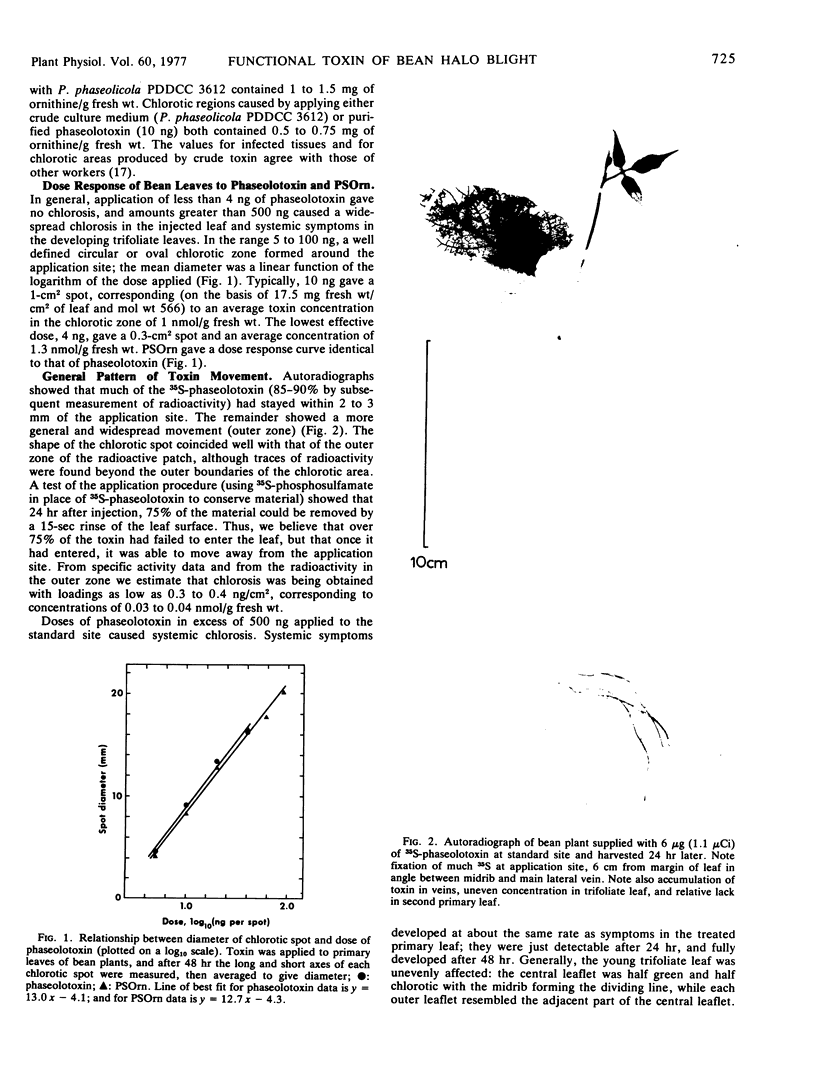
Abstract
Phaseolotoxin ([Nδ-phosphosulfamyl]ornithylalanylhomoarginine) is produced by Pseudomonas phaseolicola (Burkh.) Dows. in liquid culture. When phaseolotoxin was applied to leaves of bean (Phaseolus vulgaris L.) at 0.1 to 1 nmoles/g fresh weight of leaf by a prick-assay procedure, the characteristic “halo” symptom of bean halo blight disease developed after 24 to 48 hours. At higher concentrations (10-100 nmoles/g fresh weight) the systemic symptoms, which are commonly a feature of diseased plants, also developed after 24 to 48 hours.
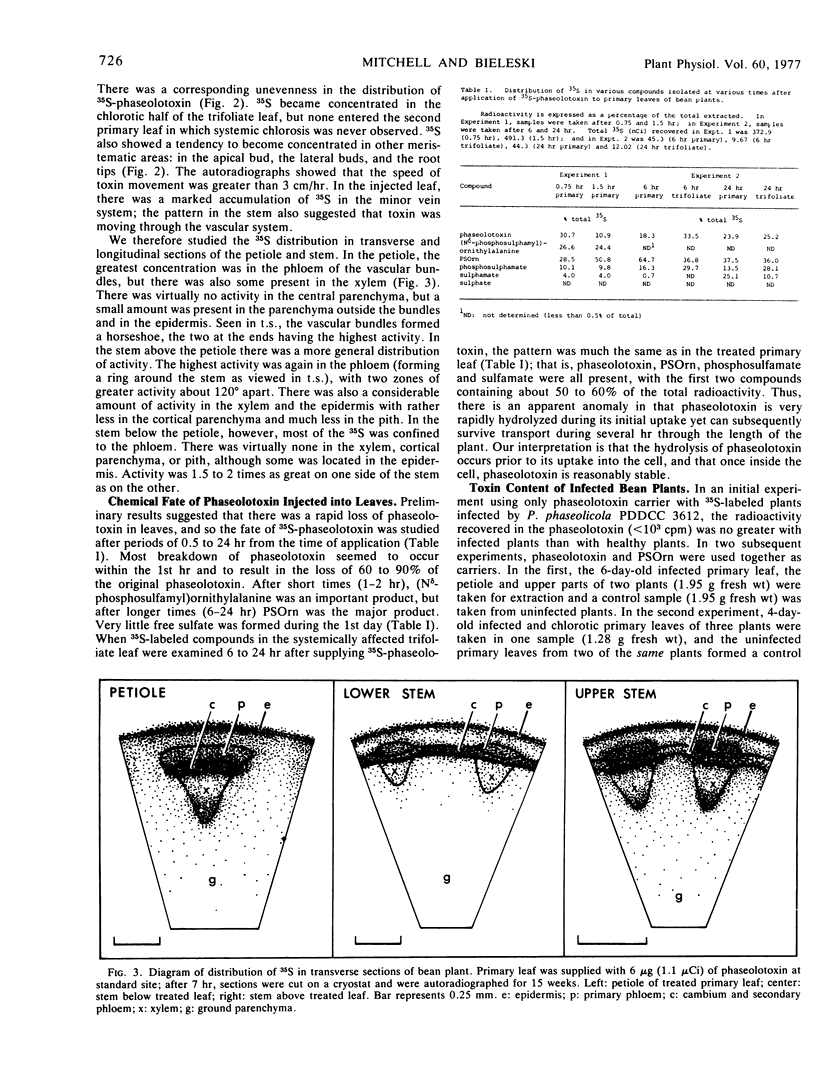
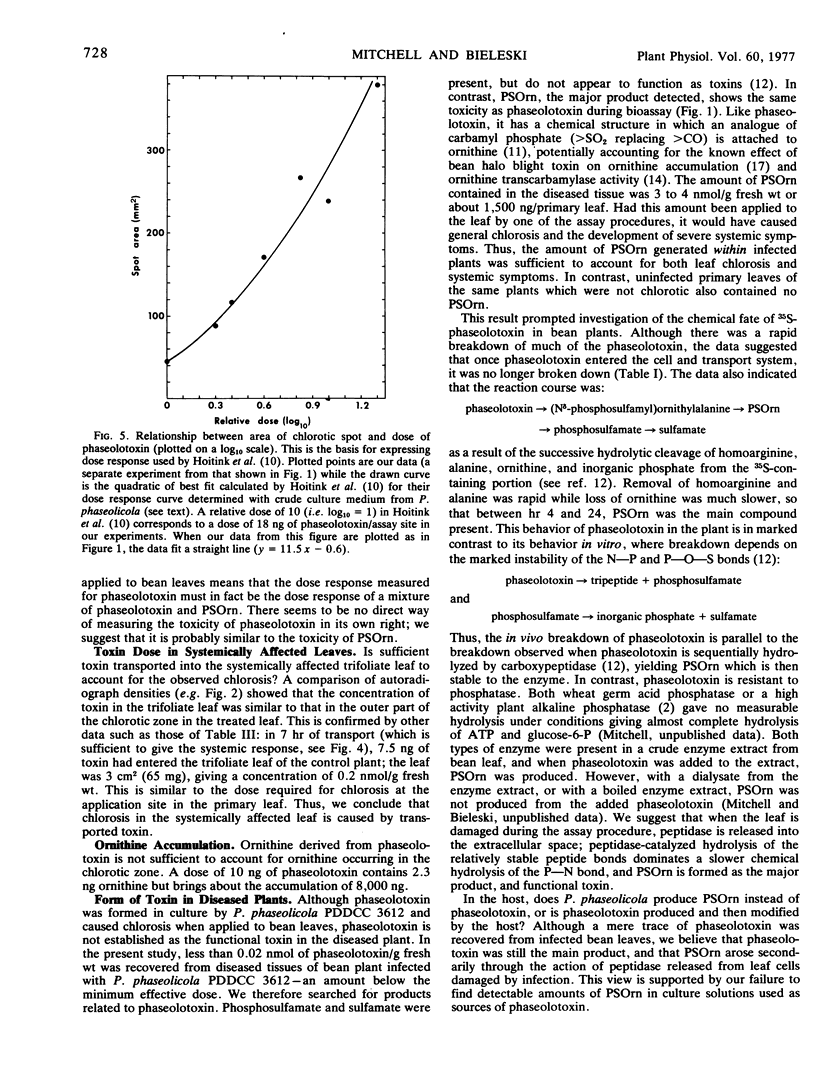
When applied to bean leaves, phaseolotoxin was rapidly broken down by the sequential removal of homoarginine and alanine. Nδ-Phosphosulfamylornithine was the major product formed, although phosphosulfamate and unreacted phaseolotoxin were also present. When P. phaseolicola infected bean plants, very little phaseolotoxin was detected within the plant, but the amount of Nδ-phosphosulfamylornithine formed was sufficient to account for the observed chlorosis, the ornithine accumulation, and the systemic symptoms. Nδ-Phosphosulfamylornithine therefore seemed to be the main functional phytotoxin of bean halo blight disease.
