Abstract
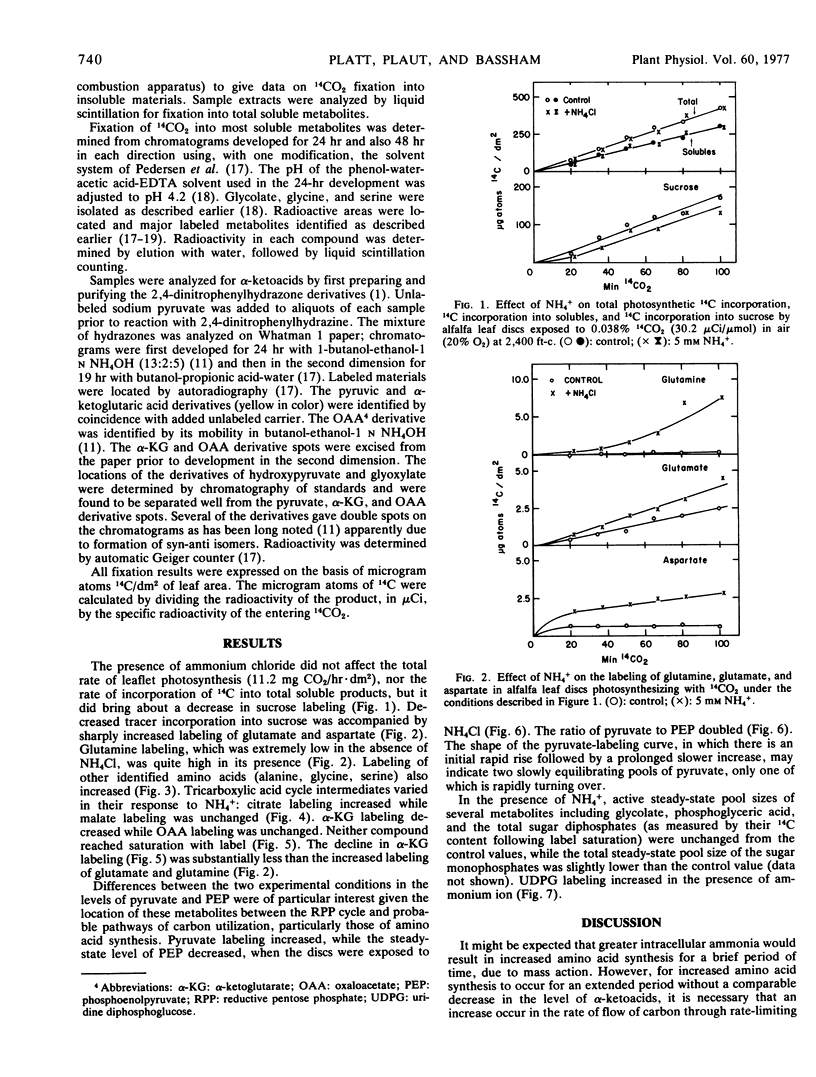
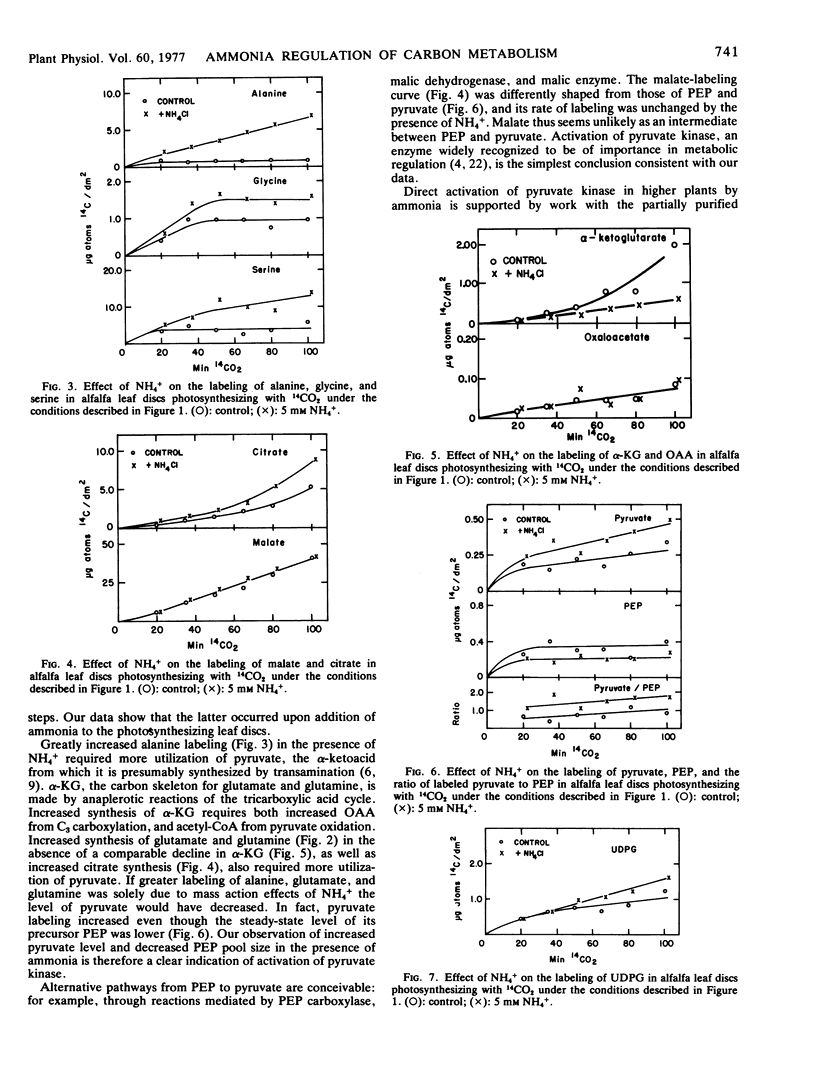
Alfalfa (Medicago sativa L., var. El Unico) leaf discs, floating on buffer containing NH4Cl and photosynthesizing with 14CO2, produced more labeled amino acid and less sucrose than did control discs (no added NH4Cl). The level of pyruvate increased and that of phosphoenolpyruvate decreased. These and other changes in levels of labeled compounds led us to conclude that pyruvate kinase was activated by ammonia, resulting in increased transfer of photosynthetically incorporated carbon to synthesis of amino acid skeletons at the expense of sucrose synthesis. Carbon flow through enzymes catalyzing the anaplerotic reactions was apparently stimulated.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Duggleby R. G., Dennis D. T. The characterization and regulatory properties of pyruvate kinase from cotton seeds. Arch Biochem Biophys. 1973 Apr;155(2):270–277. doi: 10.1016/0003-9861(73)90115-x. [DOI] [PubMed] [Google Scholar]
- Jensen R. G., Bassham J. A. Photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1095–1101. doi: 10.1073/pnas.56.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa T., Kirk M. R., Bassham J. A. Regulatory effects of ammonia on carbon metabolism in photosynthesizing Chlorella pyrenoidosa. Biochim Biophys Acta. 1970 Jun 30;205(3):401–408. doi: 10.1016/0005-2728(70)90106-4. [DOI] [PubMed] [Google Scholar]
- Kirk P. R., Leech R. M. Amino Acid Biosynthesis by Isolated Chloroplasts during Photosynthesis. Plant Physiol. 1972 Aug;50(2):228–234. doi: 10.1104/pp.50.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea P. J., Miflin B. J. Alternative route for nitrogen assimilation in higher plants. Nature. 1974 Oct 18;251(5476):614–616. doi: 10.1038/251614a0. [DOI] [PubMed] [Google Scholar]
