Abstract
Ovaleap® (XM17) is a recombinant human follicle-stimulating hormone to treat infertility by inducing ovulation or controlled ovarian stimulation for assisted reproductive technology (ART) procedures. Ovaleap® (follitropin-α) was approved by the European Medicines Agency in 2013 as a biosimilar medicinal product to the reference medicine, Gonal-f®. Information is often not easily accessible and/or publicly available regarding the rigorous manufacturing procedures for biosimilars. Objectives of the current analysis were to report on validation procedures for the Ovaleap® manufacturing process, to compare the characteristics of Ovaleap® versus Gonal-f®, and to describe the performance and consistency of Ovaleap®. Formal validation of the Ovaleap® manufacturing process was performed at full commercial scale, consisting of several consecutive fermentation and purification runs. Comparison with Gonal-f® involved numerous techniques to determine molecular structure, isoform distribution, biological activity, and product-related impurities. The stability of the multidose application system, targeted for long-term stability at ambient temperature, was assessed and demonstrated. All analyses showed the manufacturing process of Ovaleap® to be robust and consistent. Ovaleap® was found to have similar characteristics when compared with Gonal-f®. This analysis supports the role of Ovaleap® as a biosimilar to Gonal-f®, thus providing patients and clinicians with another therapeutic option during ART procedures.
Key Points
| Comprehensive physicochemical and biological nonclinical characterization of Ovaleap®, a recombinant human follicle-stimulating hormone to treat infertility and an approved biosimilar, demonstrated similar molecular characteristics and quality attributes to the reference medicinal product, Gonal-f®. |
| The Ovaleap® manufacturing process was found to be robust and reliable. |
| The Ovaleap® multidose application system was found to have long-term stability at ambient temperature, consistent with product labeling. |
Introduction
Recombinant DNA methods have been frequently used to produce proteins such as human follicle-stimulating hormone (FSH) for assisted reproductive technology (ART) procedures. Some current FSH-containing products include those with follitropin-α (e.g. Gonal-f®, Merck Serono, Feltham, UK; Bemfola®, Finox Biotech AG, Balzers, Liechtenstein; and Pergoveris®, Merck Serono, Feltham, UK [active ingredients include both follitropin-α and lutropin-α]) and those with follitropin-β (e.g. Puregon®, Organon, Oss, The Netherlands; Follistim®, Merck Sharp & Dohme B.V., Whitehouse Station, NJ, USA), as well as a long-acting FSH-containing product, corifollitropin-α (Elonva®; Merck Sharp & Dohme B.V., Whitehouse Station, NJ, USA).
Ovaleap® (XM17; Teva Pharmaceuticals Europe B.V., The Netherlands) is a recombinant human FSH (r-hFSH) product that was approved in 2013 by the European Medicines Agency (EMA) as a biosimilar medicinal product to the reference medicine Gonal-f® [1]. Ovaleap® is intended for use in women for controlled ovarian stimulation and the treatment of anovulation, for stimulation of follicular development in women with severe luteinizing hormone (LH) and FSH deficiency (in association with an LH preparation), and in men for spermatogenesis stimulation [1]. It is supplied in cartridges (300 IU/0.5 mL/22 µg; 450 IU/0.75 mL/33 µg; and 900 IU/1.5 mL/66 µg) for use with a multidose reusable pen.
The active ingredient of Ovaleap® (follitropin-α) is a heterodimeric glycoprotein hormone consisting of two subunits. The α-subunit contains 92 amino acids, 10 cysteine residues resulting in five disulfide bridges, and two N-glycosylation sites, whereas the β-subunit consists of 111 amino acids, 12 cysteine residues resulting in six disulfide bridges, and two N-glycosylation sites [1]. Each glycosylation site might vary in terms of the composition and complexity of the oligosaccharides attached, with differing levels of branching, or antennary, possible (e.g. single-branched, di-branched, tri-branched, etc.) [2]. Depending on the presence or absence of a negatively charged sialic acid residue at the termination of each branch, a number of different isoforms with varying isoelectric points (pI) can exist.