When 35S-phaseolotoxin was applied to primary leaves, 35S (assumed to be a mixture of phaseolotoxin, Nδ-phosphosulfamylornithine, and phosphosulfamate) was actively loaded into the fine veins of the leaf and moved through the plant in the vascular system at a speed greater than 3 cm/hour, particularly toward the apical and lateral buds and the root tips. Certain factors which affect pholem transport (arsenate, cold) affected toxin movement and the expression of systemic symptoms. Autoradiography suggested that the 35S was transported in the phloem.
A model for the involvement of phaseolotoxin in halo blight disease is presented.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bieleski R. L., Turner N. A. Separation and estimation of amino acids in crude plant extracts by thin-layer electrophoresis and chromatography. Anal Biochem. 1966 Nov;17(2):278–293. doi: 10.1016/0003-2697(66)90206-5. [DOI] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Patil S. S., Youngblood P., Christiansen P., Moore R. E. Phaseotoxin A: an antimetabolite from Pseudomonas phaseolicola. Biochem Biophys Res Commun. 1976 Apr 19;69(4):1019–1027. doi: 10.1016/0006-291x(76)90474-5. [DOI] [PubMed] [Google Scholar]
- WOOLLEY D. W., PRINGLE R. B., BRAUN A. C. Isolation of the phytopathogenic toxin of Pseudomonas tabaci, an antagonist of methionine. J Biol Chem. 1952 May;197(1):409–417. [PubMed] [Google Scholar]




