Abstract
The axillary reverse mapping (ARM) technique has been described as an attempt to map and preserve the upper extremity lymphatic drainage during axillary lymph node dissection (ALND) and/or SLNB. This technique is based on the hypothesis that the lymphatic pathway from the upper extremity is not involved by metastasis from primary breast cancer. The ARM node/s however, has been found, in various studies, to be involved with metastatic foci in patients with extensive axillary lymph node metastases. Therefore, the oncological safety of this procedure has not yet been determined. In this pilot study, we assessed the ARM node intraoperatively for various parameters and compared it to final HPR, to try and determine the oncologic safety of preserving the ARM node. Seventy-two breast cancer patients were screened for this prospective pilot study which was planned to recruit 20 patients. The study was initiated on May 2014, 20 patients were recruited till July 2015. Eligibility criterion was as follows: patients requiring primary axillary lymph node dissection based on a clinically positive axilla. Forty-five patients were ineligible because they had either received neoadjuvant chemotherapy or underwent previous axillary surgery or axillary radiation (exclusion criteria). Seven patients refused to give consent. ARM node identification rate was 75%. The most common location of the ARM node was lateral to the latissimus dorsi pedicle (42.10%), none of them being malignant. None of the oval or firm nodes were malignant. Tumor deposits were identified in 13%. Fine-needle aspiration cytology (FNAC) had 100% specificity, 94.4% negative predictive value, 100% positive predictive value, and 50% sensitivity. ARM is feasible using blue dye alone, with an acceptable identification rate. Location, consistency, and intraoperative FNAC of the ARM node, put together, may be reliable parameters to predict involvement of the ARM node with metastasis.
Keywords: Axillary reverse mapping, Sentinel node
Introduction
Axillary lymph node dissection (ALND) is the standard surgical treatment for breast cancer patients with clinically or pathologically involved axillary lymph nodes. Morbidity associated with ALND is significant, including lymphedema, neuropathy, and axillary seroma formation [1]. In particular, upper extremity (UE) lymphedema develops in 7–77% of patients who undergo ALND [2–5]. Several trials have shown lymphedema rates of approximately 7% even with sentinel lymph node biopsy alone [6, 7].
This lymphedema is believed to arise secondary to obstruction of upper limb lymphatics following axillary surgery.
Recently, the axillary reverse mapping (ARM) technique has been described as an attempt to map and preserve the UE lymphatic drainage during ALND and/or sentinel lymph node biopsy (SLNB) [8, 9]. This technique is based on the hypothesis that the lymphatic pathway from the UE is not involved by metastasis from primary breast cancer. The assumption is that the lymphatic drainage from the UE is different from that of the breast. The ARM technique allows safe removal of only the lymphatics of the breast and protects the lymphatic channels draining the UE during ALND or SLNB, thereby preventing lymphedema. However, several studies have shown that there are limits to the principle of nonoverlap between the breast and UE nodes. Specific concerns include (a) the ARM nodes may be involved with metastatic foci in patients with extensive axillary lymph node metastases [10–14] and (b) the SLN draining the breast may be the same as the ARM node draining the UE in some patients [11, 15–18]. Therefore, the oncological safety of this procedure has not yet been determined.
To our knowledge, there are limited reports on methods to assess the oncologic safety of preserving the ARM nodes during axillary lymph node mapping. We assessed the ARM node intraoperatively for its site, size, location, consistency, and perinodal adhesions. We also performed an intraoperative fine-needle aspiration cytology (FNAC) of the ARM node before harvesting it and sending it for histopathological examination. Comparison of the surgeon’s description of the node and intraoperative FNAC, with that of the final histopathological report, forms the basis of this study.
Materials and Methods
Seventy-two breast cancer patients were screened for this prospective pilot study which was planned to recruit 20 patients. The study was initiated on May 2014, 20 patients were recruited till July 2015. Eligibility criterion was as follows: patients requiring primary axillary lymph node dissection based on a clinically positive axilla. Forty-five patients were ineligible because they had either received neoadjuvant chemotherapy or underwent previous axillary surgery or axillary radiation (exclusion criteria). Seven patients refused to give consent. The study was approved by our Institutional Ethics Committee of and was funded by St. John’s Research Institute, a nonprofit funding body. Written informed consent was obtained from all patients that participated in the study.
