Abstract
Purpose
Endocrine disruptors are known to modulate a variety of endocrine functions and increase the risk for neoplasia. Epidemiological data reported increased prevalence of pituitary tumors in high industrial areas while genotyping studies showed that mutations in the aryl hydrocarbon receptor (AhR) interacting protein (AIP)—chaperone to the dioxin ligand AhR—gene are linked to predisposition to pituitary tumor development. Aim of the present study was to establish whether endocrine pollutants can induce cell proliferation in normal rat pituitary cells.
Methods
Pituitary primary cultures were incubated with 250, 650 and 1250 pM benzene or 2-ethyl-phthalate for up to 96 h and viability, energy content and cell proliferation assessed. Expression of pituitary tumor transforming gene (PTTG), cyclin D1 (Ccnd1), AhR and AIP was quantified by RT-qPCR.
Results
Incubation with benzene or 2-ethyl-phthalate increased viability and energy content in pituitary cells. The endocrine disruptors also increased cell proliferation as well as Ccnd1 and PTTG expression. Increased AhR and AIP expression was observed after incubation with the two pollutants.
Conclusions
Our findings indicate that benzene and 2-ethyl-phthalate activate AhR/AIP expression and stimulate proliferation in normal rat pituitary cells. This study is the first demonstration that pollutants can induce normal pituitary cells to proliferate and provides a link between epidemiological and genomic findings in pituitary tumors.
Electronic supplementary material
The online version of this article (doi:10.1007/s11102-016-0777-3) contains supplementary material, which is available to authorized users.
Keywords: Endocrine disruptor, Pituitary adenoma, Proliferation, Aryl hydrocarbon receptor (AhR), Aryl hydrocarbon receptor-interacting protein (AIP)
Introduction
Endocrine disruptors are widely distributed chemical pollutants known to affect endocrine functions [1, 2], in particular reproduction and development. Indeed, it is known since the early 1970s that breeding patterns, sex of offspring and fetal maturation are variably affected by endocrine toxicants [3, 4] as is hormonal production [2, 5].
More recently, the carcinogenic potential of endocrine disruptors has become a major research focus following epidemiological data showing an association between endocrine disruptor exposure and breast, prostate, testis and thyroid neoplasia [6–10]. In support of this evidence, in vitro studies showed that endocrine disruptors induce cell cycle deregulation, death and proliferation in breast and ovarian cancer cell lines [11–13]. Similar growth-promoting effects have also been reported for estrogen-sensitive pituitary adenoma cell lines, e.g. MtT/E2 [14, 15], GH3 [16, 17], suggesting that endocrine disruptors may be linked to pituitary tumor development. Further, in vivo models revealed a higher incidence of pituitary adenomas in rats treated with a mixture of endocrine disruptors [18]. In humans, epidemiological studies showed an increased prevalence of growth hormone (GH)-secreting pituitary tumors in high industrial density areas [19] and, possibly, higher incidence of pituitary neoplasia following the accidental spillage of dioxin [20]. An additional link between endocrine disruptors and pituitary tumorigenesis was provided by the discovery of mutations in the aryl hydrocarbon receptor interacting protein (AIP) gene in patients with pituitary tumors [21], as the aryl hydrocarbon receptor (AhR) is well-known to bind toxins and phytochemicals [22]. Indeed, the AhR pathway is called into play by several endocrine disruptors [23], both in the pituitary and in other tissues [24, 25].
Given this evidence, we decided to study whether endocrine disruptors affect normal rat pituitaries in vitro. Our findings indicate that long-term incubation with benzene and 2-ethyl-phthalate increases cell viability, energy content and proliferation in normal rat pituitary cells. Further, we observed an increase in genes associated with cell cycle progression and pituitary tumorigenesis as well as in AhR and AIP expression. Taken together, our findings show for the first time that endocrine pollutants can induce proliferation in normal pituitary cells and support the contention that endocrine disruptors play a role in pituitary tumorigenesis.
Materials and methods
Pituitary primary cultures
Rat anterior pituitaries were dissected from adult male Sprague–Dawley rats, sacrificed in accordance with animal care guidelines (National Institutes of Health, Office of Animal Cure and Use). The study was approved by the Ethical Committee of the Grant Coordinating Institution, i.e. University of Messina, Italy. Pituitaries were cultured using our usual protocol [26, 27]. Briefly, pituitaries were trypsin-digested and dispersed cells plated at 50,000 cells/well in 96 multi-well plates for cell assays and at 50,000 cells/well in 24 multi-well plates for RT-qPCR. Wells were incubated in Dulbecco’s modified medium (DMEM), 10% fetal bovine serum (FBS), antibiotics for 3–4 days (Sigma, Saint Louis MO, USA) prior to experimental procedures.
Treatments
After 3–4 days attachment, cells were washed for 1 h in Dulbecco’s modified Eagle medium (DMEM) and 0.1% bovine serum albumin (BSA) then treated with 250, 650, 1250 pM benzene (Sigma, Saint Louis MO, USA) or bis-(2-ethylhexyl)-phthalate (2-ethyl-phthalate; Sigma, Saint Louis MO, USA) for 3, 24 or 96 h. Wells were examined by light microscope prior and at the end of incubations in order to exclude fibroblast contamination; experience over the past 20 years showed that contamination with fibroblasts or stromal cells does not constitute a problem with the current cell dispersion protocol. Incubation with 250 µg/ml cycloheximide (CHX) (Sigma, Saint Louis MO, USA) served as representative control for cytotoxicity given that high doses of the protein synthesis inhibitor have been shown to be cytotoxic [28, 29]; wells incubated with DMEM + 0.1% BSA represented untreated control. Treatments were repeated in four separate experiments on quadruplicate wells.
