Abstract
Alveolar soft part sarcoma (ASPS) is a rare but distinct soft tissue tumor with unique histopathological and electron microscopic features. Orbital involvement is rare with only few reports published in the literature. ASPS have an indolent clinical course, but it is known to metastasize. Primary modality of treatment is surgery followed by adjuvant treatment. This case is a unique presentation with orbital mass with on and off bleeding. This is the largest orbital ASPS for which orbital exenteration was performed.
Keywords: Orbit, Alveolar soft part sarcoma, Pediatric
Introduction
Alveolar soft part sarcoma (ASPS) is a rare but discrete soft tissue tumor which accounts for 0.5–1 % of all soft tissue sarcomas. It has inimitable histopathological and electron microscopic features. It is a slow-growing malignant neoplasm with an uncertain histogenesis. ASPS first defined and named by Christopherson et al. in 1952 [1].Most of these tumors occur in adolescents and young adults (age 15–30 years), and females are more often affected. They are usually located in the head and neck region in infants and children, but in young adults they are predominantly found in extremities [2–4]. These tumors are extremely vascular. Involvement of orbit is rare with only few case reports published in literature [5].Although ASPS has an indolent clinical course, it is known to metastasize. Awareness of its histopathological and immunohistochemical features would help in the early diagnosis and treatment.
Case Report
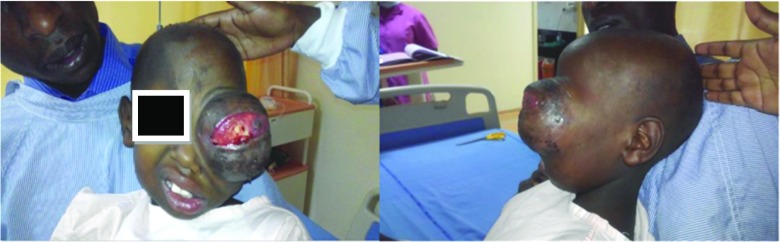
A 7-year-old girl, presented to the department of surgical oncology head and neck services with a left orbital swelling, proptosis, and a non-functional left eye with on and off bleeding from orbital mass. Past history revealed that the patient was a diagnosed case of rhabdomyosarcoma of left globe (with loss of vision) in 2012 outside and she received 5 cycles of vincristine, adraimycin, cyclophosphamide (VAC). But, there was no improvement in the signs and symptoms, rather there was a gradual increase in the orbital swelling, with a non-functional eye with on and off bleeding and very gross protrusion of globe. On examination, the mass was involving the left globe with gross proptosis with distortion of the globe with total loss of vision in the left eye without cervical lymphadenopathy. Biopsy from mass was suggestive of inflammatory pathology.
Contrast-enhanced computed tomography of the left orbit showed large exophytic protruding intensely enhancing heterogeneous mass lesion measuring 8.6 × 7.5cm with increased vascularity all around the lesion with expansion of orbit (Fig. 1). The left globe was not visualized. All the orbital walls are thinned out and eroded with no obvious intracranial extension. Bone scan was done and the scan findings are suggestive of increased activity in left maxillary and left infraorbital region -? local infiltration.
Fig. 1.
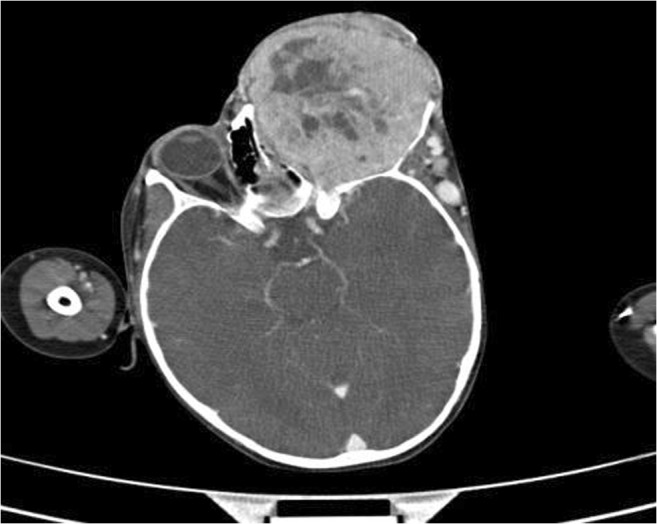
Preoperative CT scan
Further, metastatic work-up did not show any evidence of disease elsewhere in the body. In view of its unresponsiveness to chemotherapy and slow progression of the disease, it was uncertain opinion regarding the diagnosis, so after a thorough multi-departmental discussion in tumor board, the opinion was in favor of orbital exenteration in view of non-functional altered structure of the left eye, size >8 cm with globe protrusion and on and off bleeding.
