Abstract
Serous ovarian cancer (SOC) is usually diagnosed at late stage and stage-adjusted five year survival rate is low. Mortality is relatively heavy on African-Americans/Black (AA) affected with SOC compared to their Caucasian counterparts, though the cause for the disparity remains unclear. DNA damage induced by oxidative stress has been linked to ovarian cancer, but the role of oxidative stress in distinguishing differences in aggressive SOC tumors among patients is yet to be determined. This study aims to determine the levels of reactive oxygen species (ROS), malondialdehyde (MDA), reactive carbonyl groups and antioxidants in primary SOC normal, precancerous (cystadenoma, borderline) and invasive (III/IV) tissue samples obtained from AA and Caucasian subgroups. Additionally, the study seeks to investigate significant changes in the level of ROS between AA and Caucasian SOC samples. A fluorogenic probe, dichlorodihydrofluorescein (DCFH-DiOxyQ), was used to scavenge reactive oxygen species in SOC normal, precancerous and malignant stages III/IV tissue samples. Malondialdehyde (MDA), a lipid peroxidation marker, and reactive carbonyl groups were measured as indicators of oxidative injury. Moreover, antioxidant status was assessed by estimating glutathione peroxidase 3 (GPX3) enzyme levels. Results indicate ROS concentration was approximately 96% higher in the malignant tissues in comparative to the normal non-diseased controls. In addition, ROS concentration among AA women was approximately 9% higher than Caucasian women. MDA levels increased exponentially from non-disease control and precancerous tissues relative to malignant tissues. Furthermore, malignant serous ovarian samples showed significantly higher reactive carbonyl content compared to the non-disease controls (p=0.009), while GPX3 levels decreased considerably in serous cystadenoma and malignant tissue samples, and non-diseased control compared to borderline disease. The results suggest accumulation of ROS and MDA levels may be a causative factor for SOC. Elevated levels of MDA and reactive carbonyl proteins could override the GPX3 enzyme capacity therefore, initiating serous ovarian neoplasm.
Keywords: Serous Ovarian Cancer, Dichlorodihydrofluorescein, Malondialdehyde, Reactive Oxygen Species, Glutathione Peroxidase 3
1. Introduction
Ovarian cancer represents the fifth most frequent cause of cancer mortality among American women, and is by far the most aggressive form of malignancy affecting the female reproductive tract [1–3]. Interestingly, African-American women (AA) carry the heaviest mortality from the disease compared to their Caucasian counterparts, though the cause for the inequality is relatively unclear [4]. Furthermore, the etiology of serous ovarian cancer (SOC) and its’ progression is not fully understood, but could be attributed to genetic instability induced by inflammation resulting from repeated repair of ovulatory defects in the epithelium lining the ovaries and fallopian tubes [5]. Multiple studies have reported release of reactive oxygen species (ROS) at the inflammatory site could damage DNA, proteins, and lipids leading to an array of cancers [1, 3, 5]. The major adverse effect of ROS is increased oxidative stress resulting from an overproduction of reactive compounds, antioxidants exhaustion, or both. Past studies suggest cancer cells, as compared to normal cells, are under enhanced oxidative injury associated with tumor transformation, changed metabolism, and increased ROS production [6–8]. Further, a surge of ROS in cancer cells may have significant consequences such as stimulating cellular proliferation, promotion of mutations and genetic instability in addition to altering cellular sensitivity to anticancer agents [6, 7]. To counteract the oxidant effects and restore redox balance, cells must reset important homeostatic parameters. Accordingly, oxidative stress may be involved in stimulating the adaptive expression of antioxidant enzymes, such as superoxide dismutase (SOD), catalase, and glutathione-S-transferase to control cancer development [9]. Given the glutathione system (GSSG/2GSH) is the most abundant redox system in maintaining cellular homeostasis [10] and intracellular ROS depend on the amount produced versus quantity eliminated, a decrease in antioxidant enzyme activity may also result in ROS accumulation [11]. A recent study evaluating serum of SOC patient samples, found glutathione peroxidase 3 enzyme to be extremely lower in patients suffering with malignant stages III/IV and recurrent disease [11] not in cystadenoma and borderline patient samples. However, an epidemiological study reported a correlation between borderline and malignant epithelial ovarian tumors [12]. Based on these observations, it is logical to speculate the biochemical and molecular changes caused by elevated ROS could be involved in the tumorigenesis of SOC. Pathogenesis of serous ovarian cystadenoma and borderline tumors linked to invasive tumors, particularly timing of the aggressive progression of the disease among AA subgroups, would help detect early stage disease and optimize timely surgical and chemotherapeutic interventions. Thus far, there has not been a clear study which associate serous cystadenoma and borderline ovarian tumors as putative precursors to advanced carcinoma. Here we evaluate levels of ROS stress against antioxidant enzyme, glutathione peroxidase 3 (GPX3) in normal and precancerous (cystadenoma, borderline) SOC tissues in association with malignant stages III & IV samples obtained from AA and Caucasian subgroups.
