NCT01426256). All study investigators at all of the sites have maintained the proper clinical trials training and have had the appropriate research training as required by local IRB regulations. Data collected on case report forms are being in locked storage spaces, and the data are inputted in real-time into the Research Electronic Data Capture (REDCap) internet based program on University secured desktops.
Potential subjects who have been admitted as an inpatient to each of the 5 hospital sites are screened and enrolled through an informed consent process. Three consent forms 1) Consent to participate as a research subject 2) Consent to store samples 3) HIPAA compliance are explained to the subject by trained study investigators. Subjects are then engaged in active discussion regarding their understanding and given the opportunity to ask questions. All participants are given copies of their signed consent forms and contact information of the study investigators in case adverse events occur or they wish to withdraw from participation in the study.
2.14.2. Trial monitoring
An independent committee of investigators consisting of 2 pulmonologists, 1 pediatric endocrinologist and a biostatistician serves as the Data Safety Monitoring Board (DSMB). The study investigators will provide the DSMB a semi-annual report, which consists of all severe adverse events including hospitalizations and deaths, and safety laboratory data including serum and urine calcium concentrations stratified by study group. An independent biostatistician compiles and analyzes the safety data by the two study groups (Treatment A or Treatment B) for the DSMB reports. The study team biostatistician is blinded to the actual study drug allocation (vitamin D3 or placebo).
3. Discussion
The purpose of this novel study is to determine role of high dose vitamin D administered during a pulmonary exacerbation of CF. This study will be the largest randomized, double-blind, placebo-controlled, multi-center study examining vitamin D administered during an acute pulmonary exacerbation in adults with CF. Although the trial was initially powered for the primary endpoint of survival, there were very few deaths that occurred midway through the study, and thus the primary endpoint was changed to time to next pulmonary exacerbation, re-hospitalization or death. The study still has adequate power to detect differences between vitamin D and placebo with the new study endpoints. Several other biomarkers of health will be evaluated as secondary endpoints, including markers of inflammation and anti-microbial peptide concentrations as well as markers of lung function.
Vitamin D deficiency has been recognized as a major co-morbidity in the CF population with prevalence rates reaching up to 90% of adults with CF [10]. Vitamin D status is often challenging to correct in the CF population owing to the malabsorption of fat soluble vitamins such as vitamin D [28]. Other approaches such as UV light therapy have been considered as potential treatment options for correcting vitamin D status in CF; however, patients often have difficulty with adherence to these treatments [29], [30]. Much larger doses of vitamin D are often required to raise vitamin D status in patients with CF [31], [32]. Vitamin D status overall is improving with time in the CF population following updated recommendations by the CF Foundation calling for higher doses of vitamin D [31], [33]; however, the evidence for improvement in clinical outcomes in CF from vitamin D therapy is still limited.
No large, multi-center, prospective study has been conducted examining vitamin D in patients with CF. A few retrospective studies have indicated that low serum 25-hydroxyvitamin D concentration was associated with pulmonary exacerbation. Vanstone et al. reported that low vitamin D status in children was associated with increased number of pulmonary exacerbations of CF [19]. McCauley et al. having a higher serum 25-hydroxyvitamin D in children was protective of having a pulmonary exacerbation during adolescence [18]. Vitamin D may be enhancing the immune system to improve production of anti-microbial peptides to clear pathogenic bacterial from the blood and lung. Simoneau et al. reported vitamin D insufficiency was associated with history of lung colonization with Pseudomonas aeruginosa [34]. In our previously conducted study, we found that a large dose bolus dose of vitamin D given at the time of pulmonary exacerbation of CF resulted in improved 1 year survival, decreased inflammatory cytokines, and a trend towards improvement of repeat pulmonary exacerbations [24], [25].
If the bolus dose of vitamin D is demonstrated to be superior to placebo on our primary endpoints, then there may be sufficient evidence to change clinical practice to rapidly correct vitamin D status at the time of pulmonary exacerbation of CF. Additionally, more concerted efforts would be undertaken to ensure vitamin D status is optimized in all children and adults with CF. Depending on our results, our study may also provide support for the hypothesis that vitamin D enhances innate immunity by increasing production of anti-microbial peptides and dampening inflammation which play a role in improving lung function and survival in CF. This study is not designed to determine the optimal concentration of serum 25(OH)D since participants will be given vitamin D or placebo without regard to baseline 25(OH)D concentrations unless they are severely deficient or in the mid upper range of normal. In post-hoc analyses, we will explore whether baseline vitamin D status influences the response to our high dose vitamin D3 regimen. In addition, we may uncover other unknown effects of CF drug therapies on vitamin D metabolism that render vitamin D ineffective in triggering the innate immune system [35].