Similar to most recombinant proteins, production of r-hFSH generally follows a well-established development process that involves (i) transfection of cultivated mammalian cells (e.g. Chinese Hamster Ovary [CHO] cells) with the human FSH gene; (ii) clonal selection and isolation of the production cell line; (iii) cell banking to provide a supply of cell substrate from the selected clone for continued manufacture of the product; and (iv) large-scale production processes involving fermentation and purification [3]. The development of the manufacturing process starts with pilot batches for process optimization prior to validation and production of industrial-size, full-scale batches with reproducible quality.
The EMA requires that biosimilar medicinal products undergo comprehensive physicochemical and biological nonclinical characterization, including evidence of comparability to the reference medicinal product [4]. The analytical testing of Ovaleap® covers two distinct aspects. Biosimilarity testing compares molecular characteristics and quality attributes to Gonal-f® (i.e. molecular mass, primary/secondary structure, isoform distribution, biological activity, and impurity profile). The second analytical testing component relates to the development of a robust manufacturing process of Ovaleap®, specifically the performance and consistency of the process (i.e. final product stability, specific activity, and batch-to-batch variability).
Given the complexity of biosimilars compared with conventional generics, nonclinical characterization alone is insufficient to ensure safety and efficacy. Subtle structural and other differences might be difficult to detect with even the most state-of-the-art analytical techniques. Thus, even if molecular and other analyzed characteristics are highly similar between the biosimilar and reference product, the bioequivalence and clinical comparability must be confirmed. Ovaleap® has undergone several clinical studies to demonstrate safety, tolerability, and efficacy. A phase I clinical study assessed the safety, tolerability, and pharmacokinetic profile of Ovaleap®, whereas two clinical comparative studies assessed bioequivalence and therapeutic equivalence to Gonal-f® [5, 6]. The first phase I study showed that single ascending doses of Ovaleap® exhibited dose-proportional pharmacokinetics [7]. In the pivotal phase I, two-way, crossover study, Lammerich and colleagues [5] demonstrated bioequivalence of single 300 IU doses of Ovaleap® to Gonal-f® with no serious adverse events or adverse events leading to discontinuation or dose reduction. Finally, in a phase III, randomized, active-controlled study in women undergoing hyperstimulation for ART, Strowitzki and colleagues [6] showed therapeutic equivalence based on the numbers of oocytes retrieved, with 12.2 ± 6.7 oocytes in the Ovaleap® treatment group compared with 12.1 ± 6.7 oocytes in the Gonal-f® group. Ovaleap® and Gonal-f® showed favorable and comparable safety profiles with no unexpected safety findings in this phase III study. In addition, the immunogenicity of Ovaleap® was similar to that of Gonal-f® [8].
Although some details regarding the manufacturing process of biosimilar products are available only to the EMA or other regulatory bodies and cannot be published for proprietary reasons, other details are nonproprietary in nature and might be of interest to the general public but are often not published and/or not easily accessible. As such, and given the increasing presence of biosimilar products in the marketplace, we considered it important and timely to report on some of the processes involved in the manufacturing and production of one such biosimilar product, Ovaleap®.
The objectives of the current analysis were to report on validation procedures for the Ovaleap® manufacturing process, to compare molecular characteristics and quality attributes of Ovaleap® versus Gonal-f®, and to describe the performance and consistency of the Ovaleap® manufacturing process.