Cell assays
Metabolic cell energy content was measured by ATP lite (Perkin Elmer, Waltham MA, USA) according to the manufacturer’s instructions. Wells were incubated in ATPlite assay reagent at room temperature and luminescence assessed after 10 min.
Cell viability was measured by methylthiotetrazole (MTT) assay (Sigma, Saint Louis MO, USA). MTT was added to wells and cells incubated at 37 °C for 3 h. Medium was subsequently discarded and cells dissolved in 1:25 1 N HCl/100% propanol. Absorbance was read at 540 nm.
Apoptosis was tested by Caspase Glo 3–7 assay (Promega, Madison WI, USA). Wells were incubated in Caspase 3–7 reagent at room temperature and luminescence assessed after 30 min.
Proliferation was assessed by 5-bromo-2′-deoxyuridine labeling (BrdU-labeling; Roche, Mannheim, Germany). Cells were incubated with BrdU-labeling reagent for 16 h, denatured then treated with anti-BrdU-POD antibody for 120 min. Substrate reaction solution was added and reaction stopped after 30 min with 1 M H2SO4. Colorimetric signal was measured at 450 nM.
RNA extraction and RT-qPCR
RNA was extracted from pituitary primary cultures with Pure link RNA mini Kit (Life Technologies, Carlsbad CA, USA) and reverse-transcribed with SuperScriptR VILO™ cDNA Synthesis Kit (Invitrogen Life Technologies, Carlsbad CA, USA). Quantitative Real-Time PCR (qRT-PCR) for Cyclin D (Ccnd1) and pituitary transforming gene 1 (Pttg1) was performed using Platinum Quantitative PCR Supermix-UDG with premixed ROX Taqman assay (Applied Biosystem, Foster City CA, USA) for the detection of Ccnd1 probe Rn00432360_m1 and Pttg1 probe Rn00574373_m1 with hypoxanthine–guanine phosphoribosyltransferase (Hprt1; probe Rn01527840_m1) as endogenous control on a 7900 HT sequence Detection System (Applied Biosystem, Foster City CA, USA). For AhR and AIP expression, primers were designed using Beacon Designer 5.0 software (see Online Resource ESM1.pdf) and qRT-PCR performed using BioRad MiIQ Detection System (BioRad Laboratories, Hercules CA, USA) with SYBR green fluorophore. A melting curve analysis was performed following every run to ensure a single amplified product. Basal expression data (2−ΔCt) was calculated and normalized to house-keeping genes (Online Resource ESM1.pdf); expression after treatment was analyzed as 2−ΔΔCt and expressed in fold increase.
Statistical analysis
Kruskal–Wallis test was used for comparisons between treatments (Statview 5.0, Cary NC, USA) and p < 0.05 considered statistically significant. Treatment values are given relative to control and expressed as mean ± S.E.M.
Results
Exposure to benzene and 2-ethyl-phthalate modulates cell metabolism, viability and proliferation
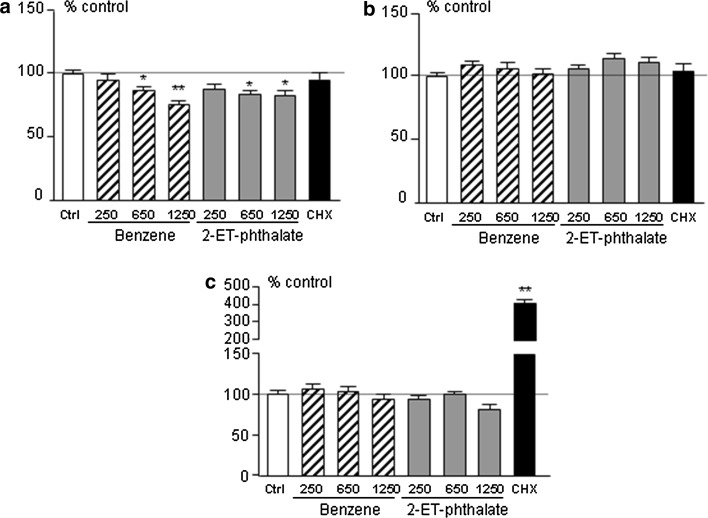
Short-term incubation, i.e. 3 h, with benzene and 2-ethyl-phthalate did not affect cell metabolism, cell viability or apoptosis in rat anterior pituitary primary cultures (see Online Resource ESM2.pdf). Conversely, 24-h incubation with benzene and 2-ethyl-phthalate decreased ATP levels (Fig. 1a), attesting to decreased intracellular energy at this time point. This was not associated with cell death, as no induction of apoptosis could be observed (Fig. 1c). On the other hand, cell viability at 24-h exhibited a slight, not significant, increase (Fig. 1b).
Fig. 1.