There remains to be a great deal of enthusiasm for the administration of supplemental high-dose vitamin D to people with CF. Vitamin D has been linked to several other co-morbid conditions common in CF including diabetes and CF bone disease. The overall results of the DISC study will inform whether vitamin D has any impact on markers of innate immunity and inflammation, recovery of lung function, recurrent pulmonary exacerbations, and survival after 1 year following a pulmonary exacerbation of CF.
Acknowledgments
The study investigators would like to acknowledge the Data Safety and Monitoring Board (DSMB) members (Chair: Laurie LeClair, Members: Octavian Ioachimescu, Eric Felner, Traci Leong) for their dedication to the oversight and safety review of the study. We would also like to acknowledge the outstanding work by the research study coordinators and CF care teams at each site which made this study possible. The study was supported by a grant from the CF Foundation Clinical Research Award Program (TANGPR11A0) and indirectly by the CF Foundation Therapeutic Drug Network and Center Grants. The National Center for Advancing Translational Sciences of the National Institutes of Health and the Atlanta Clinical and Translational Science Institute (UL1TR000454) provided additional research support at the coordinating site at Emory University.
Footnotes
Vitamin D for Enhancing the Immune System in Cystic Fibrosis Investigators
References
- 1.Cutting GR Cystic fibrosis genetics: from molecular understanding to clinical application. Nat. Rev. Genet. 2015 Jan;16(1):45–56. doi: 10.1038/nrg3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strausbaugh S.D., Davis P.B. Cystic fibrosis: a review of epidemiology and pathobiology. Clin. Chest Med. 2007;28(2):279–288. doi: 10.1016/j.ccm.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Elborn J.S. Cystic fibrosis. Lancet. 2016 Apr 29 pii: S0140–6736(16)00576-6 [Epub ahead of print] [Google Scholar]
- 4.Ode K.L., Moran A. New insights into cystic fibrosis-related diabetes in children. Lancet Diabetes Endocrinol. 2013 Sep;1(1):52–58. doi: 10.1016/S2213-8587(13)70015-9. [DOI] [PubMed] [Google Scholar]
- 5.Siwamogsatham O., Alvarez J.A., Tangpricha V. Diagnosis and treatment of endocrine comorbidities in patients with cystic fibrosis. Curr. Opin. Endocrinol. Diabetes Obes. 2014 Oct;21(5):422–429. doi: 10.1097/MED.0000000000000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schindler T., Michel S., Wilson A.W. Nutrition Management of Cystic Fibrosis in the 21st Century. Nutr. Clin. Pract. 2015 Aug;30(4):488–500. doi: 10.1177/0884533615591604. [DOI] [PubMed] [Google Scholar]
- 7.O'Reilly R., Fitzpatrick P., Leen G. Severe bone demineralisation is associated with higher mortality in children with cystic fibrosis. Ir. Med. J. 2009;102:47–49. [PubMed] [Google Scholar]
- 8.Smith P.J., Blumenthal J.A., Trulock E.P., Freedland K.E., Carney R.M., Davis R.D., Hoffman B.M., Palmer S.M. Psychosocial Predictors of Mortality Following Lung Transplantation. Am. J. Transpl. 2016 Jan;16(1):271–277. doi: 10.1111/ajt.13447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolfenden L.L., Judd S.E., Shah R., Sanyal R., Ziegler T.R., Tangpricha V. Vitamin D and bone health in adults with cystic fibrosis. Clin. Endocrinol. (Oxf) 2008 Sep;69(3):374–381. doi: 10.1111/j.1365-2265.2008.03216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chesdachai S., Tangpricha V. Treatment of vitamin D deficiency in cystic fibrosis. J. Steroid Biochem. Mol. Biol. 2015 Sep 10 doi: 10.1016/j.jsbmb.2015.09.013. pii: S0960–0760(15)30073-X [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grossmann R.E., Tangpricha V. Evaluation of vehicle substances on vitamin D bioavailability: a systematic review. Mol. Nutr. Food Res. 2010 Aug;54(8):1055–1061. doi: 10.1002/mnfr.200900578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chesdachai S., Tangpricha V. Treatment of vitamin D deficiency in cystic fibrosis. J. Steroid Biochem. Mol. Biol. 2015 Sep 10 doi: 10.1016/j.jsbmb.2015.09.013. pii: S0960–0760(15)30073-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Black P.N., Scragg R. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest. 2005;128:3792–3798. doi: 10.1378/chest.128.6.3792. [PubMed] [DOI] [PubMed] [Google Scholar]