Methods
Quality Development of Ovaleap®
The manufacturing process for Ovaleap® takes place primarily in Ulm, Germany, under full control of the manufacturer (Teva Biotech GmbH). As reported in the publicly available assessment report, the manufacturing process includes the cultivation of genetically modified CHO cells in a serum-free cell culture medium, into which the CHO cells secrete the active substance [1]. In order to secure consistent product quality and long-term supply, a two-tiered cell banking system was developed and maintained, which consists of a master cell bank of cells from the selected clone and a working cell bank obtained from culturing the master cell bank. The main steps of the Ovaleap® manufacturing process include thawing of cells and expansion in flasks, main culturing in a bioreactor, harvesting with filtration, and purification with a series of chromatography, virus inactivation, filtration, and ultrafiltration/diafiltration steps. The manufacturing process of Ovaleap® fulfilled all relevant European guidelines for sterility and viral safety available at the time of the study [9, 10].
Process Validation and Consistency
The formal validation of the Ovaleap® manufacturing process was performed at full commercial scale. The process validation program consisted of several consecutive fermentation runs and several consecutive purification runs.
Comparability with Gonal-f®
A comprehensive comparability exercise with the reference medicinal product, Gonal-f®, was performed to demonstrate biosimilarity. Molecular mass was assessed using various techniques, including matrix-assisted laser desorption ionization time of flight (MALDI-TOF) mass spectrometry and sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) with Coomassie stain. The primary structure was evaluated with peptide mapping using trypsin, endo Lys-C, and endo Glu-C, followed by electrospray ionization mass spectrometry (ESI-MS) determination of peptides. Ultraviolet spectroscopy was used to assess the secondary structure. Isoform distribution was determined via isoelectric focusing (IEF) and Serva Violet 17 staining. Biological activity was determined with several assays, including a cell-based receptor binding assay and a rat bioassay, as requested by current EMA guidelines [11, 12]. Product-related impurities, such as multimers and aggregates, were assessed utilizing techniques such as SDS-PAGE and size exclusion high-performance liquid chromatography (SE-HPLC).
Formulation and Stability
The Ovaleap® formulation was developed based on a multidose application system targeted for long-term stability at ambient temperature. Protein stability in the final product was assessed using a variety of storage conditions, including long-term conditions started by a 5-day storage period at 25 °C/60% relative humidity (RH) prior to storage at the claimed storage temperature of 5 °C without control of RH. These long-term conditions were performed alone or followed by short or long temperature excursions (Table 1).
Table 1.
Storage conditions to assess thermal stability
| Storage condition | Description |
|---|---|
| Long-term | Storage at 25 ± 2 °C/60 ± 5% RH for 5 days before storage at 5 ± 3 °C (claimed storage temperature) without control of RH |
| Long-term with short TE | Storage at 25 ± 2 °C/60 ± 5% RH for 5 days before storage at 5 ± 3 °C, followed by a final TE at 25 ± 2 °C/60 ± 5% RH for 1 month, and 28 days broached at 25 ± 2 °C/60 ± 5% RH |
| Long-term with long TE | Storage at 25 ± 2 °C/60 ± 5% RH for 5 days before storage at 5 ± 3 °C, followed by a final TE at 25 ± 2 °C/60 ± 5% RH for 3 months, and 28 days broached at 25 ± 2 °C/60 ± 5% RH |
| Accelerated | Storage at 25 ± 2 °C/65 ± 5% RH for up to 6 months |
| Stress | Storage at 40 ± 2 °C/75 ± 5% RH for up to 3 months |
TE temperature excursion, RH relative humidity
In total, seven batches (3 × 300 IU/0.5 mL, 1 × 450 IU/0.75 mL, and 3 × 900 IU/1.5 mL), produced at commercial scale, were included in the thermal stability study in order to create a bracketing design. The formulation of all dosage strengths is identical; only the fill volume of the cartridges varies [i.e. 0.5 mL (300 IU), 0.75 mL (450 IU), and 1.5 mL (900 IU)]. Stability data from the pivotal stability study of Ovaleap® under conditions described above were assessed for up to 24 months of storage for all seven batches.
Additionally, accelerated and stress storage conditions (up to 6 and 3 months, respectively) were investigated to provide supporting data on product stability, as recommended by the relevant guidelines [13], but will not be the focus of this report.
A filled-by-mass approach was used for filling cartridges (follitropin-α, 44 µg/mL). Batch-to-batch variability was assessed.