Left orbital exenteration and temporalis muscle flap reconstruction was done.
Intraoperative findings are the following: highly vascular well-encapsulated mass involving left globe with gross disfigurement and proptosis of left eye. Extraorbital extension was found in the floor of the orbit and through the lateral orbital wall. The orbit was expanded. All the walls of the orbit eroded. All the tumors with its extraorbital extensions grossly resected enbloc. The defect area was reconstructed using temporalis muscle flap and the available hypertrophied eyelids (Figs. 2, 3, 4, 5, and 6).
Fig. 2.
Preoperative pictures
Fig. 3.
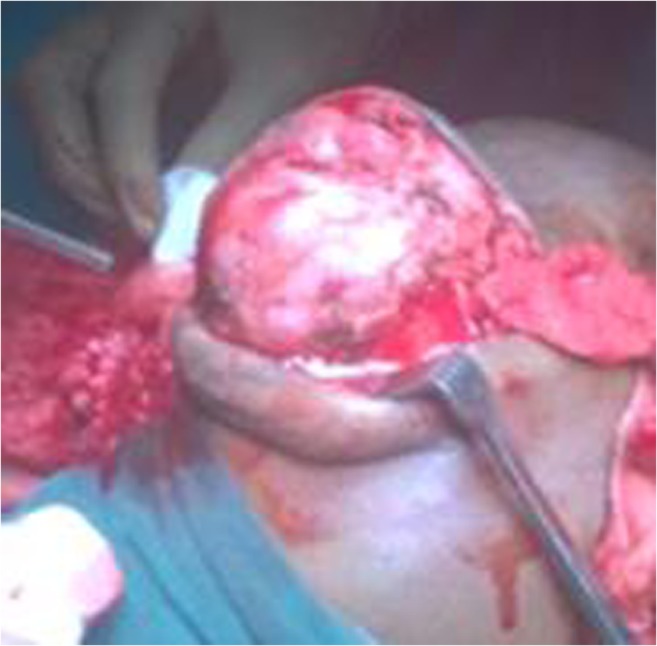
Intraoperative picture
Fig. 4.
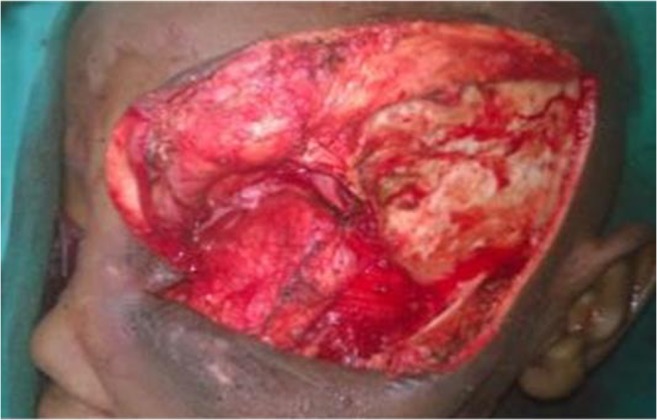
Defect closed by temporalis flap
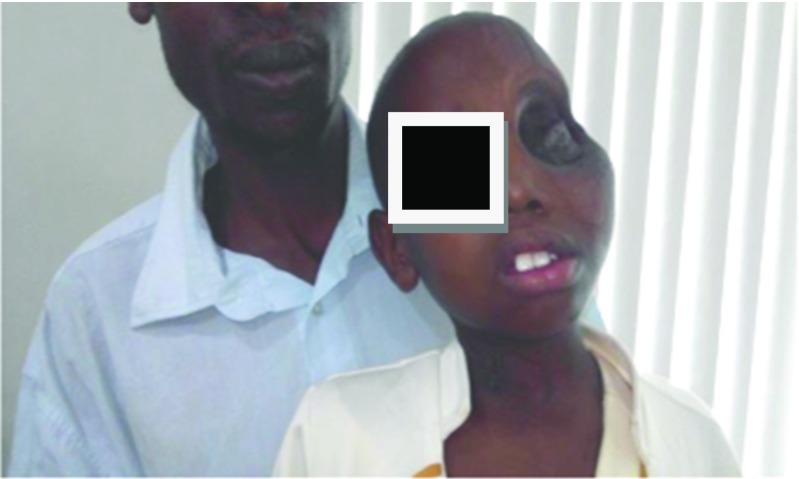
Fig. 5.