2. Materials and Methods
2.1. Study Samples
Thirty-four micro-dissected, frozen primary epithelial SOC tissues, from patients who underwent surgical resections, were obtained from the Southern Regional Cooperative Human Tissue Network. Tissue samples from patients with other gynecological syndrome, or inflammatory disease were excluded. The breakdown of the tissues were as follows: normal ovarian tissues as negative controls (n=9), benign cystadenoma (n=7), borderline (n=7), and invasive carcinoma (n=11) (Table 1). Normal controls were classified as non-disease ovarian tissues. Serous cystadenoma was defined as benign ovarian neoplasm. Serous borderline tissues were classified as benign tumors with low malignant potential [13]. Invasive serous ovarian carcinoma was defined as malignant stages III/IV. All samples were obtained from patients with a mean age of 50±16 years (Table 1). The tumors were diagnosed and histopathologically staged by pathologists based on the criteria of the International Federation of Gynecology and Obstetrics. The samples were stabilized by snap-freezing immediately after excision and dissection. The dissected tissues were placed in cryovials, immersed in liquid nitrogen before transferred to −80°C for long-term storage, as recommended for measurement of nucleic acids and proteins [14]. This study was implemented under protocols approved by Institutional Review Boards of Morehouse School of Medicine and the University of Alabama at Birmingham.
Table 1.
Serous epithelial ovarian cancer samples from AA and Caucasion subgroups.
| Characteristics | Category | Subcategory | n (%) |
|---|---|---|---|
| Ethnicity | African-American | 12 (35%) | |
| Caucasian | 20 (59%) | ||
| Unidentified | 2 (6%) | ||
| Age | >55 | African-American | 6 (37.5%) |
| Caucasian | 10 (62.5%) | ||
| Unidentified | 0 | ||
| Total | 16 | ||
| <55 | African-American | 6 (33%) | |
| Caucasian | 10 (56%) | ||
| Unidentified | 2 (11%) | ||
| Total | 18 | ||
| Tumor | |||
| Classification | Normal (benign) | 9 (26%) | |
| Cystadenoma(benign) | 7 (21%) | ||
| Borderline (premalignant) | 7 (21%) | ||
| Malignant (Stages III/IV) | 11 (32%) |
2.2. Measurement of ROS
Intracellular ROS levels were determined using the OxiSelect in Vitro ROS/RNS assay Kit (Cell Biolabs, Inc.) based on manufacturer’s instructions. The assay measured total ROS/RNS free radical activity through the use of a specific ROS/RNS proprietary fluorogenic probe, dichlorodihydrofluorescein (DCFH-DiOxyQ), that bind to the reactive compounds emitting a relative fluorescence directly linear to the sum of ROS/RNS in the sample. Briefly, DCF standards (200μl of 0μM-10μM) were added to a 96-well microtiter plate and fluorescence was measured with a fluorescent microplate reader at 480 nm excitation and 530 nm emission. SOC tissues (10mg) were washed in 500μL of ice cold PBS, homogenized on ice and centrifuged at 10,000 x g for 5 minutes to remove insolubles. Samples lysate (50μl) and Catalyst (50μl) were pipetted into 96-well microtiter plate, mixed and incubated at room temperature for 5 minutes. After plate incubation, the prepared DCFH probe (100μl) was added to each well, the plate was covered with aluminum foil and incubated for additional 45 minutes. Fluorescence was measured with a fluorescent microplate reader at 480 nm excitation and 530 nm emission. Concentration of ROS in samples were determined using DCF standard curve, and concentration was calculated by linear regression.
2.2.1. Lipid Peroxidation
To detect lipid peroxide malonialdehyde (MDA) as an indicator of oxidative stress within the tissue samples, the lipid peroxidation MDA assay kit (Abcam, ab118970) was used following the previously described protocol by Kalghatgi et al. [15]. Briefly, 10 mg of SOC tissues was homogenized on ice with MDA lysis buffer (300 μl) and 3 ul of BHT (100X) after washing with 500 μl of PBS. To eradicate all insoluble substance, the mixture was centrifuged at 13,000 x g for 10 minutes. TBA reagent (600 μl) was added to the samples (200 μl) and standards (200 μl of 0, 4, 8, 12, 16, 20 nmol MDA), incubated at 95°C for one hour and cooled on an ice bath for 10 minutes. The samples and standards (200μl) were added to a 96 well microtiter plate and absorbance was read using Spectrophotometer microplate reader at 532nm. The standard curve was generated according to the manufacturer’s protocol and used to calculate the concentration of MDA in sample tissues.
2.2.2. Protein Carbonyl Assay
The dinitrophenylhydrazine (DNPH) spectrophotometric assay was used to quantify oxidized proteins within the samples as previously described by Mesquita et al [16] with some modifications. The assay permits detection of carbonyl proteins modified by ROS as an indication of elevated oxidative damage. During the interaction ROS interact with amino acid residues such as (histidine, arginine, lysine), carbonyl groups to form DNPH adducts. The DNPH adduct levels are direct manifestation of the oxidized protein concentration within tissues [17]. Briefly, DNA free homogenized SOC tissues (100μl) were derivatized with 200 μl of 10mM DNPH (Sigma-Aldrich) dissolved in 2 M HCl, the controls were treated with 2mL of 2 M HCl. Samples and controls were incubated at 37 °C for 10 minutes. Following incubation 100 μl of 6M NaOH was added, solution mixed, and a second incubation of 5 minutes. The solution cooled to room temp for approximately 5 minutes. Samples and controls (200 μl) were immediately pipetted into a 96 well microtiter plate to obtain a pooled reading for accurate data analysis. The plate was read at 450 nm on spectrophotometer [14]. The carbonyl protein concentration was calculated according to the protocol using the Beer-Lambert law with estimation coefficient 22,000M−1 cm−1 [14].