Cell energy content (a), viability (b) and apoptosis (c) in rat anterior pituitary primary cultures treated with 250, 650, 1250 pM benzene (Ben, striped bars) or bis-(2-ethylhexyl)-phthalate (2-ET, grey bars), 250 µg/ml of cycloheximide (CHX, black bar) for 24 h. White bars represent control wells treated with plain medium (Ctrl). Data were normalized to control values and expressed as percentage of control; bars represent mean ± SEM from four separate experiments
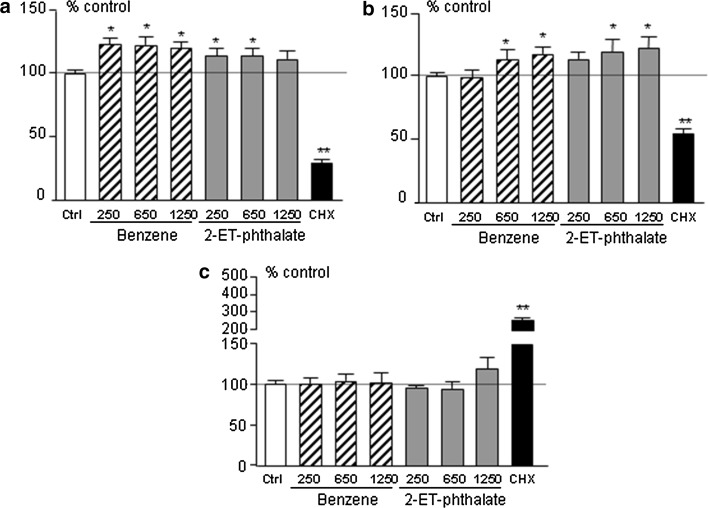
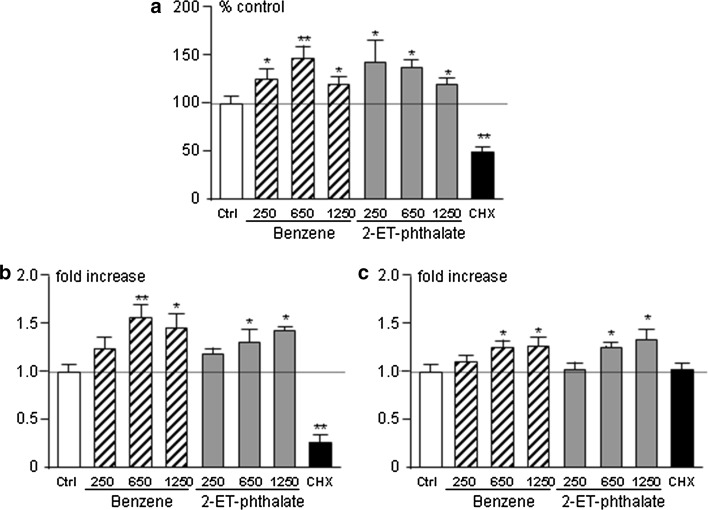
Given these results, we tested the effect of prolonged, e.g. 96 h, incubation on rat anterior pituitary primary cultures and observed an increase in cell energy content (Fig. 2a) as well as increased cell viability (Fig. 2b). We therefore decided to assess proliferation by Brd-U incorporation and Cyclin D1 expression, a marker of cell cycle progression. The percentage of Brd-U positive cells was increased in wells treated with benzene or 2-ethyl-phthalate compared to control wells (Fig. 3a) as was Ccnd1 expression (Fig. 3b), attesting to increased proliferation of rat anterior pituitary cells after 96-h incubation. In view of these effects, we evaluated expression of pituitary tumor transforming gene (PTTG), a protooncogene implicated in pituitary tumorigenesis [30], and, indeed, could observe an increase in Pttg1 expression in wells treated with benzene and 2-ethyl-phthalate (Fig. 3c). As expected, cyclohexamide reduced cell metabolism, viability and proliferation and induced cell apoptosis (Figs. 2, 3).
Fig. 2.
Cell energy content (a), viability (b) and apoptosis (c) in rat anterior pituitary primary cultures treated with 250, 650, 1250 pM benzene (Ben, striped bars) or 2-ethyl-phthalate (2-ET, grey bars), 250 µg/ml of cycloheximide (CHX, black bar) for 96 h. White bars represent control wells treated with plain medium (Ctrl). Data were normalized to control values and expressed as percentage of control; bars represent mean ± SEM from four separate experiments
Fig. 3.
Cell proliferation (a) and Ccnd1 (b) and Pttg1 expression (c) in rat anterior pituitary primary cultures. Cells were treated with 250, 650 pM benzene (Ben, striped bars) or 2-ethyl-phthalate (2-ET, grey bars), 250 µg/ml of cycloheximide (CHX, black bar) in proliferation experiments and with 650, 1250 pM benzene (Ben, striped bars) or 2-ethyl-phthalate (2-ET, grey bars) for mRNA quantification experiments. Both experiments were carried out for 96 h. White bars represent control wells treated with plain medium (Ctrl). Data were normalized to control values and expressed as percentage of control in proliferation experiments and fold-increase in gene expression experiments; bars represent mean ± SEM from three separate experiments
Exposure to benzene and 2-ethyl-phthalate increases AhR/AIP expression
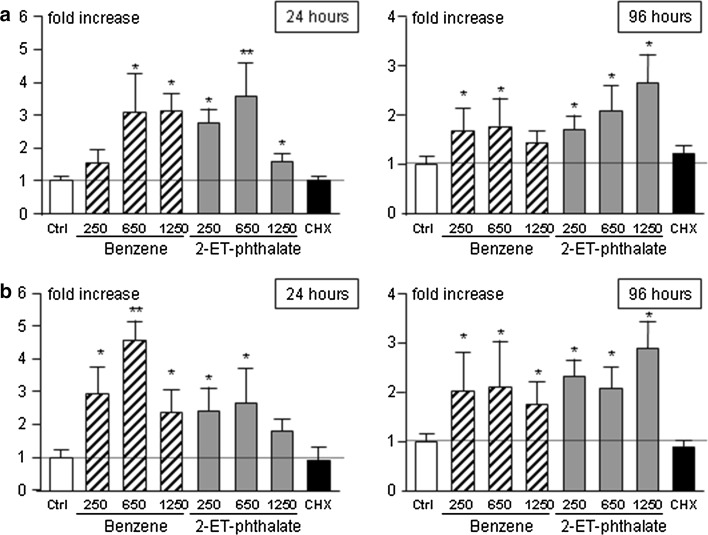
Given the role of AhR as a mediator of endocrine disruptors [23] and of AIP, its chaperone protein, in pituitary tumorigenesis [21] we decided to study whether incubation with benzene or 2-ethyl-phthalate affects expression of either gene. No effect of the two endocrine disruptors were observed after 3 h whereas a clear-cut increase in both AhrR and AIP expression was apparent after 24- and 96-h incubation (Fig. 4).