Study protocol is shown in Fig. 1.
Fig. 1.
Two independent teams of pathologists were involved in reporting the ARM FNAC slides and final HPR of the ARM nodes. Both the teams were blinded to the findings of each other
Results
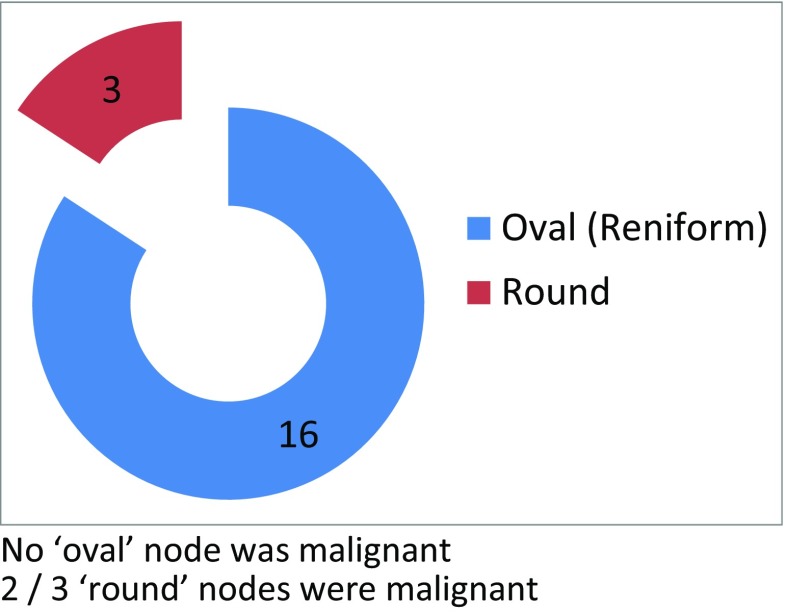
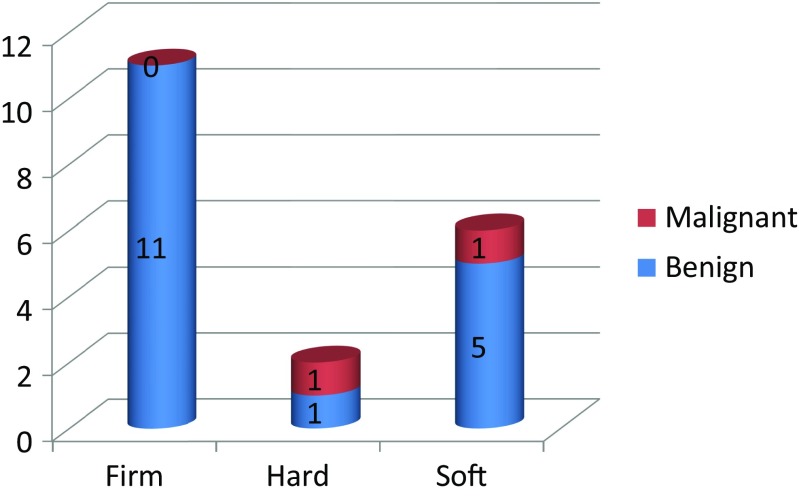
Among the 20 patients enrolled in the study, the most common stage of breast cancer was T2N1 (11/20) and the most common location of the primary was in the upper outer quadrant (10/20). The ARM node was identified in 15/20 patients (75% identification rate), with the mean ARM node per patient being 1.26 (19 nodes in 15 patients). The smallest and the largest node (along the short and long axes) were 1 × 0.5 and 3 × 1.5 cm, respectively. The operating surgeon described the shape of the ARM node as oval (16/19) or round (3/19). None of the nodes described as oval were malignant and two of the three round nodes turned out to be malignant (Fig. 2). Consistency of the ARM node was described by the operating surgeon as either firm (11/19), soft (6/19), or hard (2/19). None of the firm nodes were malignant. Of the two hard nodes, one had malignancy and of the six soft nodes, one had malignancy (Fig. 3). Three of the 19 identified ARM nodes were described by the operating surgeon, as having perinodal adhesions, but none of them were malignant on final histopathology.
Fig. 2.
Shape of ARM node—surgeon’s description. No “oval” node was malignant. Two out of three “round” nodes were malignant
Fig. 3.