Results
Process Validation and Consistency
In the Ovaleap® fermentation process, all analyzed batch samples demonstrated that the manufacturing process is robust. The characteristics of all validation runs of intermediate cultures and main cultures in the production bioreactor were consistent and comparable.
The purification process, as measured by step recovery and yield, allowed for consistent and reproducible manufacturing of Ovaleap®. The r-hFSH produced in this process passed all in-process and release specifications in all validation runs. The consistency of the Ovaleap® purification process was demonstrated by highly comparable chromatogram profiles, reproducible elution positions of product, and removal of contaminants from chromatographic steps. The hygiene status of the overall purification process was well-controlled, as is shown by the absence of bioburden and endotoxin in the product intermediates and purified bulks of all validation batches.
Comparability with Gonal-f®
A summary of the results of the extensive program for characterization of Ovaleap® and comparability with Gonal-f® is shown in Table 2. Structural characteristics and product-related impurities were similar between Ovaleap® and Gonal-f®, and no substantive differences were found with respect to molecular mass, primary structure, secondary structure, biological activity, and product-related impurities.
Table 2.
Overview of extended characterization program of Ovaleap® and comparability with Gonal-f®
| Characteristic | Method | Ovaleap® | Gonal-f® | |
|---|---|---|---|---|
| Molecular mass | ||||
| Molecular mass (Daltons) | Nonreduced | SDS-PAGE Coomassie staining | Main band with approximately 40 kDa | Main band with approximately 40 kDa |
| Reduced | Main band with approximately 20 kDa; additional faint bands between 16 and 20 kDa | Main band with approximately 20 kDa; additional faint bands between 16 and 20 kDa | ||
| α-Subunit (native) | MALDI-TOF MS | 14.6 kDa | 14.6 kDa | |
| β-Subunit (native) | 17.8/18.3 kDaa | 17.8/18.3 kDaa | ||
| Primary structure | ||||
| Peptide mapping | Peptide mapping using trypsin, endo Lys-C, and endo Glu-C followed by ESI-MS determination of peptides | All determined masses of peptides correspond with expected masses; 100% sequence coverage achieved | All determined masses of peptides correspond with expected masses; 100% sequence coverage achieved | |
| Similar peptide maps obtained with Ovaleap® and Gonal-f® | ||||
| Secondary structure | ||||
| Structural topology | Far-UV circular dichroism spectroscopy | Samples have identical structural conformations and folding, which are superimposable with those of FSH reference standard | Samples have identical structural conformations and folding, which are superimposable with those of FSH reference standard | |
| Isoform distribution | ||||