- MCARDLE B. The quantitative estimation of pyruvic and alpha-oxoglutaric acids by paper chromatography in blood, urine and cerebrospinal fluid. Biochem J. 1957 May;66(1):144–148. doi: 10.1042/bj0660144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Evans H. J. The Influence of Salts on Pyruvate Kinase from Tissues of Higher Plants. Plant Physiol. 1957 Jul;32(4):346–354. doi: 10.1104/pp.32.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C. A., Stocking C. R. Kinetics and Energetics of Light-driven Chloroplast Glutamine Synthesis. Plant Physiol. 1975 Jan;55(1):59–63. doi: 10.1104/pp.55.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt S. G., Bassham J. A. Separation of 14 C-labeled glycolate pathway metabolites from higher plant photosynthate. J Chromatogr. 1977 Mar 21;133(2):396–401. doi: 10.1016/s0021-9673(00)83504-9. [DOI] [PubMed] [Google Scholar]
- Platt S. G., Plaut Z., Bassham J. A. Analysis of steady state photosynthesis in alfalfa leaves. Plant Physiol. 1976 Jan;57(1):69–73. doi: 10.1104/pp.57.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt S. G., Plaut Z., Bassham J. A. Steady-state photosynthesis in alfalfa leaflets: effects of carbon dioxide concentration. Plant Physiol. 1977 Aug;60(2):230–234. doi: 10.1104/pp.60.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson J. D., Turner J. F. Pyruvate kinase of higher plants. Biochim Biophys Acta. 1973 Nov 2;329(1):128–139. doi: 10.1016/0304-4165(73)90015-9. [DOI] [PubMed] [Google Scholar]