Fig. 4.
Quantification of AhR (a) and AIP (b) expression in rat anterior pituitary primary cultures treated with 250, 650, 1250 pM benzene (Ben, striped bars) or 2-ethyl-phthalate (2-ET, grey bars), 250 µg/ml of cycloheximide (CHX, black bar) for 3, 24 and 96 h. White bars represent control wells treated with plain medium (Ctrl). Expression data were analyzed as 2−∆∆Ct in three independent experiments and expressed as fold increase over control
Discussion
Our results show that benzene and 2-ethyl-phthalate stimulate rat anterior pituitary cell proliferation, an important finding given the increasing evidence of endocrine-disruptor induced tumorigenesis [3]. In fact, although endocrine disruptors were initially discovered due their adverse effect on reproduction and fetal development, subsequent studies demonstrated a role in an variety of endocrine disorders and, eventually, endocrine-related cancers [4, 31]. The mechanisms underlying endocrine disruptor-induced carcinogenesis are varied and as yet not fully understood but appear to comprise receptor agonism or antagonism, activation of oncogenes and/or repression of tumor suppressor genes, changes in intracellular signaling pathways and DNA methylation patterns. These effects are associated with alterations ranging from hyperplasia to carcinoma [32–34], increased risk of cancer and, ultimately, increased cancer-related mortality [3, 10].
Pituitary tumors are common intracranial neoplasias with site-related symptoms and systemic morbidity due to hormonal excess. Most are sporadic, slow-growing and diagnosed in middle-aged to older individuals [35]. Epidemiological studies are few but recent reports of increased prevalence of pituitary adenomas in high industrialized areas [19] and, possibly, after toxic spillage [20] suggested a link to environmental causes.
Experimental data provided support for this association as endocrine pollutants have been shown to exert several effects in cell lines derived from rat pituitary neoplasms, most notably estrogen-sensitive somatotropes, i.e. GH3, and mammosomatotropes, i.e. MtT/E-2. Bisphenol A, genistein, o,p′-DDT, cadmium and endosulfan have all been shown to increase proliferation in either cell line [14–17, 36]. Further, increased GH and prolactin synthesis and release has been observed with toxaphene, bisphenol A, dioxin and other alkyl-phenols [37–40].
Our study shows for the first time that endocrine pollutants can stimulate proliferation in normal adult pituitary cells. Our finding is of particular relevance given that the abovementioned studies have been performed on tumoral pituitary cell lines, thus unsuitable to study the development of pituitary tumors. So far, only cadmium, a heavy metal with long half-life and estrogen-like activity, has been studied in the normal pituitary in vitro and increased cell growth was observed after 96-h incubation [36]. In this context, it has been reported that perinatal administration of endocrine disruptors is associated with increased incidence of pituitary tumors in grown rats [18] and, interestingly, that cats with acromegaly present higher plasma concentrations of halogenated contaminants, such as polychlorinated biphenyls, polybrominated diphenyl ethers and dichlorophenyl ethane, compared to non-acromegalic cats [41]. Altogether, it appears that the normal pituitary is indeed sensitive to the proliferative effect of endocrine contaminants.
Endocrine disruptors were first identified as compounds with estrogenic potential [42] and, as such, act via the estrogen/androgen receptor pathway [43, 44]. Indeed, the estrogen receptor is involved also in stimulation of proliferation and transcription in pituitary cell lines [14, 36, 39, 45], mainly via the ERK pathway [16]. Current evidence demonstrates that disruptors call several additional pathways into play including the AhR–AIP–ARNT system [23]. AhR is a cytosolic transcription factor first identified through its dioxin-binding capacity and, indeed, mediates a variety of responses to toxic halogenated aromatic hydrocarbons [22]. AIP acts as chaperone to AhR and facilitates activation of AhR; in turn, activated AhR translocates into the nucleus, heterodimerizes with AhR-nuclear translocator (ARNT) and acts upon target genes [46]. The role of this pathway in carcinogenesis is the focus of increasing interest [23] and, indeed, a link to pituitary tumorigenesis was recently detected as germline mutations in AIP were shown to predispose to development of pituitary adenomas [21]. Several studies followed upon this first report in an attempt to clarify the pathogenesis of AIP-mutated pituitary tumors but the exact mechanism remains elusive [47, 48]. In fact, expression and cellular localization of AIP, AhR and ARNT appear variable with some tumors presenting low AIP, absent nuclear AhR staining and loss of ARNT expression, others increased AIP expression or nuclear AhR staining [49–51]. A most recent study in fibroblasts from patients with four different AIP mutations showed that AhR expression was unaffected but that AhR target genes, i.e. CYP1B1, AhR repressor (AHRR), were either reduced or increased depending on the AIP variant [52]. From a clinical viewpoint, patients carrying AIP mutations are more often young, male and with large GH- or mixed GH- and prolactin-secreting tumors [48, 53, 54]. Interestingly, the AhR gene itself appears to contribute to severity of acromegaly as polymorphisms and variants in AhR have been associated with more aggressive disease [55, 56].