Postoperative picture
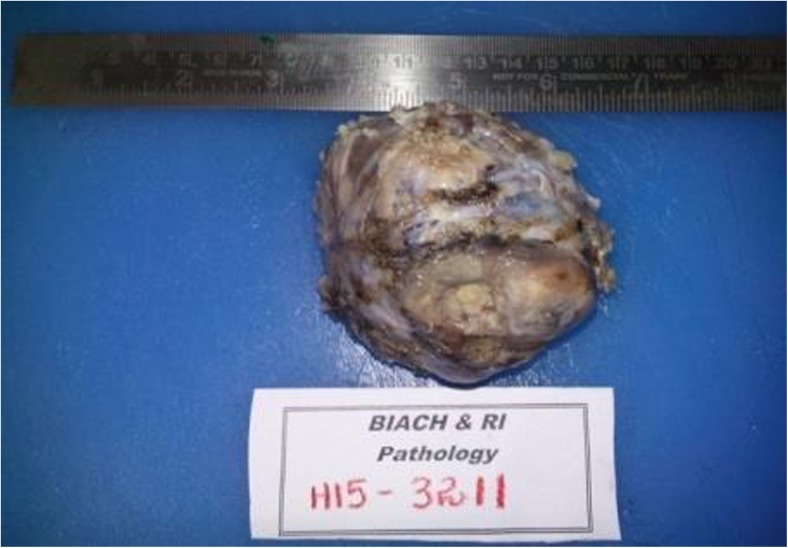
Fig. 6.
Left orbital exenteration specimen measuring 8 ×7 × 5.5cms. Cut surface shows gray white nodular tumor measuring 6.5 × 6.5 × 5.5cm with areas of hemorrhage. There is infiltration into the posterior segment of the eye ball
Pathology:
Grossfindings
Microscopic Findings
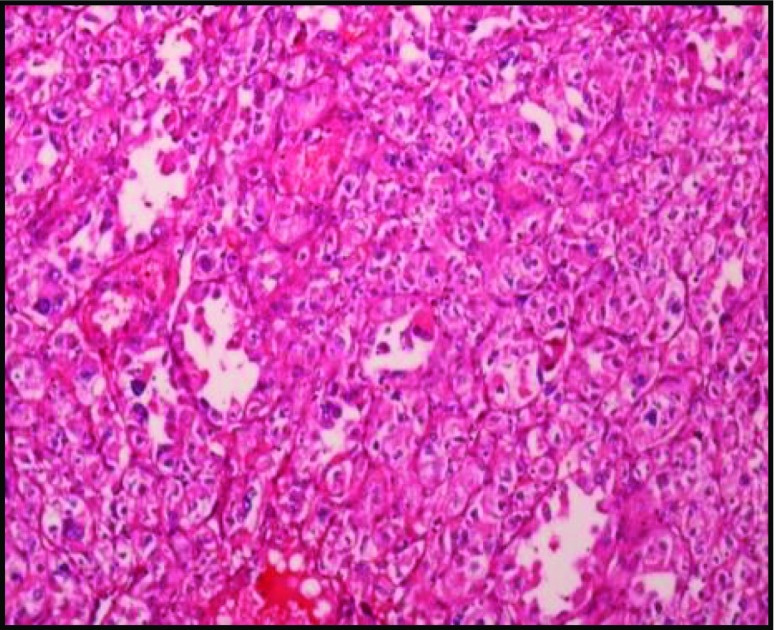
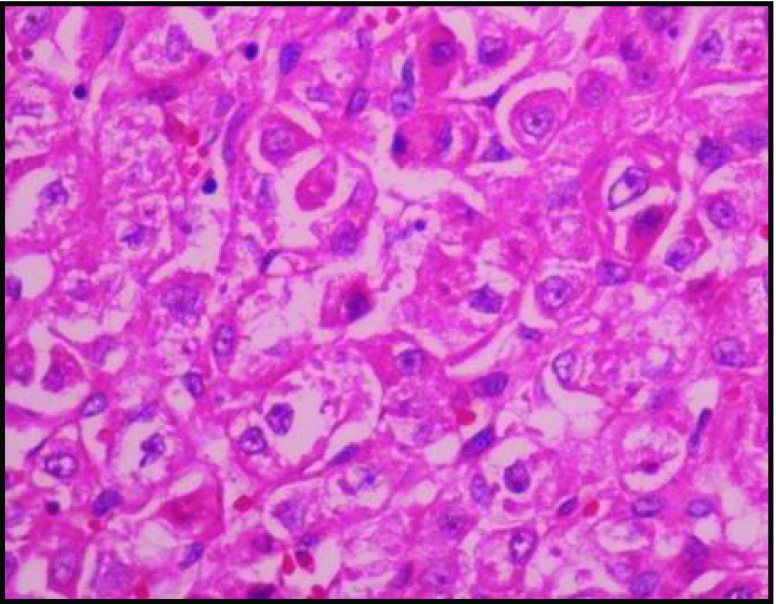
Sections reveal highly cellular lesion composed of alveolar nests and aggregates of oval to polygonal cells with vesicular nuclei abundant clear to eosinophilic cytoplasm, and mitotic figures are seen. There is focal tubular arrangement of cells with hobnailing of cells. There is focal tubulocystic pattern with few clusters lying loose in the lumen. The neoplastic cell clusters are separated by broad fibrous bands. There are interspersed branching vascular channels. The cornea is free of tumors. The retinal pigment epithelium, is uninvolved by the tumor (Figs. 7 and 8).