| Isoform distribution profile | Isoelectric focusing (Serva Violet 17 staining) | Nine isoforms detectable with pI values between 3.6 and 5.3 | Nine isoforms detectable with pI values between 3.6 and 5.3 | |
| High similarity with respect to isoform distribution between Ovaleap® and Gonal-f® | ||||
| Biological activity | ||||
| In vitro bioassay | Cell-based receptor binding assay | Similar specific activities and dose–response curves obtained for Ovaleap® and Gonal-f® | ||
| In vivo bioassay | Rat bioassay | Similar specific activities determined for Ovaleap® and Gonal-f® | ||
| Product-related impurities | ||||
| Nonmonomeric r-hFSH (multimers and aggregate) | SE-HPLC | No aggregates detectable | No aggregates detectable | |
| Nonreducing SDS-PAGE Western immunoblot | No aggregates detectable; expected main band of heterodimer, additional faint bands of free subunits | No aggregates detectable; expected main band of heterodimer, additional faint bands of free subunits | ||
| Ovaleap® truncated forms | Reducing SDS-PAGE Western immunoblot | No truncated forms detectable; expected bands of free subunits | No truncated forms detectable; expected bands of free subunits | |
ESI-MS electrospray ionization mass spectrometry, FSH follicle-stimulating hormone, kDa kilodalton, MALDI-TOF MS matrix-assisted laser desorption ionization time of flight mass spectrometry, pI isoelectric points, r-hFSH recombinant human FSH, SDS-PAGE sodium dodecyl sulfate polyacrylamide gel electrophoresis, SE-HPLC size exclusion high-performance liquid chromatography, UV ultraviolet
aAfter harsh denaturation and reducing conditions
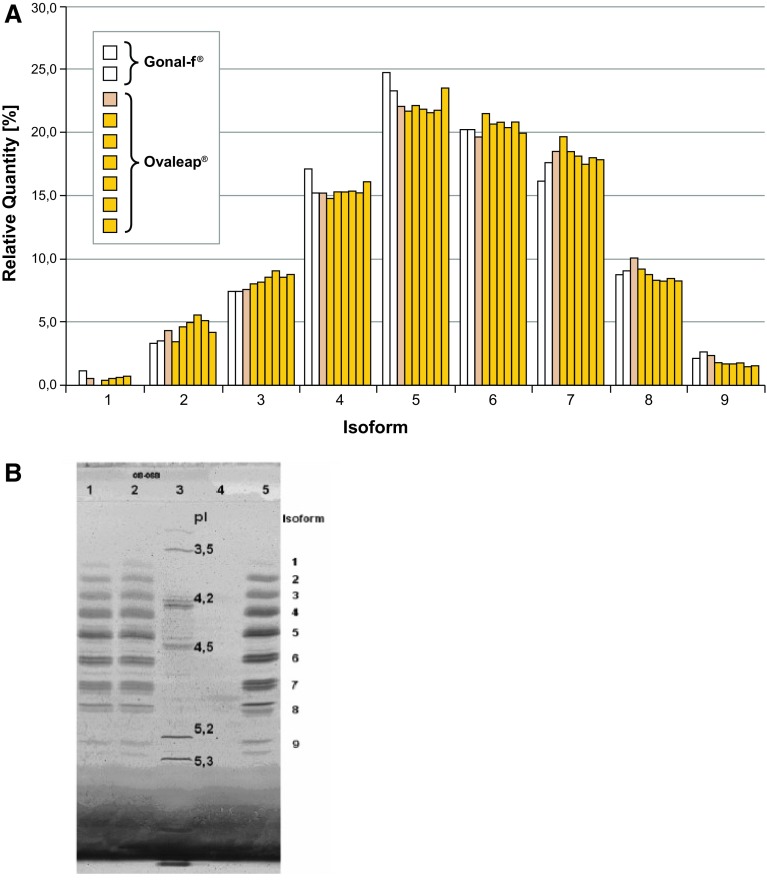
The pattern of isoform distribution as analyzed by IEF was comparable between different batches of Ovaleap® consistent with high batch-to-batch consistency (Fig. 1). In addition, the batches of Ovaleap® show a very similar distribution pattern of charged isoforms to those of Gonal-f®. Nine isoforms were detectable with pI values between 3.5 and 5.3 for both Ovaleap® and Gonal-f®. As charge differences mainly derive from differentially glycosylated follitropin, these results do not give any indication that there are relevant physicochemical differences between Ovaleap® and Gonal-f®.
Fig. 1.
Isoform distribution of Ovaleap® versus Gonal-f® by isoelectric focusing, as shown by a bar graph and b gel (Serva Violet 17 stain). In b, the decimal place is represented by a comma. Lane assignments are as follows: lanes 1 and 2 Gonal-f®; lane 3 pI marker; lane 4 empty; and lane 5 Ovaleap®. pI isoelectric points
Formulation and Stability
The active substance is formulated with sodium dihydrogen phosphate dihydrate (buffer), sodium hydroxide (pH-adjusting agent), mannitol (tonicity-adjusting agent), l-methionine (antioxidant), polysorbate 20 (solubilizer), benzyl alcohol (preservative—multidose preparation), benzalkonium chloride (preservative—multidose preparation) and water for injection (vehicle). These excipients are commonly used for biopharmaceutical preparations.