Altogether, it is clear that the AhR–AIP pathway is involved in pituitary tumorigenesis and our findings shed further light into this concept. We observed an increase in AhR and AIP expression during treatment with benzene and 2-ethyl-phthalate, which represents the first evidence for upregulation of AhR/AIP gene expression in normal pituitaries in vitro. Data in pituitary cell lines showed that some AhR ligands, e.g. ß-naphtoflavone, prothioconazole, reduce AhR expression [57] and function [24] while other AhR ligands, e.g. 2-methyl-4-chlorophenoxyacetic acid, tau-fluvalinate, stimulate AhR activity [24]. In contrast, dioxin, the main AhR ligand, failed to affect AhR expression in pituitary cells in vitro [58] and no changes in anterior pituitary AhR expression up to 4 weeks after dioxin administration were observed in vivo [59]. As regards benzene and 2-ethyl-phthalate, both act via AhR in different cell models [60, 61] and our evidence now shows that these endocrine disruptors modulate the pituitary AhR/AIP pathway.
Last, one word of comment on our research protocol. As mentioned above, our study evaluated the effects of two specific pollutants, benzene and 2-ethyl-phthalate, rather than a mixture of pollutants, as usually occurs for both routine and occupational exposure. Indeed, research strategies into the impact of endocrine disruptors encourage testing with a variety of chemicals at different dosages in distinct stages of development [62]. In order for this research to prove significant, however, there has to be some evidence on the effect of one or another pollutant in a given tissue. Our findings prove that benzene and 2-ethyl-phthalate stimulate proliferation in adult rat pituitary cells and provide the basis for further studies aimed at expanding upon our results, e.g. susceptibility in adult vs early life, effect of low-dose chemical mixtures, multigenerational studies in exposed areas [3].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Funding
This work was supported by the Italian Ministry for Teaching, University and Research (PRIN 2010–2011 Grant Number 2010TYCL9B_004).
Compliance with ethical standards
Conflict of interest
The Authors declare that there is no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s11102-016-0777-3) contains supplementary material, which is available to authorized users.
References
- 1.Kabir ER, Rahman MS, Rahman I. A review on endocrine disruptors and their possible impacts on human health. Environ Toxicol Pharmacol. 2015;40:241–258. doi: 10.1016/j.etap.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Gore AC. Neuroendocrine targets of endocrine disruptors. Hormones. 2015;9:16–27. doi: 10.14310/horm.2002.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT. EDC-2: the endocrine society’s second scientific statement on endocrine-disrupting chemicals. Endocr Rev. 2015;36:E1–E150. doi: 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schug TT, Johnson AF, Birnbaum LS, Colborn T, Guillette LJ, Jr, Crews DP, Collins T, Soto AM, Vom Saal FS, McLachlan JA, Sonnenschein C, Heindel JJ. Minireview: endocrine disruptors: past lessons and future directions. Mol Endocrinol. 2016;30:833–847. doi: 10.1210/me.2016-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hampl R, Kubatova J, Starka L. Steroids and endocrine disruptors—history, recent state of art and open questions. J Steroid Biochem Mol Biol. 2016;155:217–223. doi: 10.1016/j.jsbmb.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Saracci R, Kogevinas M, Bertazzi PA, Bueno de Mesquita BH, Coggon D, Green LM, Kauppinen T, L’Abbe KA, Littorin M, Lynge E. Cancer mortality in workers exposed to chlorophenoxy herbicides and chlorophenols. Lancet. 1991;338:1027–1032. doi: 10.1016/0140-6736(91)91898-5. [DOI] [PubMed] [Google Scholar]
- 7.Rudel RA, Fenton SE, Ackerman JM, Euling SY, Makris SL. Environmental exposures and mammary gland development: state of the science, public health implications, and research recommendations. Environ Health Perspect. 2011;119:1053–1061. doi: 10.1289/ehp.1002864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warner M, Mocarelli P, Samuels S, Needham L, Brambilla P, Eskenazi B. Dioxin exposure and cancer risk in the Seveso Women’s Health Study. Environ Health Perspect. 2011;119:1700–1705. doi: 10.1289/ehp.1103720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hemminki K, Li X. Cancer risks in second-generation immigrants to Sweden. Int J Cancer. 2002;99:229–237. doi: 10.1002/ijc.10323. [DOI] [PubMed] [Google Scholar]
- 10.Akthar FZ, Garabrant DH, Ketchum NS, Michalek JE. Cancer in US Air Force veterans of the Vietnam war. J Occup Environ Med. 2004;46:123–136. doi: 10.1097/01.jom.0000111603.84316.0f. [DOI] [PubMed] [Google Scholar]