2.2.3. Immunoblotting
In order to evaluate the antioxidant capacity in normal ovarian tissue vs precancerous and malignant SOC tissues, Western blot analyses were performed. Briefly, lysates from SOC tissues were prepared by using the MPER Mammalian Protein Extraction Reagent according to the manufacturers’ instructions (Thermo Scientific). Protein concentration was determined using the Bradford Assay (Bio-Rad). Equal amounts of total protein (30μg) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. Nitrocellulose membranes were blocked with 5% non-fat milk for approximately 45 minutes. The membranes were then incubated overnight with mouse anti-GPX3 antibody (1:500, Thermofisher). Following incubation time, the nitrocellulose membrane was washed with PBST (900 mL ddH20, 100mL 10% PBS, 1mL Tween) probed with appropriate secondary HRP-conjugated anti-rabbit antibodies (Thermo Scientific) for 2 hours, and the immune complexes were visualized using enhanced SuperSignal West Pico chemiluminescent substrate according to the manufacturer’s protocol (Thermo Scientific). Protein bands were quantified with Image J digitizing system (NIH) and the results were expressed as arbitrary units.
3. Statistical Analysis
Analysis of Variance (One Way ANOVA), Paired t test and T-test were used to determine differences in ROS, MDA, and Reactive Carbonyl Protein levels between normal non-disease control, precancerous and advanced stage serous ovarian tumors. Values were expressed as mean ± SEM where n=number of tissue samples. Statistical significance was justified at p<0.05.
4. Results
4.1. Total ROS Concentration
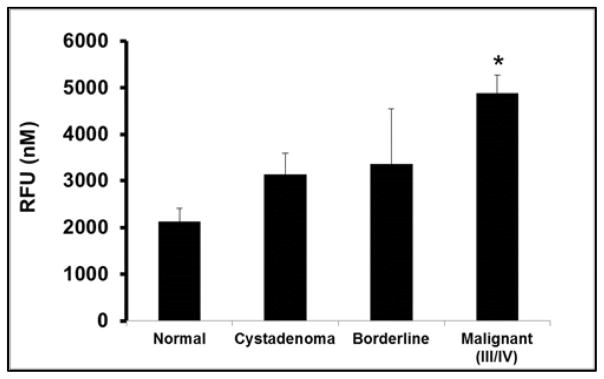
To determine ROS concentration, a dichlorodihydrofluorescein probe was used to scavenge the compounds in precancerous and malignant serous ovarian tumors comparative to normal ovarian tissues. Fluorescent intensity was measured with a standard fluorescent plate reader that generated a standard curve used to calculate levels of ROS within the samples. The results of ROS from normal non-disease control, precancerous and progressive stages of SOC neoplasms are summarized in Figure 1. Overall fluorescent emission from the reactive compounds, increased exponentially from normal non-disease control and precancerous samples to the progressive stages of SOC neoplasms (Figure 1). Within the malignant tissues ROS concentration was approximately 96% higher compared to the normal non-disease control with statistical significance of (p=0.007, Figure 1). Additionally, serous cystadenoma tissue samples had about 50% intensification in ROS emission from normal negative control, while serous borderline tissue samples revealed a 57% increase (Figure 1). Although there was not substantial difference shown in ROS among serous cystadenoma and borderline tissue samples, when compared to malignant samples, there was a noticeable increase of 33% and 24% levels of the reactive compounds respectively (Figure 1). Our data suggest ROS levels appreciably increased as serous ovarian tumors progressed.
Figure 1.
ROS concentration among different histological subclass of SOC.
Endogenous ROS activity was measured fluorimetrically using a fluorescent microplate reader at 480 nm excitation and 530 nm emission in Normal, non-disease negative control; Cystadenoma, benign neoplasm; Borderline, premalignant stage; Malignant stages III/IV, advanced tumor stages III/IV SOC subclasses. Each value represent the mean ± S.E.M. of duplicate determinants from representative experiment. * Indicates significant difference when compared to normal tissue (p=0.007; One-Way ANOVA test, n=34 samples). There was nearly a 96% increase in ROS intensity from normal non-disease control to invasive stages III/IV serous ovarian tumors. Cystadenoma and borderline subclasses showed 33% and 24% increase in ROS levels compard to invasive stage III/IV SOC. When compared to normal control, cystadenoma had a 49% increase in ROS levels while ROS in borderline tissue samples increased by 57% though there was no significance difference. Experiments were repeated twice with similar results and representative data shown.