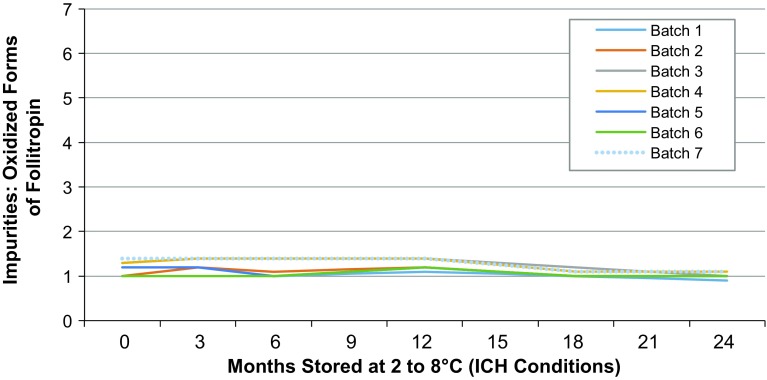
The developed formulation resulted in high protein stability. Data on storage for up to 24 months under long-term conditions alone and under long-term conditions with either long or short temperature excursions were within the specified ranges. Ovaleap® oxidized by RP-HPLC resulted in values between <1.0 and 1.4% for all seven batches when stored over 24 months at 2–8 °C (Fig. 2). Similarly, results of Ovaleap® monomer/aggregates by SE-HPLC for all seven batches indicated 100% monomers and no aggregates, and these results were constant over 24 months of storage at 25 °C. No trend was observed, therefore no statistical evaluation was applicable and all values were within Ovaleap® specification.
Fig. 2.
Summary of RP-HPLC results for oxidized Ovaleap® during stability testing. ICH International Conference on Harmonisation, RP-HPLC reversed phase high-performance liquid chromatography
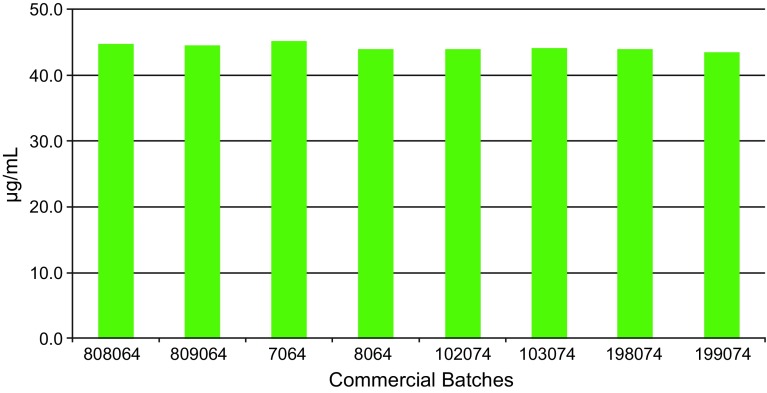
Eight commercial batches were examined for protein content (batch numbers 808064, 809064, 7064, 8064, 102074, 103074, 198074, 199074). Filling by mass led to consistent dosage strength with very low batch-to-batch variability (Fig. 3).
Fig. 3.
Protein content in eight commercial batches
Discussion
Ovaleap®, approved by the EMA in 2013 as a biosimilar to Gonal-f®, is produced via a robust and safe manufacturing process completely under the control of the manufacturer. Ovaleap® is manufactured using a CHO cell line, an extensively studied and widely utilized cell line for biopharmaceutical manufacturing processes with mammalian cell culture technology. The cell line and process, which includes a state-of-the-art, serum-free cell culture media, adhere to all relevant guidelines regarding viral safety in order to exclude any viral or prion contamination.