- 11.Dairkee SH, Luciani-Torres MG, Moore DH, Goodson WH., III Bisphenol-A-induced inactivation of the p53 axis underlying deregulation of proliferation kinetics, and cell death in non-malignant human breast epithelial cells. Carcinogenesis. 2013;34:703–712. doi: 10.1093/carcin/bgs379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mlynarcikova A, Macho L, Fickova M. Bisphenol A alone or in combination with estradiol modulates cell cycle- and apoptosis-related proteins and genes in MCF7 cells. Endocr Regul. 2013;47:189–199. doi: 10.4149/endo_2013_04_189. [DOI] [PubMed] [Google Scholar]
- 13.Kang NH, Hwang KA, Lee HR, Choi DW, Choi KC. Resveratrol regulates the cell viability promoted by 17beta-estradiol or bisphenol A via down-regulation of the cross-talk between estrogen receptor alpha and insulin growth factor-1 receptor in BG-1 ovarian cancer cells. Food Chem Toxicol. 2013;59:373–379. doi: 10.1016/j.fct.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 14.Maruyama S, Fujimoto N, Yin H, Ito A. Growth stimulation of a rat pituitary cell line MtT/E-2 by environmental estrogens in vitro and in vivo. Endocr J. 1999;46:513–520. doi: 10.1507/endocrj.46.513. [DOI] [PubMed] [Google Scholar]
- 15.Fujimoto N. Effects of environmental estrogenic compounds on growth of a transplanted estrogen responsive pituitary tumor cell line in rats. Food Chem Toxicol. 2003;41:1711–1717. doi: 10.1016/S0278-6915(03)00198-4. [DOI] [PubMed] [Google Scholar]
- 16.Vinas R, Watson CS. Mixtures of xenoestrogens disrupt estradiol-induced non-genomic signaling and downstream functions in pituitary cells. Environ Health. 2013;12:26–36. doi: 10.1186/1476-069X-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cossette LJ, Gaumond I, Martinoli MG. Combined effect of xenoestrogens and growth factors in two estrogen-responsive cell lines. Endocrine. 2002;18:303–308. doi: 10.1385/ENDO:18:3:303. [DOI] [PubMed] [Google Scholar]
- 18.Isling LK, Boberg J, Jacobsen PR, Mandrup KR, Axelstad M, Christiansen S, Vinggaard AM, Taxvig C, Kortenkamp A, Hass U. Late-life effects on rat reproductive system after developmental exposure to mixtures of endocrine disrupters. Reproduction. 2014;147:465–476. doi: 10.1530/REP-13-0448. [DOI] [PubMed] [Google Scholar]
- 19.Cannavò S, Ferraù F, Ragonese M, Curtò L, Torre ML, Magistri M, Marchese A, Alibrandi A, Trimarchi F. Increased prevalence of acromegaly in a highly polluted area. Eur J Endocrinol. 2010;163:509–513. doi: 10.1530/EJE-10-0465. [DOI] [PubMed] [Google Scholar]
- 20.Pesatori AC, Baccarelli A, Consonni D, Lania A, Beck-Peccoz P, Bertazzi PA, Spada A. Aryl hydrocarbon receptor-interacting protein and pituitary adenomas: a population-based study on subjects exposed to dioxin after the Seveso, Italy, accident. Eur J Endocrinol. 2008;159:699–703. doi: 10.1530/EJE-08-0593. [DOI] [PubMed] [Google Scholar]
- 21.Vierimaa O, Georgitsi M, Lehtonen R, Vahteristo P, Kokko A, Raitila A, Tuppurainen K, Ebeling TM, Salmela PI, Paschke R, Gündogdu S, De Menis E, Mäkinen MJ, Launonen V, Karhu A, Aaltonen LA. Pituitary adenoma predisposition caused by germline mutations in the AIP gene. Science. 2006;312:1228–1230. doi: 10.1126/science.1126100. [DOI] [PubMed] [Google Scholar]
- 22.Poland A, Knutson J, Glover E. Studies on the mechanism of action of halogenated aromatic hydrocarbons. Clin Physiol Biochem. 1985;3:147–154. [PubMed] [Google Scholar]
- 23.Safe S. Molecular biology of the Ah receptor and its role in carcinogenesis. Toxicol Lett. 2001;120:1–7. doi: 10.1016/S0378-4274(01)00301-0. [DOI] [PubMed] [Google Scholar]
- 24.Ghisari M, Long M, Tabbo A, Bonefeld-Jorgensen EC. Effects of currently used pesticides and their mixtures on the function of thyroid hormone and aryl hydrocarbon receptor in cell culture. Toxicol Appl Pharmacol. 2015;284:292–303. doi: 10.1016/j.taap.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Tran C, Richmond O, Aaron L, Powell JB. Inhibition of constitutive aryl hydrocarbon receptor (AhR) signaling attenuates androgen independent signaling and growth in (C4-2) prostate cancer cells. Biochem Pharmacol. 2013;85:753–762. doi: 10.1016/j.bcp.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pecori Giraldi F, Cavagnini F. Corticotropin-releasing hormone is produced by rat corticotropes and modulates ACTH secretion in a paracrine/autocrine fashion. J Clin Invest. 1998;101:2478–2484. doi: 10.1172/JCI443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pecori Giraldi F, Pesce S, Maroni P, Pagliardini L, Lasio G, Losa M, Cavagnini F. Inhibitory effect of preproTRH(178-199) on ACTH secretion by human corticotrope tumours. J Neuroendocrinol. 2010;22:294–300. doi: 10.1111/j.1365-2826.2010.01959.x. [DOI] [PubMed] [Google Scholar]