4.2. Lipid Peroxidation Marker
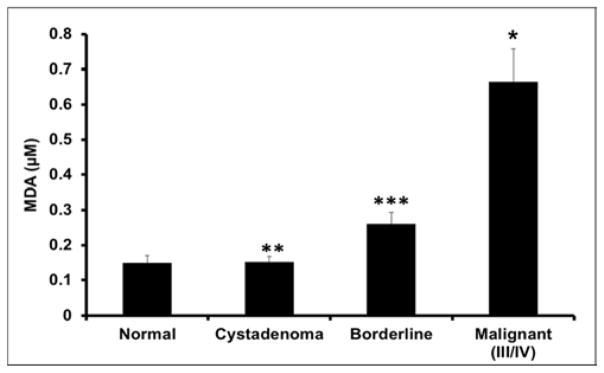
The levels of MDA adducts were measured as an indicator of oxidative damage due to elevated ROS. MDA levels were appreciably amplified among normal non-disease control and precancerous tissues relative to the malignant tissues (Figure 2). Notably, there was about 5 fold less MDA adducts within the non-disease control tissues relative to the malignant tumor samples (p=0.019, Figure 2). Further, t-test analysis comparing serous cystadenoma and malignant stages III/IV tissue samples revealed a 5-fold decrease in MDA levels within cystadenoma samples (p=0.018, Figure 2). When compared to the malignant tissue samples, borderline samples showed a 4-fold reduction (p=0.041, Figure 2). There was no change seen in MDA levels between normal non-disease control and cystadenoma tissues. Consequently, there was a slight increase in MDA compounds in borderline disease relative to serous cystadenoma and normal non-disease controls, however it was not statistically significance.
Figure 2.
Malondialdehyde (MDA) Concentration.
Differential MDA levels in serous ovarian subclasses were determined spectrophotometrically at 532nm. Subclasses included; Normal, non-disease negative control; Cystadenoma, benign neoplasm; Borderline, premalignant stage; Malignant stage III/IV, advanced tumor stages III/IV. Each value represent the mean ± S.E.M. of duplicate determinants from representative experiment. * Indicates significant difference when compared to normal non-disease tissues (p=0.019 One-Way ANOVA test, n=26 samples). ** Indicates significant difference when compared to malignant tissues (p=0.018; T-test, n=26 samples). *** Indicates significant difference when compared to malignant tissues (p=0.041; Paired t-test, n=26). MDA adducts increased 5-fold in advanced tumor stages III/IV comparative to the normal disease control and cystadenoma tissues. While reactive oxygen compounds within borderline tissues decreased by 4-fold compared to advance stage serous ovarian tumors. There was no difference seen in ROS levels between negative control, cystadenoma and borderline samples. Experiments were repeated twice with similar results and representative data shown.
4.3. Reactive Carbonyl Proteins
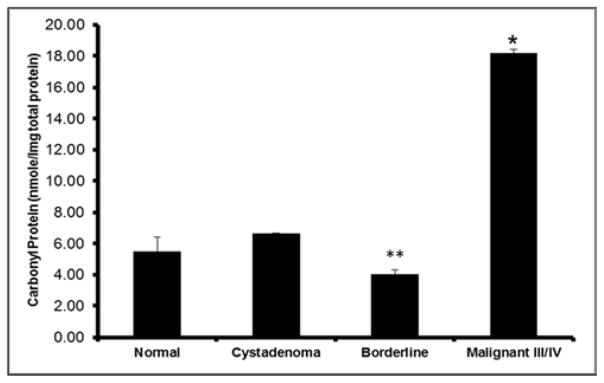
Oxidized protein carbonyl groups were also measured as a second oxidative stress marker to determine ROS damage. The results revealed an overall exponential incline in the reactive protein groups amid the normal non-disease control and the SOC precancerous stage cystadenoma (Figure 3). The expression of reactive carbonyl protein groups were shown to be appreciably higher (6.22 nmole) in the SOC malignant tissue samples in comparison to the normal non-disease control tissues (p=0.009, Figure 3). There was no statistically difference in carbonylation of proteins within serous cystadenoma tissues when compared to serous borderline and malignant tissue samples. Noteworthy, we observed a significant decrease in the reactive proteins within the borderline samples of 5.75 nmole in comparison to the malignant tissue samples (p=0.020). The results suggest MDA adducts and oxidized proteins could be indicative of ROS damage in woman at risk of SOC.
Figure 3.
Reactive Carbonyl Levels.
ROS react with residues of amino acids in proteins to produce oxidized carbonyl groups used as an indicator of increased oxidative damage. Ratios of protein carbonyl groups to total protein concentration in serous ovarian tissue samples groups was determined by spectrophotometric method, 450nm. Normal, non-disease negative control; Cystadenoma, benign neoplasm; Borderline, premalignant stage; Malignant stages III/IV, advanced tumor stages III/IV. Each value represent the mean ± S.E.M. of duplicate determinants from representative experiment. * Indicates significant difference when compared to normal tissue (p=0.009; T-test, n=25 samples). ** Indicates significant difference when compared to malignant tissue samples (p=0.020). Progressively increasing levels of carbonyl groups were observed in the lysates of derivatized samples from SOC subclasses. A three-fold decline in oxidized proteins was observed in normal non-disease control and borderline tissue samples comparative to advanced tumor stages III/IV samples. Cystadenoma samples decreased by 2-fold compared to invasive tumors as noted. Experiments were repeated twice with similar results and representative data shown.