Fig. 7.
H & E 100 ×
Fig. 8.
H & E 400 ×
Discussion
Alveolar soft part sarcoma(ASPS) is rare soft-tissue sarcomas with 0.5–1 % incidence. It has a characteristic and invariable histological appearance. They are caused by a specific unbalanced translocation, der(16)t(X:17)(p11;p25), which results in the formation of an ASPL–TFE3 fusion gene [6–11]. Involvement of orbit is very rare. Orbital ASPS has been described to occur in both adults and children. The commonest presenting feature is non-pulsatile and non-reducible proptosis. Pain can also be a presenting feature [12]. Restricted ocular motility is not a universal feature [13]. Compression of optic nerve might lead to decreased vision. In the present case scenario, the patient presented with 8.6 × 7.5 cm mass which was non-functional, painful, and bleeding eye with hypertrophic eyelids. This is the largest alveolar soft part sarcoma of the orbit reported till now.
The clinical differential diagnosis of ASPS is a schwannoma, neurofibroma, or hemangioma because of circumscribed nature of the lesion, orbital location, and closeness to the rectus muscle or optic nerve [6, 14].
Pathologically the differential diagnosis includes malignant melanoma, paraganglioma, alveolar rhabdomyosarcoma, and granular cell tumor.
ASPS are usually negative for epithelial markers EMA and CK, neuroendocrine markers synaptophysin and chromogranin A, and melanocytic markers HMB45 and melan A (Tables 1 and 2). Expression for vimentin, NSE, SMA, skeletal muscle actin, and desmin has been documented in up to 50 % of ASPS. MyoD1 and TFE-3 have been reported to be expressed strongly in ASPS [14–21].
Table 1.
IHC
| INI−1 | Negative |
| CD 99 | Focal positive |
| S-100 | Negative |
| TFE-3 | Positive |
| CD 31 | Positive |
| Ki-67 | 12–15 % |
| CD 34 | Positive |
| Desmin | Focal positive |
| Vimentin | Focal positive |
| Melan A | Negative |
| CD 56 | Negative |
| CD 68 | Focal positve |
| EMA | Negative |
| Myogenin | Negative |
| Pancytokeratin | Negative |
Table 2.
Pathological differential diagnosis
| Diagnosis | EMA | HMB 45 | Melan A | S-100 |
|---|---|---|---|---|
| ASPS | − | − | N/A | ± |
| Paraganglioma | − | − | ± | + |
| Melanoma | − | + | + | + |
| Granular cell tumor | − | − | − | + |
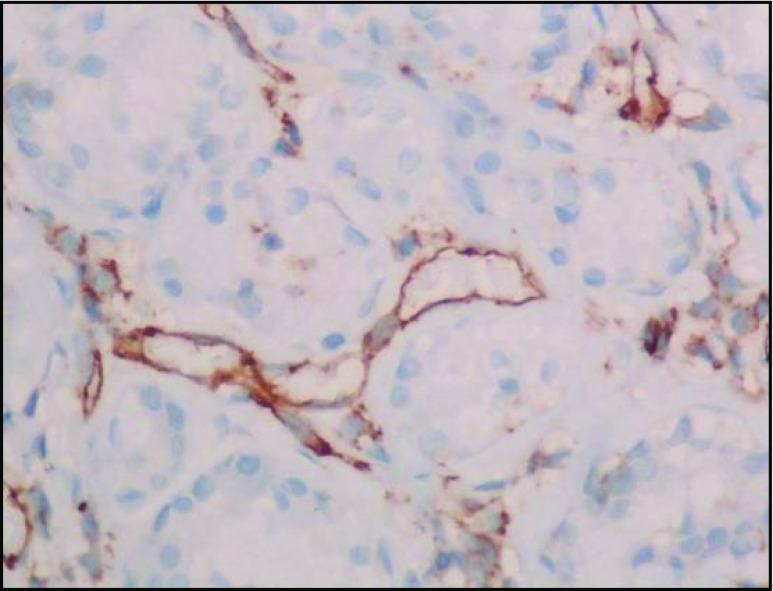
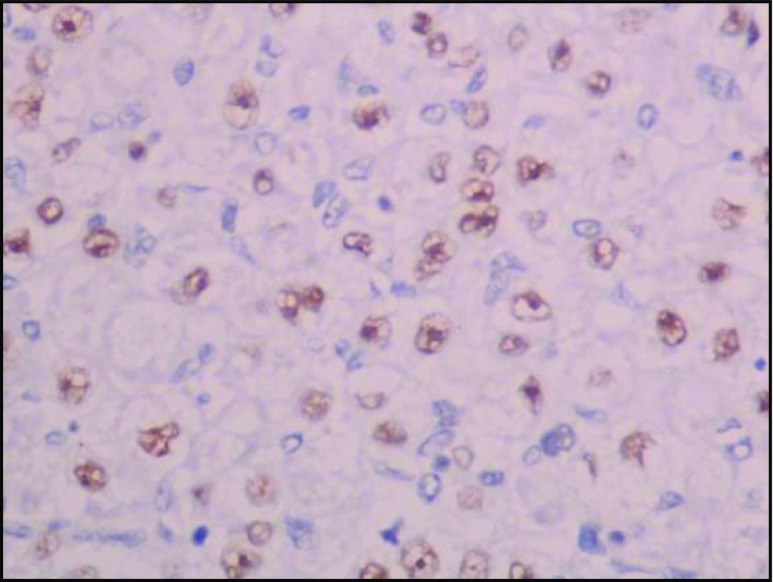
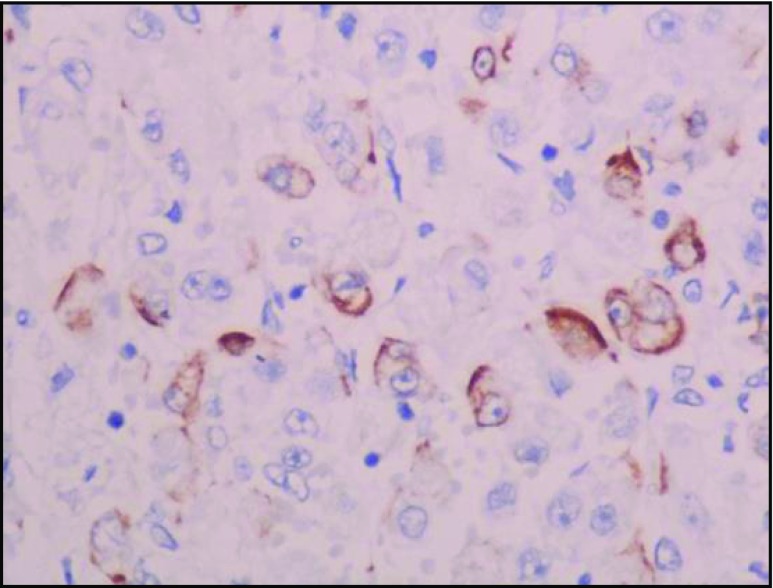
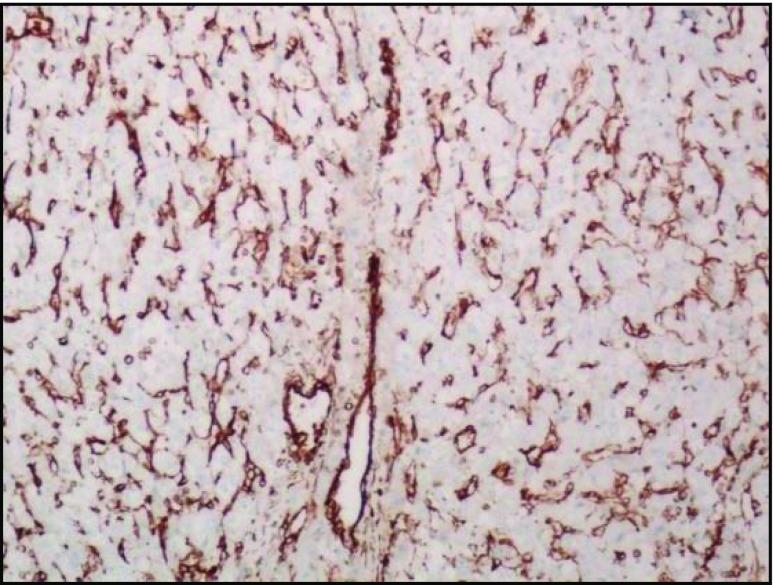
In our study, IHC analysis showed TFE-3 positivity, vimentin, desmin, CD 68, and CD 99 focal positivity. CD 34 and CD 31 positivity highlights the vascular nature of the tumor. Ki-67 positivity was ranging from 12 to 15 % which shows the slow and indolent nature of the disease (Figs. 9, 10, 11, and 12).
Fig. 9.
CD 34 positive
Fig. 10.