- 14.Kearns M.D., Alvarez J.A., Seidel N., Tangpricha V. Impact of vitamin D on infectious disease. Am. J. Med. Sci. 2015 Mar;349(3):245–262. doi: 10.1097/MAJ.0000000000000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finklea J.D., Grossmann R.E., Tangpricha V. Vitamin D and chronic lung disease: a review of molecular mechanisms and clinical studies. Adv. Nutr. 2011 May;2(3):244–253. doi: 10.3945/an.111.000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stephenson A., Brotherwood M., Robert R., Atenafu E., Corey M., Tullis E. Cholecalciferol significantly increases 25-hydroxyvitamin D concentrations in adults with cystic fibrosis. Am. J. Clin. Nutr. 2007 May;85(5):1307–1311. doi: 10.1093/ajcn/85.5.1307. [DOI] [PubMed] [Google Scholar]
- 17.Pincikova T., Nilsson K., Moen I.E., Karpati F., Fluge G., Hollsing A., Knudsen P.K., Lindblad A., Mared L., Pressler T., Hjelte L. Scandinavian Cystic Fibrosis Study Consortium. Inverse relation between vitamin D and serum total immunoglobulin G in the Scandinavian Cystic Fibrosis Nutritional Study. Eur. J. Clin. Nutr. 2011 Jan;65(1):102–109. doi: 10.1038/ejcn.2010.194. [DOI] [PubMed] [Google Scholar]
- 18.McCauley L.A., Thomas W., Laguna T.A., Regelmann W.E., Moran A., Polgreen L.E. Vitamin D deficiency is associated with pulmonary exacerbations in children with cystic fibrosis. Ann. Am. Thorac. Soc. 2014 Feb;11(2):198–204. doi: 10.1513/AnnalsATS.201208-068OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vanstone M.B., Egan M.E., Zhang J.H., Carpenter T.O. Association between serum 25-hydroxyvitamin D level and pulmonary exacerbations in cystic fibrosis. Pediatr. Pulmonol. 2015 May;50(5):441–446. doi: 10.1002/ppul.23161. [DOI] [PubMed] [Google Scholar]
- 20.Dimitrov V., White J.H. Species-specific regulation of innate immunity by vitamin D signaling. Steroid Biochem. Mol. Biol. 2015 Sep 11 doi: 10.1016/j.jsbmb.2015.09.016. pii: S0960-0760(15) 30075–3. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez J.A., Chowdhury R., Jones D.P., Martin G.S., Brigham K.L., Binongo J.N., Ziegler T.R., Tangpricha V. Vitamin D status is independently associated with plasma glutathione and cysteine thiol/disulphide redox status in adults. Clin. Endocrinol. (Oxf) 2014 Sep;81(3):458–466. doi: 10.1111/cen.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foong R.E., Bosco A., Jones A.C., Gout A., Gorman S., Hart P.H., Zosky G.R. The effects of in utero vitamin D deficiency on airway smooth muscle mass and lung function. Am. J. Respir. Cell Mol. Biol. 2015 Nov;53(5):664–675. doi: 10.1165/rcmb.2014-0356OC. [DOI] [PubMed] [Google Scholar]
- 23.Britt R.D., Jr., Thompson M.A., Freeman M.R., Stewart A.L., Pabelick C.M., Prakash Y.S. Vitamin D Reduces Inflammation-induced Contractility and Remodeling of Asthmatic Human Airway Smooth Muscle. Ann. Am. Thorac. Soc. 2016 Mar;13(Suppl 1):S97–S98. doi: 10.1513/AnnalsATS.201508-540MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grossmann R.E., Zughaier S.M., Liu S., Lyles R.H., Tangpricha V. Impact of vitamin D supplementation on markers of inflammation in adults with cystic fibrosis hospitalized for a pulmonary exacerbation. Eur. J. Clin. Nutr. 2012 Sep;66(9):1072–1074. doi: 10.1038/ejcn.