4.4. Antioxidant Capacity
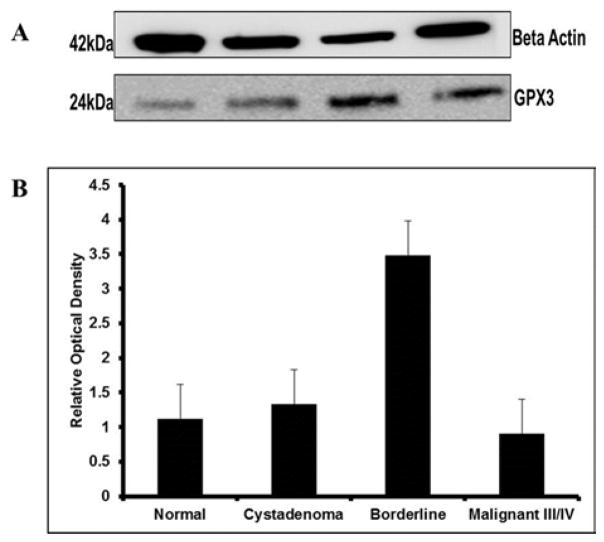
To evaluate whether antioxidant capacity in the precancerous of SOC could promote advance tumors, GPX3 expression was measured and normalized to housekeeping gene, beta actin. Our findings showed a gradual increase in the expression of GPX3 in normal non-disease and serous cystadenoma tissue samples though there was no statistical significance noted between the groups. Serous borderline tissue samples indicated a 2.5-fold increase in GPX3 expression relative to non-disease control and serous cystadenoma samples (Figure 4). Consequently, GPX3 protein expression decreased considerably, 3-fold, in malignant stages III/IV samples comparative to borderline disease as indicated by western blot profile (Figure 4) though mean difference was obvious, but not statistically relevant. Further, there was a slight decrease in GPX3 intensity from normal non-disease control and serous cystadenoma tissue specimens compared to the malignant specimens (Figure 4).
Figure 4.
Glutathione Peroxidase 3 Expression (GPX3).
A. Western blot analysis was used to measure GPX3 expression in lysates of Cystadenoma, benign neoplasm; Borderline, premalignant stage; Malignant stage III/IV, advanced tumor stages III/IV compared to Normal, non-disease negative control SOC subclasses. Glutathione Peroxidase 3 was normalized to Beta Actin. B. Protein bands were quantified using relative optical density calculated using NIH Image J software. The average fold increase in GPX3 activity in borderline tissue samples compared to normal non-disease control and cystadenoma samples were approximately 2.5 as indicated. While there was a 3-fold increase in protein expression in borderline disease when compared to malignant samples. There was a slight decline in GPX3 enzyme from normal non-disease control and cystadenoma samples when compared to malignant tissues though there was no significant differences. The result is representative of three independent experiments with similar results and representative data shown.
4.5. Racial Differences in ROS Levels Between AA and Caucasians SOC Samples
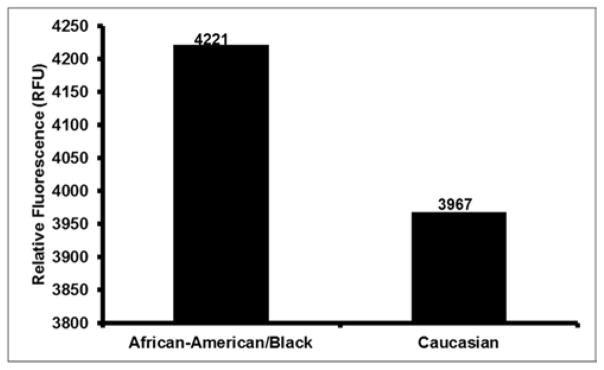
To determine whether there were racial differences in ROS levels among study samples, we separated the SOC tissue samples measured with the dichlorodihydrofluorescein probe (Figure 1) based on AA and Caucasian subgroups (Table 1). Fluorescent intensity was measured with a standard fluorescent plate reader that generated a standard curve used to calculate levels of ROS within the samples. Results are indicated in Figure 5. ROS concentrations from AA suffering with SOC were higher, 4221 relative fluorescence (RFU) compared to SOC tissues obtained from Caucasian with 3967 RFU.
Figure 5.
Levels of ROS concentration in SOC tissues from AA and Caucasian Subgroups.
Levels of ROS concentration in Normal, non-disease negative control and serous subtype ovarian cancer tissues in African-American/Black and Caucasian women as measured by the fluorometric method (n=32). For African-Americans, there were notable differences in ROS levels in comparison to Caucasian women although there was no significant differences between the two groups.