TFE 3 positive
Fig. 11.
Desmin focal positive
Fig. 12.
Vimentin focal positive
The intracytopasmic PAS-positive, diastase resistant needle shaped crystals are present in only 80 % of all cases of ASPS. Hence, absence of crystals does not rule out ASPS [22]. ASPS is characterized by a specific chromosomal alteration, der(16)t(X:17)(p11:q25), resulting in fusion of the transcription factor E3 (TFE3) with alveolar soft part sarcoma critical region 1 (ASPSCR1) at 17q25. This translocation is diagnostically useful because the tumor nuclei are positive for TFE3 by immunohistochemistry. ASPS is consistently positive for an antibody that detects the carboxyl terminal portion of the transcription factor E3 (TFE3) gene retained in the fusion protein. The pattern of expression is strong nuclear staining [23].
In the present case scenario where it was misdiagnosed and treated as rhabdomyosarcoma for almost 3 years, shows the indolent nature of the disease. Pathological features help in instigating the distinction between the rhabdomyosarcoma and ASPS (Table 3).
Table 3.
IHC comparison of ASPS and rhabdomyosarcoma
| IHC Markers | ASPS | Rhabdomyosarcoma |
|---|---|---|
| Vimentin | + | ± |
| Desmin | + | + |
| Myogenin | − | + |
| MyoD1 | + (Cytoplasmic only) | + (Strongly positive) |
| Cytokeratin | − | ± |
| Synaptophysin/ Chromogranin A |
− | ± |
| TFE-3 | + | − |
Surgery remains the primary treatment of choice followed by postoperative radiotherapy (33# 66Gy) [24–26] which helps to attain a good local control rate of up to 90 %. Complete surgical resection is necessary for better survival.
The exact role of neoadjuvant chemotherapy is unclear. Some studies show that there is an advantage of an extended 3-year disease specific survival. The most commonly used chemotherapy agents are doxorubicin and ifosfamide. Some show that there is no benefit [27–31]. The tumor can metastasize late in the course of the disease (median 6 years) with 38 % of metastases appearing 10 years after the diagnosis. Metastasis usually occurs in the lung, brain, or skeletal bone [32, 33]. In general, non-orbital ASPS tend to have poorer prognosis than those with tumors in the orbit. Tumors that occur in younger patients seem to have a better outcome. Poor prognostic factors include increasing age, tumors larger than 5 cm and metastatic disease at initial presentation [34, 35].
Conclusion
Alveolar soft part sarcoma is an uncommon soft tissue tumor, which rarely involves the orbit. This young girl presented with the largest alveolar soft part sarcoma of the orbit reported till now in the literature with 8.6 × 7.5 cm. Understanding its characteristic microscopic and ultra structural features would facilitate in early diagnosis. Prompt diagnosis with microscopic and immunohistochemistry helps in early diagnosis and better treatment. Surgery remains the primary modality followed by adjuvant therapy in the form of radiotherapy and chemotherapy.
Contributor Information
G kranthi kumar, Email: kranthikumargangiti@gmail.com.
Hemant Nemade, Phone: +91 9494715011, Email: drhemantnemade@gmail.com, Email: honemade@gmail.com.
Krishnamohan, Email: mvtkm@yahoo.com.
Daphne Fonseca, Email: daf_doc_2005@hotmail.com.
L.M.Chandra Sekhara Rao, Email: drlmcsraos@gmail.com.
T.Subramanyeshwar Rao, Email: subramanyesh@gmail.com.