Consistency of node—surgeon’s description
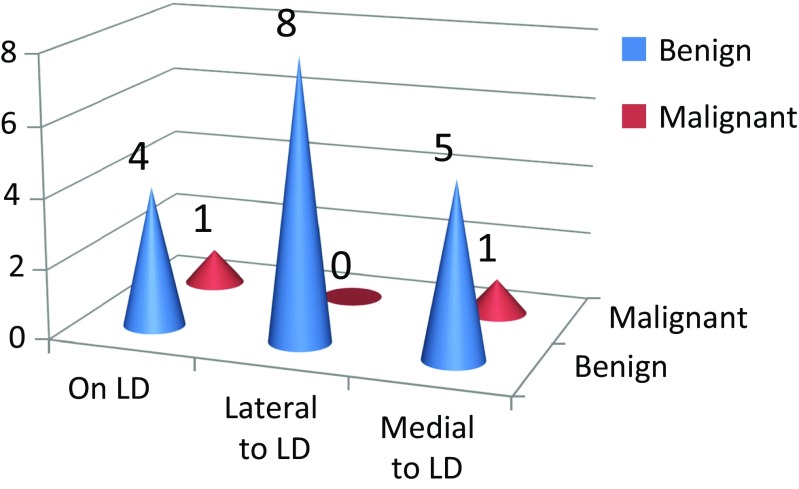
The most common position of the ARM node was lateral to the latissimus dorsi (LD) pedicle (8/19 42%). None of the nodes lateral to the LD pedicle had malignancy whereas one of the five nodes situated on the pedicle and one of the six nodes situated medial to the pedicle had malignancy (Fig. 4). Tumor deposits were identified in 2/15 patients (13%) and both were associated with extensive axillary LN metastasis (pN2 and pN3).
Fig. 4.
Location of ARM node and outcome
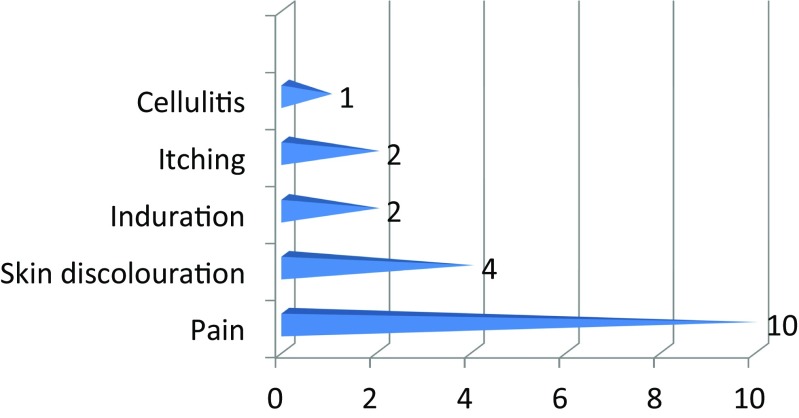
The most common complication of injecting methylene blue was pain at the site of injection with the maximum pain score being 2/10 on the visual analog scale. The duration of pain varied from 2 days to 2 weeks. The other complications being cellulitis 1, induration at site of injection 2, itching 2, and skin discoloration 4 (Fig. 5).
Fig. 5.
Complications
FNAC had a specificity of 100%, sensitivity of 50%, NPV of 94.4%, and a PPV of 100%.
Discussion
The concept of ARM was developed to map the upper limb draining lymphatics and lymph nodes with the help of blue dye and/or radioisotope colloid and then created the road map for preservation of these lymphatics and lymph nodes. Since lymphedema following ALND is believed to be caused as a result of removal of these lymphatics and lymph nodes, therefore by identifying and preserving these lymph nodes, the surgeons should be able to reduce lymphedema. However, little data is available regarding the oncological safety of preserving these ARM nodes. Noguchi et al. [1] had demonstrated the presence of metastasis in the ARM nodes. Therefore, in our study, we attempted to identify clinical and pathological criteria that could identify metastasis in the lymph nodes during the upper extremity using blue dye alone.