5. Discussion
The aim of the study was to show how ROS and antioxidant capacity could improve our understanding of serous epithelial ovarian tumor pathogenesis. There is considerable speculation relating to the association of serous cystadenoma and borderline tumors as part of the continuum of progressive changes from benign to malignant tumors [18] and supported by our unpublished sequence data. This arises because of the difficulties in accessing ovarian tissues and other anatomical histology appearances. Given that early stage ovarian cancer has a greater five year survival status than advanced presentation, the difficulty lies in the detection of early malignant or premalignant changes in the ovaries [1, 3]. ROS are short lived oxygen-containing derivatives developed as by-products of normal cellular respiration that help to maintain cellular homeostasis along with the body’s innate antioxidant system [1,16]. Oxidative damage is a major factor inducing the adaptive expression of antioxidant enzymes, such as superoxide dismutase (SOD), catalase, and enzymes of the glutathione system which could aid in our understanding of signaling cascades involved in promoting normal ovarian cells to malignant cells [9]. Many studies show continuous release of ROS induce oxidative reactions which damage nucleic acids, proteins, and lipids [19–21]. Further, oxidative stress has been associated with the promotion of many human diseases including various cancers [1, 2, 16, 17, 18]. Specifically, ovarian tumors have known to secrete excessive amounts of ROS leading to increase tumor size [3, 11]. Moreover, the microenvironment generated as a result of oxidative injury may help the compounds evade normal checkpoints to trigger human disease [22]. Consequently, the stage-specific ROS levels could be used to establish potential markers involved in the progression of ovarian carcinoma. Also past studies reported active oxygen molecules such as those generated during oxidative phosphorylation, were directly involved in altering enzymes and maturing cells, processes usually seen with aging [23–25]. Perhaps, more importantly variations in the mitochondrial genome due to elevated ROS could increase the risk of developing an age related disease like ovarian cancer.
In the current study a proprietary fluorogenic probe, dichlorodihydrofluorescein (DCFH-DiOxyQ) was used to measure ROS in SOC tissue samples. We reported notable elevation in the reactive compounds between the normal non-disease control and cancerous tissue samples. Furthermore, there was a progressive increase in ROS levels between the precancerous and cancerous tissue samples (Figure 1). The relative fluorescence emitted from the advanced tumor samples was significantly higher when compared to the precancerous and normal negative control (Figure 1). An association between late stage tumors and high reactive species has been well documented in several research studies [26]. Conversely, this is the first study to our knowledge to show an increase in ROS levels between different serous epithelial ovarian histological subtypes. Results from this study correlates with the Incessant Ovulation Theory proposed by Fathalla of 1971, which reported the etiology of SOC to be associated with years of inflammation and ROS production from continuous ovulations [25]. Similarly, our findings parallel with the “free radical theory of aging” study proposed by D. Harman which suggests persistent ROS augmentation is highly associated with the pathogenesis of countless diseases as well as aging [27]. Harman indicated that irreversible damages were a causative of ROS build-up over time. Harman’s theory, further clarifies the role of ROS in triggering cancer progression with advance age [27]. Thereby the paradigm from the two models support our theory suggesting oxidative stress could contribute to the progression of tumors such as SOC.
Further, our study evaluated the negative effects of increased ROS within different stages of serous ovarian tumors through the measurement of several commonly used oxidative stress biomarkers. MDA has been well established as a positive marker for increased oxidative damage [28]. Correspondingly, an amplification in MDA adducts have been reported in different types of cancer [26, 29]. Nathan et al., reported findings of increased MDA adducts in body fluids of patients suffering from bone and soft tissue sarcoma. According to the study oxidative stress markers MDA and reactive carbonyl proteins along with decrease in antioxidant enzymes SOD and catalase were associated with bone and soft tissue sarcoma formation [19]. Further Gonenc’s group reported increase in MDA concentration in the plasma, serum and triglycerides of breast and lung cancer patients that could serve helpful in treating patients with elevated lipid peroxides [29]. Here, we observed an exponential increase in the levels of MDA adducts within the different stages of serous ovarian tumors (Figure 2). Higher levels of MDA adducts were observed in the invasive stages of serous ovarian tumors relative to the precancerous serous ovarian tumors and normal non-disease control (Figure 2). The resulting adduct could function as a tumor promoter and hinder key antioxidant enzymes. Although previous studies reported similar findings, our data was the first to demonstrate increased oxidative damage markers and reduced antioxidant capacity in diseased human tissues directly derived from the original tumor. Our results suggest that MDA adducts levels could be an indicator of one of the risk factors for the development of serous ovarian cancer.
Proteins are major targets for ROS compounds. The reactive compounds attack the residual groups of proteins changing the tertiary structure leading to change in function [2]. In the current study, oxidized protein compounds were measured as a second indicator of oxidative damage. The results revealed a progressive increase in levels of oxidized protein groups which could play a role in advancing serous ovarian tumors. Our observed results were consistent with previous findings published by our lab demonstrating reactive carbonyl groups increased with advanced serous ovarian tumors [2]. Noteworthy, our data showed serous borderline tissues had a significant reduction in reactive oxidative carbonyl groups compared to the normal non-disease control and cystadenoma samples (Figure 3). This observation could be attributed to the percentage of tumor within the serous borderline tissue specimens, which ranged from 10–50%. According to findings reported by Burkholz et al., a distinguished trait of borderline tumors is the ability to implant in an area opposite from the ovary in the underlying tissues therefore preventing detection of malignant cells [30]. The lower percentage of tumor coupled with the ability to evade discovery may also be attributed to the decrease in oxidized proteins detected within the pooled SOC borderline samples in this study. Conversely, ROS are major effectors in the innate immune response high levels of reactive carbonyl groups may be involved in the progressive stages of serous ovarian tumors.