- 28.Nagami K, Kawashima Y, Kuno H, Kemi M, Matsumoto H. In vitro cytotoxicity assay to screen compounds for apoptosis-inducing potential on lymphocytes and neutrophils. J Toxicol Sci. 2002;27:191–203. doi: 10.2131/jts.27.191. [DOI] [PubMed] [Google Scholar]
- 29.Rodrigues RM, Bouhifd M, Bories G, Sacco MG, Gribaldo L, Fabbri M, Coecke S, Whelan MP. Assessment of an automated in vitro basal cytotoxicity test system based on metabolically-competent cells. Toxicol In Vitro. 2013;27:760–767. doi: 10.1016/j.tiv.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Heaney AP, Melmed S. Pituitary tumour transforming gene: a novel factor in pituitary tumour formation. Bailliere’s Clin Endocrinol Metab. 1999;13:367–380. doi: 10.1053/beem.1999.0028. [DOI] [PubMed] [Google Scholar]
- 31.Maqbool F, Mostafalou S, Bahadar H, Abdollahi M. Review of endocrine disorders associated with environmental toxicants and possible involved mechanisms. Life Sci. 2016;145:265–273. doi: 10.1016/j.lfs.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 32.Penaglotidou E, Zerva S, Mitsiou DJ, Alexis MN, Kitraki E. Perinatal exposure to low-dose bisphenol A affects the neuroendocrine stress response. J Endocrinol. 2014;220:207–218. doi: 10.1530/JOE-13-0416. [DOI] [PubMed] [Google Scholar]
- 33.Murray TJ, Maffini MV, Ucci AA, Sonnenschein C, Soto AM. Induction of mammary gland ductal hyperplasias and carcinoma in situ following fetal bisphenol A exposure. Reprod Toxicol. 2007;23:383–390. doi: 10.1016/j.reprotox.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prins GS, Birch L, Tang WY, Ho SM. Developmental estrogen exposures predispose to prostate carcinogenesis with aging. Reprod Toxicol. 2007;23:374–382. doi: 10.1016/j.reprotox.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aflorei ED, Korbonits M. Epidemiology and etiopathogenesis of pituitary adenomas. J Neurooncol. 2014;117:379–394. doi: 10.1007/s11060-013-1354-5. [DOI] [PubMed] [Google Scholar]
- 36.Ronchetti SA, Miler EA, Duvilanski BH, Cabilla JP. Cadmium mimics estrogen-driven cell proliferation and prolactin secretion from anterior pituitary cells. PLoS ONE. 2013;8:e81101–e81113. doi: 10.1371/journal.pone.0081101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinmetz R, Brown NG, Allen DL, Bigsby RM, Ben JN. The environmental estrogen bisphenol A stimulates prolactin release in vitro and in vivo. Endocrinology. 1997;138:1780–1786. doi: 10.1210/endo.138.5.5132. [DOI] [PubMed] [Google Scholar]
- 38.Graham M, Cossette L, Gelinas S, Martinoli MG. In vitro modulation of prolactin mRNA by toxaphene and 3,3′,4,4′-tetrachlorobiphenyl. Environ Res. 2003;92:207–212. doi: 10.1016/S0013-9351(02)00093-2. [DOI] [PubMed] [Google Scholar]
- 39.Dang VH, Nguyen TH, Lee GS, Choi KC, Jeung EB. In vitro exposure to xenoestrogens induces growth hormone transcription and release vie estrogen-receptor dependent pathways in rat pituitary GH3 cells. Steroids. 2009;74:707–714. doi: 10.1016/j.steroids.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Elango A, Shepherd B, Chen T. Effects of endocrine disrupters on the expression of growth hormone and prolactin mRNA in the rainbow trout pituitary. Gen Comp Endocrinol. 2006;145:116–127. doi: 10.1016/j.ygcen.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Dirtu AC, Niessen SJM, Jorens PG, Covaci A. Organohalogenated contaminants in domestic cats’ plasma in relation to spontaneous acromegaly and type 2 diabetes mellitus: a clue for endocrine disruption in humans? Environ Int. 2013;57–58:60–67. doi: 10.1016/j.envint.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 42.Feldman D, Krishnan A. Estrogens in unexpected places: possible implications for researchers and consumers. Environ Health Perspect. 1995;103(Suppl 7):129–133. doi: 10.1289/ehp.95103s7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kiyama R, Wada-Kiyama Y. Estrogenic endocrine disruptors: molecular mechanisms of action. Environ Int. 2015;83:11–40. doi: 10.1016/j.envint.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 44.Kumar N, Sharan S, Srivastava S, Roy P. Assessment of estrogenic potential of diethyl phthalate in female reproductive system involving both genomic and non-genomic actions. Reprod Toxicol. 2014;49:12–26. doi: 10.1016/j.reprotox.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 45.Dang VH, Nguyen TH, Choi KC, Jeung EB. A calcium-binding protein, Calbindin-D9k, is regulated through an estrogen-receptor-mediated mechanism following xenoestrogen exposure in the GH3 cell line. Toxicol Sci. 2007;92:408–415. doi: 10.1093/toxsci/kfm120. [DOI] [PubMed] [Google Scholar]
- 46.Mulero-Navarro S, Fernandez-Salguero PM. New trends in aryl hydrocarbon receptor biology. Front Cell Dev Biol. 2016;4:45–59. doi: 10.3389/fcell.2016.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trivellin G, Korbonits M. AIP and its interacting partners. J Endocrinol. 2011;210:137–155. doi: 10.1530/JOE-11-0054. [DOI] [PubMed] [Google Scholar]