In the current analysis, the process validation studies effectively demonstrated that the fermentation and purification processes are consistent and reproducible in delivering high-quality, intermediate, and purified bulk Ovaleap® product. Results of the extended characterization program comparing Ovaleap® with Gonal-f® were as expected. Ovaleap® was shown to have a high degree of similarity to Gonal-f® with respect to molecular mass, primary structure, secondary structure, isoform distribution, biological activity, and product-related impurities.
In terms of product stability, no change in quality or consistency was observed when Ovaleap® was stored for up to 24 months at 5 ± 3 °C in multidose cartridges, representing the primary form of packaging. Additionally, data obtained during storage under long-term conditions with both long and short temperature excursions (+3 months at 25 °C +28 days broached at 25 °C, and +1 month at 25 °C +28 days broached at 25 °C, respectively) prior to removal from storage provided evidence that the quality of Ovaleap® would not be adversely affected by storage at 25 °C for 1–3 months (+28 days broached at 25 °C). Therefore, a shelf-life of 24 months while stored at 2–8 °C was supported by the available data. Furthermore, commercial batches show consistent dosage strength with very low batch-to-batch variability.
The formulation of Ovaleap® was developed based on a multidose application system. Ovaleap® is presented as a sterile, clear solution for subcutaneous injection at the concentration of 600 IU/44 µg follitropin-α per mL. The Ovaleap® cartridge can be stored for up to 3 months at room temperature (≤25 °C) and for up to an additional 28 days at room temperature (≤25 °C) once in the pen.
Benefits of the Ovaleap® pen device include reusability, compatibility with all three dosing cartridges (300, 450, and 900 IU), flexible dosing (12.5 IU dosing increments), reduced residual medication, easy changing of cartridges, and an environmentally friendly package. Other attributes of the pen device include the dial-back function, which allows for correction in case of over-rotation for dosing; the large window, which allows for easy reading of the dose to be displayed; the clear cartridge housing, which allows for quick confirmation of medication in the cartridge; and the release button on the side of the pen, which allows for an automatic release of the entire dose after touching this button (semi-automatic) for ease of use and comfort.
Conclusions
The current physicochemical and biological nonclinical analysis demonstrates the robust and rigorous nature of the Ovaleap® manufacturing process. The study confirms the reproducible quality of Ovaleap® and the high similarity to Gonal-f® on a molecular/physicochemical level. Due to the complexity of biosimilar products such as Ovaleap®, an heterodimeric glycoprotein composed of 92 amino acids in the α-subunit and 111 amino acids in the β-subunit, even state-of-the-art analytical techniques might be insufficient to detect subtle differences compared with the reference product. The results of these comparative nonclinical analyses are further supported by a clinical development program, including a pharmacokinetic bioequivalence and a clinical comparability study, which have shown bioequivalence and therapeutic equivalence of Ovaleap® compared with Gonal-f® [5, 6]. As a product biosimilar to Gonal-f®, Ovaleap® provides consumers and clinicians with an additional option when planning for ART procedures.
Acknowledgements
The authors wish to thank Rebecca Miles, PhD, of MedVal Scientific Information Services, LLC, for providing professional writing and editorial assistance. This manuscript was prepared according to the International Society for Medical Publication Professionals’ ‘Good Publication Practice for Communicating Company-Sponsored Medical Research: the GPP2 Guidelines’, and the International Committee of Medical Journal Editors’ ‘Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals’.
Author contributions
RW headed the team that did most of the analytical assays during process validation and comparability, provided input to the manuscript in several iterative rounds of writing, and approved the final article. JW headed the team that performed the process validation, provided input to the manuscript in several iterative rounds of writing, and approved the final article. BG headed the overall project team for the development of Ovaleap®, performed the nonclinical and clinical development, and approved the final article. AM provided input to the manuscript in several iterative rounds of writing and approved the final article. HA provided input to the manuscript in several iterative rounds of writing and approved the final article.
Compliance with Ethical Standards
Funding
This study was sponsored by BioGeneriX AG, a member of the Teva group. Medical writing assistance was provided by MedVal Scientific Information Services, LLC, Princeton, NJ, USA, and was funded by Teva Branded Pharmaceutical Products R&D, Inc. (Frazer, PA, USA). Teva provided a full review of the article.