References
- 1.Christopherson WM, Foote FW, Jr, Stewart FW. Alveolar soft-part sarcomas; structurally characteristic tumors of uncertain histogenesis. Cancer. 1952;5:100–111. doi: 10.1002/1097-0142(195201)5:1<100::AID-CNCR2820050112>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 2.Lieberman PH, Brennan MF, Kimmel M, et al. Alveolar soft-part sarcoma. A clinico-pathologic study of half a century. Cancer. 1989;63:1–13. doi: 10.1002/1097-0142(19890101)63:1<1::AID-CNCR2820630102>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 3.Pappo AS, Parham DM, Cain A, et al. Alveolar soft part sarcoma in children and adolescents: clinical features and outcome of 11 patients. Med PediatrOncol. 1996;26:81–84. doi: 10.1002/(SICI)1096-911X(199602)26:2<81::AID-MPO2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 4.Simmons WB, Haggerty HS, Ngan B, Anonsen CK. Alveolar soft part sarcoma of the head and neck. A disease of children and young adults. Int J Pediatr Otorhinolaryngol. 1989;17:139–153. doi: 10.1016/0165-5876(89)90089-X. [DOI] [PubMed] [Google Scholar]
- 5.Altamirano–Dimas M, Albores–Saavedra J. Alveolar soft part sarcoma of the orbit. Arch Ophthalmol. 1966;75:496–499. doi: 10.1001/archopht.1966.00970050498010. [DOI] [PubMed] [Google Scholar]
- 6.Lasundry J, Heimann P. Cytogenetic analysis of rare orbital tumours: further evidence for diagnostic implication. Orbit. 2000;19:87–95. doi: 10.1076/0167-6830(200006)1921-PFT087. [DOI] [PubMed] [Google Scholar]
- 7.Abrahams IW, Fenton RH, Vidone R. Alveolar soft-part sarcoma of the orbit. Arch Ophthalmol. 1968;79:185–188. doi: 10.1001/archopht.1968.03850040187016. [DOI] [PubMed] [Google Scholar]
- 8.Coupland SE, Heimann H, Hoffmeister B, Lee WR, Foerster MH, Gross U. Immunohistochemical examination of an orbital alveolar soft part sarcoma. Graefes Arch ClinExpOphthalmol. 1999;237:266–272. doi: 10.1007/s004170050231. [DOI] [PubMed] [Google Scholar]
- 9.Font RL. Jurco S 3 rd, Zimmerman E. Alveolar soft part sarcoma of the orbit: a clinicopathological analysis of seventeen cases and a review of the literature. Hum Pathol. 1982;13:569–579. doi: 10.1016/S0046-8177(82)80273-6. [DOI] [PubMed] [Google Scholar]
- 10.Ladanyi M, Lui MY, Antonescu CR, et al. The der(17)t(X;17)(p11;q25) of human alveolar soft part sarcoma fuses the TFE3 transcription factor gene to ASPL, a novel gene at 17q25. Oncogene. 2001;20:48–57. doi: 10.1038/sj.onc.1204074. [DOI] [PubMed] [Google Scholar]
- 11.Argani P, Lal P, Hutchinson B, et al. Aberrant nuclear immunoreactivity for TFE3 in neoplasms with TFE3 gene fusions: a sensitive and specific immunohistochemical assay. Am J SurgPathol. 2003;27:750–761. doi: 10.1097/00000478-200306000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Mukherjee PK, Agarwal S. Alveolar soft part sarcoma of the orbit. Indian J Ophthalmol. 1979;27:15–17. [PubMed] [Google Scholar]
- 13.Khan AO, Burke MJ. Alveolar soft-part sarcoma of the orbit. J PediatrOphthalmol Strabismus. 2004;41:245–246. doi: 10.3928/0191-3913-20040701-16. [DOI] [PubMed] [Google Scholar]
- 14.Grant GD, Shields JA, Flanagan JC, Horowitz P. The ultrasonographicand radiologic features of a histologically proven case of alveolar soft part sarcoma of the orbit. Am J Ophthalmol. 1979;87:773–777. doi: 10.1016/0002-9394(79)90352-0. [DOI] [PubMed] [Google Scholar]
- 15.Daigeler A, Kuhnen C, Hauser J, et al. (2008) Alveolar soft part sarcoma: clinicopathological findings in a series of 11 cases. World J Surg Oncol 6:71. doi:10.1186/1477-7819-6-71 [DOI] [PMC free article] [PubMed]
- 16.Rosai J, Dias P, Parham DM, et al. MyoD1 protein expression in alveolar soft part sarcoma as confirmatory evidence of its skeletal muscle nature. Am J SurgPathol. 1991;15:974–981. doi: 10.1097/00000478-199110000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Tallini G, Parham DM, Dias P, et al. Myogenic regulatory protein expression in adult soft tissue sarcomas. A sensitive and specific marker of skeletal muscle differentiation. Am J Pathol. 1994;144:693–701. [PMC free article] [PubMed] [Google Scholar]
- 18.Wang NP, Bacchi CE, Jiang JJ, et al. Does alveolar soft-part sarcoma exhibit skeletal muscle differentiation? An immunocytochemical and biochemical study of myogenic regulatory protein expression. Mod Pathol. 1996;9:496–506. [PubMed] [Google Scholar]