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grossmann R.E., Zughaier S.M., Kumari M., Seydafkan S., Lyles R.H., Liu S., Sueblinvong V., Schechter M.S., Stecenko A.A., Ziegler T.R., Tangpricha V. Pilot study of vitamin D supplementation in adults with cystic fibrosis pulmonary exacerbation: A randomized, controlled trial. Dermatoendocrinol. 2012 Apr 1;4(2):191–197. doi: 10.4161/derm.20332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tirouvanziam R., Gernez Y., Conrad C.K., Moss R.B., Schrijver I., Dunn C.E., Davies Z.A., Herzenberg L.A., Herzenberg L.A. Profound functional and signaling changes in viable inflammatory neutrophils homing to cystic fibrosis airways. Proc. Natl. Acad. Sci. U. S. A. 2008 Mar 18;105(11):4335–4339. doi: 10.1073/pnas.0712386105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tirouvanziam R., Diaz D., Gernez Y., Laval J., Crubezy M., Makam M. An integrative approach for immune monitoring of human health and disease by advanced flow cytometry methods. In: Tuchin V.F., editor. Advanced Optical Flow Cytometry: Methods and Disease Diagnoses. Wiley-VCH Verlag GmbH & co. KGaA; Weinheim, Germany: 2011. pp. 333–362. [Google Scholar]
- 28.Lark R.K., Lester G.E., Ontjes D.A., Blackwood A.D., Hollis B.W., Hensler M.M., Aris R.M. Diminished and erratic absorption of ergocalciferol in adult cystic fibrosis patients. Am. J. Clin. Nutr. 2001 Mar;73(3):602–606. [Google Scholar]
- 29.Khazai N.B., Judd S.E., Jeng L., Wolfenden L.L., Stecenko A., Ziegler T.R., Tangpricha V. Treatment and prevention of vitamin D insufficiency in cystic fibrosis patients: comparative efficacy of ergocalciferol, cholecalciferol, and UV light. J. Clin. Endocrinol. Metab. 2009 Jun;94(6):2037–2043. doi: 10.1210/jc.2008-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gronowitz E., Larkö O., Gilljam M., Hollsing A., Lindblad A., Mellström D., Strandvik B. Ultraviolet B radiation improves serum levels of vitamin D in patients with cystic fibrosis. Acta Paediatr. 2005 May;94(5):547–552. doi: 10.1111/j.1651-2227.2005.tb01937.x. [DOI] [PubMed] [Google Scholar]
- 31.Tangpricha V., Kelly A., Stephenson A., Maguiness K., Enders J., Robinson K.A., Marshall B.C., Borowitz D. Cystic Fibrosis Foundation Vitamin D Evidence-Based Review Committee. An update on the screening, diagnosis, management, and treatment of vitamin D deficiency in individuals with cystic fibrosis: evidence-based recommendations from the Cystic Fibrosis Foundation. J. Clin. Endocrinol. Metab. 2012 Apr;97(4):1082–1093. doi: 10.1210/jc.2011-3050. [DOI] [PubMed] [Google Scholar]
- 32.Ferguson J.H., Chang A.B. Vitamin D supplementation for cystic fibrosis. Chrane Database Syst. Rev. 2014 May 14;(5):CD007298. doi: 10.1002/14651858.CD007298.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siwamogsatham O., Dong W., Binongo J.N., Chowdhury R., Alvarez J.A., Feinman S.J., Enders J., Tangpricha V. Relationship between fat-soluble vitamin supplementation and blood concentrations in adolescent and adult patients with cystic fibrosis. Nutr. Clin. Pract. 2014 Apr 17;29(4):491–497. doi: 10.1177/0884533614530170. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simoneau T., Bazzaz O., Sawicki G.S., Gordon C. Vitamin D status in children with cystic fibrosis. Associations with inflammation and bacterial colonization. Ann. Am. Thorac. Soc. 2014 Feb;11(2):205–210. doi: 10.1513/AnnalsATS.201306-171BC. [DOI] [PubMed] [Google Scholar]
- 35.Chesdachai S., Zughaier S.M., Hao L., Kempker R.R., Blumberg H.M., Ziegler T.R., Tangpricha V. The effects of first-line anti-tuberculosis drugs on the actions of vitamin D in human macrophages. J. Clin. Transl. Res. 2016 Dec;6:23–29. doi: 10.1016/j.jcte.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]