- 48.Beckers A, Aaltonen LA, Daly AF, Karhu A. Familial isolated pituitary adenomas (FIPA) and the pituitary adenoma predisposition due to mutations in the aryl hydrocarbon receptor interacting protein (AIP) gene. Endocr Rev. 2013;34:239–277. doi: 10.1210/er.2012-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jaffrain-Rea ML, Angelini M, Gargano D, Tichomirowa MA, Daly AF, Vanbellinghen JF, D’Innocenzo E, Barlier A, Giangaspero F, Esposito V, Ventura L, Arcella A, Theodoropoulou M, Naves LA, Fajardo C, Zacharieva S, Rohmer V, Brue T, Gulino A, Cantore G, Alesse E, Beckers A. Expression of aryl hydrocarbon receptor (AHR) and AHR-interacting protein in pituitary adenomas: pathological and clinical implications. Endocr Relat Cancer. 2009;16:1029–1043. doi: 10.1677/ERC-09-0094. [DOI] [PubMed] [Google Scholar]
- 50.Heliövaara E, Raitila A, Launonen V, Paetau A, Arola J, Lehtonen H, Sane T, Weil RJ, Vierimaa O, Salmela P, Tuppurainen K, Mäkinen M, Aaltonen LA, Karhu A. The expression of AIP-related molecules in elucidation of cellular pathways in pituitary adenomas. Am J Pathol. 2009;175:2501–2507. doi: 10.2353/ajpath.2009.081131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hernandez-Ramirez LC, Martucci F, Morgan RM, Trivellin G, Tilley D, Ramos-Guajardo N, Iacovazzo D, D’Acquisto F, Prodromou C, Korbonits M. Rapid proteasomal degradation of mutant proteins is the primary mechanism leading to tumorigenesis in patients with missense AIP mutations. J Clin Endocrinol Metab. 2016;101:3144–3154. doi: 10.1210/jc.2016-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lecoq AL, Viengchareun S, Hage M, Bouligand J, Young J, Boutron A, Zizzari P, Lombes M, Chanson P, Kamenicky P. AIP mutations impair AhR signaling in pituitary adenoma patients fibroblasts and in GH3 cells. Endocr Relat Cancer. 2016;23:433–443. doi: 10.1530/ERC-16-0041. [DOI] [PubMed] [Google Scholar]
- 53.Cazabat L, Guillaud-Bataille M, Bertherat J, Raffin-Sanson ML. Mutations of the gene for the aryl hydrocarbon receptor-interacting protein in pituitary adenomas. Horm Res. 2009;71:132–141. doi: 10.1159/000197869. [DOI] [PubMed] [Google Scholar]
- 54.Martucci F, Trivellin G, Korbonits M. Familial isolated pituitary adenomas: an emerging clinical entity. J Endocrinol Invest. 2012;35:1003–1014. doi: 10.1007/BF03346742. [DOI] [PubMed] [Google Scholar]
- 55.Cannavò S, Ferrau F, Ragonese M, Romeo PD, Torre ML, Puglisi S, De Menis E, Arnaldi G, Salpietro C, Cotta OR, Albani A, Ruggeri RM, Trimarchi F. Increased frequency of the rs2066853 variant of aryl hydrocarbon receptor gene in patients with acromegaly. Clin Endocrinol. 2014;81:249–253. doi: 10.1111/cen.12424. [DOI] [PubMed] [Google Scholar]
- 56.Cannavò S, Ragonese M, Puglisi S, Romeo PD, Torre ML, Alibrandi A, Scaroni C, Occhi G, Ceccato F, Regazzo D, De Menis E, Sartorato P, Arnaldi G, Trementino L, Trimarchi F, Ferrau F. Acromegaly is more severe in patients with AHR or AIP gene variants living in highly polluted areas. J Clin Endocrinol Metab. 2016;101:1872–1879. doi: 10.1210/jc.2015-4191. [DOI] [PubMed] [Google Scholar]
- 57.Moran TB, Brannick KE, Raetzman LT. Aryl-hydrocarbon receptor activity modulates prolactin expression in the pituitary. Toxicol Appl Pharmacol. 2012;265:139–145. doi: 10.1016/j.taap.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang P, Ceccatelli S, Håkansson H, Grandison L, Rannung A. Constitutive and TCDD-induced expression of Ah receptor-responsive genes in the pituitary. NeuroToxicology. 2002;23:783–793. doi: 10.1016/S0161-813X(02)00040-2. [DOI] [PubMed] [Google Scholar]
- 59.Huang P, Rannung A, Ahlbom E, Håkansson H, Ceccatelli S. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the expression of cytochrome P450 1A1, the aryl hydrocarbon receptor, and the aryl hydrocarbon receptor nuclear translocator in rat brain and pituitary. Toxicol Appl Pharmacol. 2000;169:159–167. doi: 10.1006/taap.2000.9064. [DOI] [PubMed] [Google Scholar]
- 60.Mankidy R, Wiseman S, Ma H, Giesy JP. Biological impact of phthalates. Toxicol Lett. 2013;217:50–58. doi: 10.1016/j.toxlet.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 61.Yoon BI, Hirabayashi Y, Kawasaki Y, Kodama Y, Kaneko T, Kanno J, Kim DY, Fujii-Kuriyama Y, Inoue T. Aryl hydrocarbon receptor mediates benzene-induced hematotoxicity. Toxicol Sci. 2002;70:150–156. doi: 10.1093/toxsci/70.1.150. [DOI] [PubMed] [Google Scholar]
- 62.Gore AC, Crews D, Doan LL, La Merril M, Patisaul H, Zota A (2014) Introduction to endocrine disrupting chemicals (EDC). A guide for public interest organizations and policy-makers. The Endocrine Society and IPEN, pp 1–69. http://ipen.org/documents/introduction-endocrine-disrupting-chemicals-edcs
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.