Antioxidant enzymes are major protective compounds that help to decrease ROS. Even though the mechanism for ROS induced disease is not well understood and the effects of ROS are too complex to predict patient outcome, it is well established that ROS act as second messengers for cellular signaling [1, 3]. Several studies have reported reduction in the antioxidant scavenging ability with an aggressive increase in ROS induce diseases such as thyroid and gastrointestinal malignancies [10, 11]. Given that GPX3 is part of the glutathione antioxidant mechanism to maintain homeostasis within a cell [10], a decrease in the enzyme has been associated with advancing tumors compared to benign tumors [11]. Our results as shown Figure 4 are consistent with a previous study measuring GPX3 in the serum of clear cell and papillary serous ovarian patients [11]. However, we report differential levels of GPX3 in cystadenoma, borderline and malignant stages III/IV serous ovarian tumors. The increase in antioxidant enzymes within the borderline tissues followed by a significant decrease in antioxidant capacity in the malignant samples could be due to the body’s attempt to restore normal homeostasis prior to cancer cell mass production [20].
Interestingly, our study also showed levels of ROS in SOC tissue samples obtained from AA patients were higher compared to Caucasian patients as shown in Figure 5. A study has associated risk factors of SOC such as ethnicity, obesity, decreased oral contraceptive use and tubal ligation to be higher in AA compared to Caucasian women [31]. Moreover, AA patients with SOC disease carry the heaviest burden comparative to Caucasian patients [3, 4, 27]. Given these findings in combination with risk factors [3, 4, 27], it is logical to speculate that the biochemical and molecular changes caused by ROS may contribute to the aggressiveness of SOC within this subgroup during disease progression.
6. Conclusion
In summary, the present study has shown an association between higher levels of ROS, MDA adducts and reactive carbonyl proteins among serous ovarian invasive tumor samples as compared to cystadenomas, borderline and normal specimens that are statistically significant. Also, we observed a substantial reduction in glutathione peroxidase 3 expression in normal, cystadeoma and invasive disease compare to the borderline SOC tissues. Additionally we reported differences in ROS compounds between AA and Caucasian women which may aid in gaining an insight in explaining the health disparity for SOC. Taken together, these results demonstrate a correlation of clinically stage disease progression with increased oxdative damage to proteins in SOC tissues, suggesting the participation of reactive oxygen species in the pathogenesis and progression of this disease. Our results provide a theoretical basis for the use of higher antioxidant compounds that could not be overriden by elevated ROS and MDA adducts in the chemoprevention of serous ovarian tumors. However, results should be cautiously interpreted due to the relative small sample size used in this study.
Acknowledgments
This work was supported by Grants NIH-NCI CA15031, CA150039-01 and NIH GM099663-(NIGMS) awarded to Dr. Aikhionbare. Also, fund was provided by NIMHD Grants no. 8G12MD007602, 8U54MD007588 and RISE 2R25GM058268 that supported S. Cohen. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH-NIMHD.
List of Abbreviations
- SOC
Serous Ovarian Cancer
- ROS/RNS
Reactive Oxygen Species/Reactive Nitrogen Species
- DNPH
2,4-Dinitrophenylhydrazine
- DCFH-DiOxyQ
fluorogenic probe, dichlorodihydrofluorescein
- DCF
dichlorodihydrofluorescein
- GPX3
glutathione peroxidase 3
- MDA
Malondialdehyde
- GSSG/GSH
oxidized glutathione/reduced glutathione
- SOD
superoxide dismutase
References
- 1.Mehrabi S, Partridge EE, Seffens W, Yao X, Aikhionbare FO. Oxidatively modified proteins in the serous subtype of ovarian carcinoma. Biomed Res Int. 2014;2014:585083. doi: 10.1155/2014/585083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta D, Lis CG. Role of CA125 in predicting ovarian cancer survival - a review of the epidemiological literature. J Ovarian Res. 2009;2:13. doi: 10.1186/1757-2215-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aikhionbare FO, Mehrabi S, Kumaresan K, Zavareh M, Olatinwo M, Odunsi K, Partridge EE. Mitochondrial DNA sequence variants in epithelial ovarian tumor subtypes and stages. J Carcinog. 2007;6:1. doi: 10.1186/1477-3163-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins Y, Holcomb K, Chapman-Davis E, Khabele D, Farley JH. Gynecologic cancer disparities: a report from the Health Disparities Taskforce of the Society of Gynecologic Oncology. Gynecologic Oncology. 2014;133(2):353–361. doi: 10.1016/j.ygyno.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jasen P. From the “Silent Killer” to the “Whispering Disease”: Ovarian Cancer and the Uses of Metaphor. Med Hist. 2009;53(4):489–512. doi: 10.1017/s0025727300000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toyokuni S, Okamoto K, Yodoi J, Hiai H. Persistent oxidative stress in cancer. FEBS Letters. 1995;358(1):1–3. doi: 10.1016/0014-5793(94)01368-b. [DOI] [PubMed] [Google Scholar]