Conflicts of interest
Rainer Winstel, Herman Allgaier, and Juergen Wieland are employees of Teva Biotech GmbH, Member of the Teva Group, Ulm, Germany. Arnd Mueller and Beate Gertz are employees of Merckle GmbH, Member of the Teva Group, Ulm, Germany.
Research involving human participants and/or animals
For this type of study, formal consent is not required.
References
- 1.European Medicines Agency. Ovaleap (follitropin alfa) [assessment report]. 31 July 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002608/WC500152908.pdf. Accessed 16 Dec 2016.
- 2.Andersen CY, Westergaard LG, van Wely M. FSH isoform composition of commercial gonadotrophin preparations: a neglected aspect? Reprod Biomed Online. 2004;9:231–236. doi: 10.1016/S1472-6483(10)62135-9. [DOI] [PubMed] [Google Scholar]
- 3.Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol. 2004;22:1393–1398. doi: 10.1038/nbt1026. [DOI] [PubMed] [Google Scholar]
- 4.European Medicines Agency. Guideline on similar biological medicinal products. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/10/WC500176768.pdf. Accessed 16 Dec 2016.
- 5.Lammerich A, Mueller A, Bias P, Phase I. two-way, crossover study to demonstrate bioequivalence and to compare safety and tolerability of single-dose XM17 vs Gonal-f® in healthy women after follicle-stimulating hormone downregulation. Reprod Biol Endocrinol. 2015;13:130. doi: 10.1186/s12958-015-0124-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strowitzki T, Kuczynski W, Mueller A, Bias P. Randomized, active-controlled, comparative phase 3 efficacy and safety equivalence trial of Ovaleap® (recombinant human follicle-stimulating hormone) in infertile women using assisted reproduction technology (ART) Reprod Biol Endocrinol. 2016;14:1. doi: 10.1186/s12958-015-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lammerich A, Bias P, Gertz B. Phase 1 safety, tolerability, and pharmacokinetic study of single ascending doses of XM17 (recombinant human follicle-stimulating hormone) in downregulated healthy women. Int J Womens Health. 2015;7:707–716. doi: 10.2147/IJWH.S83418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou L, Mueller A, Gertz B, Weiss H, Liu P. Immunogenicity assessment of Ovaleap® (XM17) recombinant human follicle-stimulating hormone in infertile women undergoing assisted reproductive technology (ART). In: Poster presented at the 17th World Congress of Gynecological Endocrinology, 2–5 March 2016; Florence, Italy.
- 9.ICH Expert Working Group. ICH harmonised tripartite guideline: viral safety evaluation of biotechnology products derived from cell lines of human or animal origin Q5A(R1). International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use; 23 Sep 1999. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q5A_R1/Step4/Q5A_R1__Guideline.pdf. Accessed 16 Dec 2016.
- 10.European Directorate for the Quality of Medicines & Healthcare (EDQM). Biological Tests - Sterility. In: European Pharmacopoeia, 8th ed. Strasbourg, France: Council of Europe, 2013. pp. 175–8.
- 11.European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP). Guideline on non-clinical and clinical development of similar biological medicinal products containing recombinant human follicle stimulating hormone (r-hFSH). http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/03/WC500139624.pdf. Accessed 16 Dec 2016.
- 12.Steelman SL, Pohley FM. Assay of the follicle stimulating hormone based on the augmentation with human chorionic gonadotropin. Endocrinology. 1953;53:604–606. doi: 10.1210/endo-53-6-604. [DOI] [PubMed] [Google Scholar]
- 13.ICH Expert Working Group. ICH harmonised tripartite guideline: quality of biotechnological products: stability testing of biotechnological/biological products Q5C. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use; 30 Nov 1995. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q5C/Step4/Q5C_Guideline.pdf. Accessed 16 Dec 2016.