In our study, we found the ARM node identification rate of 75%. This is very similar to the identification rates of Nos C et al. [8] 71%, Thompson et al. [9] 61%, and Ponzone et al. [14] 50%. We found the risk of metastasis in the ARM nodes to be 13%. This is again similar to many published data like that of Nos et al. [8] 14%, Kang SH et al. [11] 8.9%, and Ponzone et al. [14] 11%. We found the most common location of the ARM node to be lateral in relation to the LD pedicle (42%).
None of the nodes situated lateral to the LD pedicle have metastasis. However, we found metastasis in one of the six nodes situated medial to the pedicle and one of the five nodes situated over the pedicle. This description of the location of the ARM nodes in relation to the LD pedicle and risk of metastasis are unique to our study. We have not found any other study trying to describe these aspects. Also, we found that the rounded shape of the node (as described by the surgeon) predicted metastasis, as two of the three nodes described as round in our study had metastasis, whereas all the nodes described as oval were free of metastasis. However, the size of the lymph node in our study was not a good predictor of metastasis because nodes as small as 1 × 0.5 cm were found to be positive for metastasis, whereas nodes as large as 2.5 cm were found to be normal. Also, the consistency, as described by us at the time of surgery, was not a good predictor. Therefore, one of the positive ARM nodes was actually described as soft. Therefore, when size, shape, and consistency were taken into account, we found shape to be a better predictor of the risk of metastasis than size and consistency. We could not find any other study which had specifically looked into size, shape, and consistency of the ARM nodes as a predictive risk factor.
We used intraoperative FNAC to predict the risk of metastasis. FNAC was found to have 50% sensitivity only. That is, one of the two ARM nodes with metastasis was missed by FNAC. However, specificity of FNAC was 100% and the negative predictive value of intraoperative FNAC was 94.4%. In our review of literature, we could not find any other study which utilized intraoperative FNAC to predict the risk of metastasis in ARM nodes. Therefore, we do not have data to compare this.
Conclusion
ARM is feasible using blue dye alone, with an acceptable identification rate.
Location, consistency, and intraoperative FNAC of the ARM node, put together, may be reliable parameters to predict involvement of ARM node with metastasis. One can consider sparing the ARM node which is
lateral to the LD pedicle,
oval in shape, and
intraoperative FNAC is benign.
When any one of the above parameters is not met, the oncologic safety of leaving behind the ARM node is yet to be established.
Compliance with Ethical Standards
The study was approved by our Institutional Ethics Committee of and was funded by St. John’s Research Institute, a nonprofit funding body. Written informed consent was obtained from all patients that participated in the study.
References
- 1.Noguchi M, Miwa K, Michigishi T, Yokoyama K, Nishijima H, Takanaka T, Kawashima H, Nakamura S, Nonomura A. The role of axillary lymph node dissection in breast cancer management. Breast Cancer. 1997;4(3):143–153. doi: 10.1007/BF02967068. [DOI] [PubMed] [Google Scholar]
- 2.Blanchard DK, Donohue JH, Reynolds C, Grant CS. Relapse and morbidity in patients undergoing sentinel lymph node biopsy alone or with axillary dissection for breast cancer. Arch Surg. 2003;138(5):482–488. doi: 10.1001/archsurg.138.5.482. [DOI] [PubMed] [Google Scholar]
- 3.Leidenius M, Leivonen M, Vironen J, von Smitten K. The consequences of long-time arm morbidity in node-negative breast cancer patients with sentinel node biopsy or axillary clearance. J Surg Oncol. 2005;92(1):23–31. doi: 10.1002/jso.20373. [DOI] [PubMed] [Google Scholar]
- 4.Haid A, Koberle-Wuhrer R, Knauer M, Burtscher J, Fritzsche H, Peschina W, Jasarevic Z, Ammann M, Hergan K, Sturn H, Zimmermann G. Morbidity of breast cancer patients following complete axillary dissection or sentinel node biopsy only: a comparative evaluation. Breast Cancer Res Treat. 2002;73(1):31–36. doi: 10.1023/A:1015234318582. [DOI] [PubMed] [Google Scholar]