- 7.Kang D, Hamasaki N. Mitochondrial oxidative stress and mitochondrial DNA. Clin Chem Lab Med. 2003;41(10):1281–1288. doi: 10.1515/CCLM.2003.195. [DOI] [PubMed] [Google Scholar]
- 8.Behrend L, Henderson G, Zwacka RM. Reactive oxygen species in oncogenic transformation. Biochemical Society Transactions. 2003;31(6):1441–1444. doi: 10.1042/bst0311441. [DOI] [PubMed] [Google Scholar]
- 9.Marrs KA. The Functions and Regulations of Glutathione S-Transferases in Plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:127–158. doi: 10.1146/annurev.arplant.47.1.127. [DOI] [PubMed] [Google Scholar]
- 10.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30(11):1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 11.Agnani D, Camacho-Vanegas O, Camacho C, Lele S, Odunsi K, Cohen S, Dottino P, Martignetti Decreased levels of serum glutathione peroxidase 3 are associated with papillary serous ovarian cancer and disease progression. J Ovarian Res. 2011;4:18. doi: 10.1186/1757-2215-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adami HO, Hsieh CC, Lambe M, Trichopoulos D, Leon D, Persson I, Ekborn A, Janson PO. Parity, age at first childbirth, and risk of ovarian cancer. a population-based study in Sweden. Lancet. 1994;344(8932):1250–1254. doi: 10.1016/s0140-6736(94)90749-8. [DOI] [PubMed] [Google Scholar]
- 13.Kurman RJ, Trimble CL. The behavior of serous tumors of low malignant potential: are they ever malignant? Int J Gynecol Pathol. 1993;12(2):120–127. doi: 10.1097/00004347-199304000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Evans P, Lyras L, Halliwell B. Measurement of protein carbonyls in human brain tissue. Methods Enzymol. 1999;300:145–156. doi: 10.1016/s0076-6879(99)00122-6. [DOI] [PubMed] [Google Scholar]
- 15.Kalghatgi S, Spina CS, Costello JC, Liesa M, Morones-Ramirez JR, Slomovic S, Molina A, Shirihai OS, Collins JJ. Bactericidal antibiotics induce mitochondrial dysfunction and oxidative damage in Mammalian cells. Sci Transl Med. 2013;5(192):192ra85. doi: 10.1126/scitranslmed.3006055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mesquita CS, Oliveira R, Bento F, Geraldo D, Rodrigues JV, Marcos JC. Simplified 2,4-dinitrophenylhydrazine spectrophotometric assay for quantification of carbonyls in oxidized proteins. Anal Biochem. 2014;458:69–71. doi: 10.1016/j.ab.2014.04.034. [DOI] [PubMed] [Google Scholar]
- 17.Chevion M, Berenshtein E, Stadtman ER. Human studies related to protein oxidation: protein carbonyl content as a marker of damage. Free Radical Research. 2000;33:S99–108. [PubMed] [Google Scholar]
- 18.Kurman RJ, Shih I. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34(3):433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog. 2006;5:14. doi: 10.1186/1477-3163-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 21.Costa A, Scholer-Dahirel A, Mechta-Grigoriou F. The role of reactive oxygen species and metabolism on cancer cells and their microenvironment. Sem in Cancer Biol. 2014;25:23–32. doi: 10.1016/j.semcancer.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11(2):85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 23.Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997;272(33):20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi R, Goto S. Alteration of aminoacyl-tRNA synthetase with age: heat-labilization of the enzyme by oxidative damage. Arch Biochem Biophys. 1990;277(2):228–233. doi: 10.1016/0003-9861(90)90573-h. [DOI] [PubMed] [Google Scholar]
- 25.Fathalla MF. Incessant ovulation--a factor in ovarian neoplasia? Lancet. 1971;2(7716):163. doi: 10.1016/s0140-6736(71)92335-x. [DOI] [PubMed] [Google Scholar]
- 26.Nathan FM, Singh VA, Dhanoa A, Palanisamy UD. Oxidative stress and antioxidant status in primary bone and soft tissue sarcoma. BMC Cancer. 2011;11:382. doi: 10.1186/1471-2407-11-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harman D. Free radical theory of aging. Triangle. 1973;12(4):153–158. [PubMed] [Google Scholar]
- 28.Chole RH, Patil RN, Basak A, Palandurkar K, Bhowate R. Estimation of serum malondialdehyde in oral cancer and precancer and its association with healthy individuals, gender, alcohol, and tobacco abuse. J Cancer Res Ther. 2010;6(4):487–491. doi: 10.4103/0973-1482.77106. [DOI] [PubMed] [Google Scholar]
- 29.Gönenç A, Özkan Y, Torun M, Şimşek B. Plasma malondialdehyde (MDA) levels in breast and lung cancer patients. J Clin Pharm Ther. 2001;26(2):141–144. doi: 10.1046/j.1365-2710.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- 30.Burkholz KJ, Wood BP, Zuppan C. Best cases from the AFIP: Borderline papillary serous tumor of the right ovary. Radiographics. 2005;25(6):1689–1692. doi: 10.1148/rg.256055015. [DOI] [PubMed] [Google Scholar]
- 31.Moorman PG, Palmieri RT, Akushevich L, Berchuck A, Schildkraut JM. Ovarian cancer risk factors in African-American and white women. Am J Epidemiol. 2009;170(5):598–606. doi: 10.1093/aje/kwp176. [DOI] [PMC free article] [PubMed] [Google Scholar]