- 19.Gomez JA, Amin MB, Ro JY, et al. Immunohistochemical profile of myogenin and MyoD1 does not support skeletal muscle lineage in alveolar soft part sarcoma. Arch Pathol Lab Med. 1999;123:503–507. doi: 10.5858/1999-123-0503-IPOMAM. [DOI] [PubMed] [Google Scholar]
- 20.Cessna MH, Zhou H, Perkins SL, et al. Are myogenin and MyoD1 expression specific for rhabdomyosarcoma? A study of 150 cases, with emphasis on spindle cell mimics. Am J Surg Pathol. 2001;25:1150–1157. doi: 10.1097/00000478-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Cullinane C, Thorner PS, Greenberg ML, et al. Molecular genetic, cytogenetic, and immunohistochemical characterization of alveolar soft-part sarcoma. Implications for cell of origin. Cancer. 1992;70:2444–2450. doi: 10.1002/1097-0142(19921115)70:10<2444::AID-CNCR2820701010>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 22.Mannan R, Bhasin TS, Kaur P. Manjari M et al prominent intracytoplasmic crystals in alveolar soft part sarcoma (ASPS): an aid in cytological diagnosis. J Clin Diagn Res. 2014;8(2):145–146. doi: 10.1111/crj.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaber O, Kirby P. Alveolar soft part sarcoma. Arch Pathol Lab Med. 2015;11:1459–1462. doi: 10.5858/arpa.2014-0385-RS. [DOI] [PubMed] [Google Scholar]
- 24.Yang JC, Chang AE, Baker AR, Sindelar WF, Danforth DN, Topalian SL, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J ClinOncol. 1998;16:197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]
- 25.O’Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359:2235–2241. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 26.Gortzak E, Azzarelli A, Buesa J, Bramwell VH, Van Coevordon F, Van Geel AN, et al. A randomised phase II study on neo-adjuvant chemotherapy for ‘high-risk’ adult soft-tissue sarcoma. Eur J Cancer. 2001;37:1096–1103. doi: 10.1016/S0959-8049(01)00083-1. [DOI] [PubMed] [Google Scholar]
- 27.Grobmyer SR, Maki RG, Demetri GD, Mazumdar M, Riedel E, Brennan MF, et al. Neo-adjuvant chemotherapy for primary high-grade extremity soft tissue sarcoma. Ann Oncol. 2004;15:1667–1672. doi: 10.1093/annonc/mdh431. [DOI] [PubMed] [Google Scholar]
- 28.Sauer R, Schuchardt U, Hohenberger W, Wittekind C, Papadopoulas T, Grabenbauer GG, et al. Neoadjuvant radiochemotherapy in soft tissue sarcomas. Optimization of local functional tumor control. Strahlenther Onkol. 1999;175:259–266. doi: 10.1007/BF02743576. [DOI] [PubMed] [Google Scholar]
- 29.Edmonson JH, Petersen IA, Shives TC, Mahoney MR, Rock MG, Haddock MG, et al. Chemotherapy, irradiation, and surgery for function-preserving therapy of primary extremity soft tissue sarcomas: initial treatment with ifosfamide, mitomycin, doxorubicin, and cisplatin plus granulocyte macrophage-colony-stimulating factor. Cancer. 2002;94:786–792. doi: 10.1002/cncr.10259. [DOI] [PubMed] [Google Scholar]
- 30.DeLaney TF, Spiro IJ, Suit HD, Gebhardt MC, Hornicek FJ, Mankin HJ, et al. Neoadjuvant chemotherapy and radiotherapy for large extremity soft-tissue sarcomas. Int J Radiat Oncol Biol Phys. 2003;56:1117–1127. doi: 10.1016/S0360-3016(03)00186-X. [DOI] [PubMed] [Google Scholar]
- 31.Kraybill WG, Harris J, Spiro IJ, Ettinger DS, DeLaney TF, Blum RH, et al. Phase II study of neoadjuvant chemotherapy and radiation therapy in the management of high-risk, high-grade, soft tissue sarcomas of the extremities and body wall: Radiation Therapy Oncology Group Trial 9514. J Clin Oncol. 2006;24:619–625. doi: 10.1200/JCO.2005.02.5577. [DOI] [PubMed] [Google Scholar]
- 32.Cordier JF, Bailly C, Tabone E, Cheix F, Brune J, Touraine R. Alveolar soft part sarcoma presenting as asymptomatic pulmonary nodules: report of a case with ultrastructural diagnosis. Thorax. 1985;40:203–204. doi: 10.1136/thx.40.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Portera CA, Jr, Ho V, Patel SR, Hunk KK, Feig BW, Respondek PM, et al. Alveolar soft part sarcoma: clinical course and patterns of metastasis in 70 patients treated at a single institution. Cancer. 2001;91:585–591. doi: 10.1002/1097-0142(20010201)91:3<585::AID-CNCR1038>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 34.Henderson JW. Miscellaneous Orbital Tumors. Philadelphia, Lippincott: WilliamsandWilkins; 2007. pp. 361–363. [Google Scholar]
- 35.Morris WR, Padgett D, Osborn FD, Fleming JC. Pathologic quiz case: an orbital mass in a 45-year-old woman. Arch Pathol Lab Med. 2005;129:534–536. doi: 10.5858/2005-129-534-PQCAOM. [DOI] [PubMed] [Google Scholar]