- 5.Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, Yiangou C, Horgan K, Bundred N, Monypenny I, England D, Sibbering M, Abdullah TI, Barr L, Chetty U, Sinnett DH, Fleissig A, Clarke D, Ell PJ. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. JNCI. 2006;98(9):599–609. doi: 10.1093/jnci/djj158. [DOI] [PubMed] [Google Scholar]
- 6.Sakorafas GH, Peros G, Cataliotti L, Vlastos G. Lymphedema following axillary lymph node dissection for breast cancer. Surg Oncol. 2006;15(3):153–165. doi: 10.1016/j.suronc.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Wilke LG, McCall LM, Posther KE, Whitworth PW, Reintgen DS, Leitch AM, Gabram SG, Lucci A, Cox CE, Hunt KK, Herndon JE, II, Giuliano AE. Surgical complications associated with sentinel lymph node biopsy: results from a prospective international cooperative group trial. Ann Surg Oncol. 2006;13(4):491–500. doi: 10.1245/ASO.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Nos C, Lesieur B, Clough KB, Lecuru F. Blue dye injection in the arm in order to conserve the lymphatic drainage of the arm in breast cancer patients requiring an axillary dissection. Ann Surg Oncol. 2007;14(9):2490–2496. doi: 10.1245/s10434-007-9450-4. [DOI] [PubMed] [Google Scholar]
- 9.Thompson M, Korourian S, Henry-Tillman R, Adkins L, Mumford S, Westbrook KC, et al. Axillary reverse mapping (ARM): a new concept to identify and enhance lymphatic preservation. Ann Surg Oncol. 2007;14(6):1890–1895. doi: 10.1245/s10434-007-9412-x. [DOI] [PubMed] [Google Scholar]
- 10.Bedrosian I, Babiera GV, Mittendorf EA, Kuerer HM, Pantoja L, Hunt KK, Krishnamurthy S, Meric-Bernstam FM. A phase I study to assess the feasibility and oncologic safety of axillary reverse mapping in breast cancer patients. Cancer. 2010;116(11):2543–2548. doi: 10.1002/cncr.25096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang SH, Choi JE, Jeon YS, Lee SJ, Bae YK. Preservation of lymphatic drainage from arm in breast cancer surgery: is it safe? Cancer Res. 2009;69(Suppl 2):87s. [Google Scholar]
- 12.Noguchi M, Yokoi M, Nakano Y. Axillary reverse mapping with indocyanine fluorescence imaging in patients with breast cancer. J Surg Oncol. 2010;101(3):217–221. doi: 10.1002/jso.21473. [DOI] [PubMed] [Google Scholar]
- 13.Nos C, Kaufmann G, Clough KB, Collignon M-A, Zerbib E, Cusumano P, Lecuru F. Combined axillary mapping (ARM) technique for breast cancer patients requiring axillary dissection. Ann Surg Oncol. 2008;15(9):2550–2555. doi: 10.1245/s10434-008-0030-z. [DOI] [PubMed] [Google Scholar]
- 14.Ponzone R, Cont NT, Maggiorotto F, Cassina E, Mininanni P, Biglia N, Sismondi P. Extensive nodal disease may impair axillary reverse mapping in patients with breast cancer. J Clin Oncol. 2009;27(33):5547–5551. doi: 10.1200/JCO.2009.22.1846. [DOI] [PubMed] [Google Scholar]
- 15.Boneti C, Korourian S, Diaz Z, Santiago C, Mumford S, Adkins L, Klimberg VS. Scientific Impact Award: axillary reverse mapping (ARM) to identify and protect lymphatics draining the arm during axillary lymphadenectomy. Am J Surg. 2009;198(4):482–487. doi: 10.1016/j.amjsurg.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Britton TB, Solanki CK, Pinder SE, Mortimer PS, Peters AM, Purushotham AD. Lymphatic drainage pathways of the breast and the upper limb. Nucl Med Comm. 2009;30(6):427–430. doi: 10.1097/MNM.0b013e328315a6c6. [DOI] [PubMed] [Google Scholar]
- 17.Noguchi M. Axillary reverse mapping for breast cancer. Breast Cancer Res Treat. 2009;119:529–535. doi: 10.1007/s10549-009-0578-8. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda K, Ogawa Y, Komatsu H, Mori Y, Ishikawa A, Nakajima T, Oohira G, Tokunaga S, Fukushima H, Inoue T. Evaluation of the metastatic status of lymph nodes identified using axillary reverse mapping in breast cancer patients. World Journal of Surgical Oncology. 2012;10:233. doi: 10.1186/1477-7819-10-233. [DOI] [PMC free article] [PubMed] [Google Scholar]