Abstract
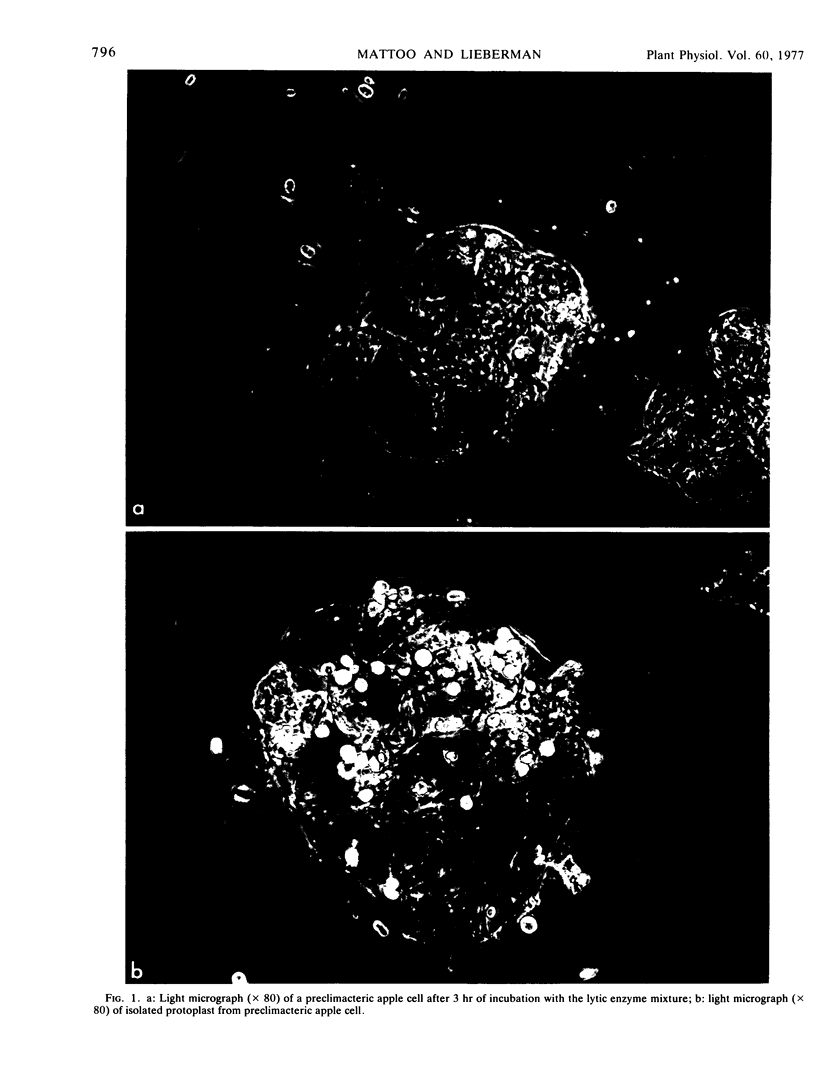
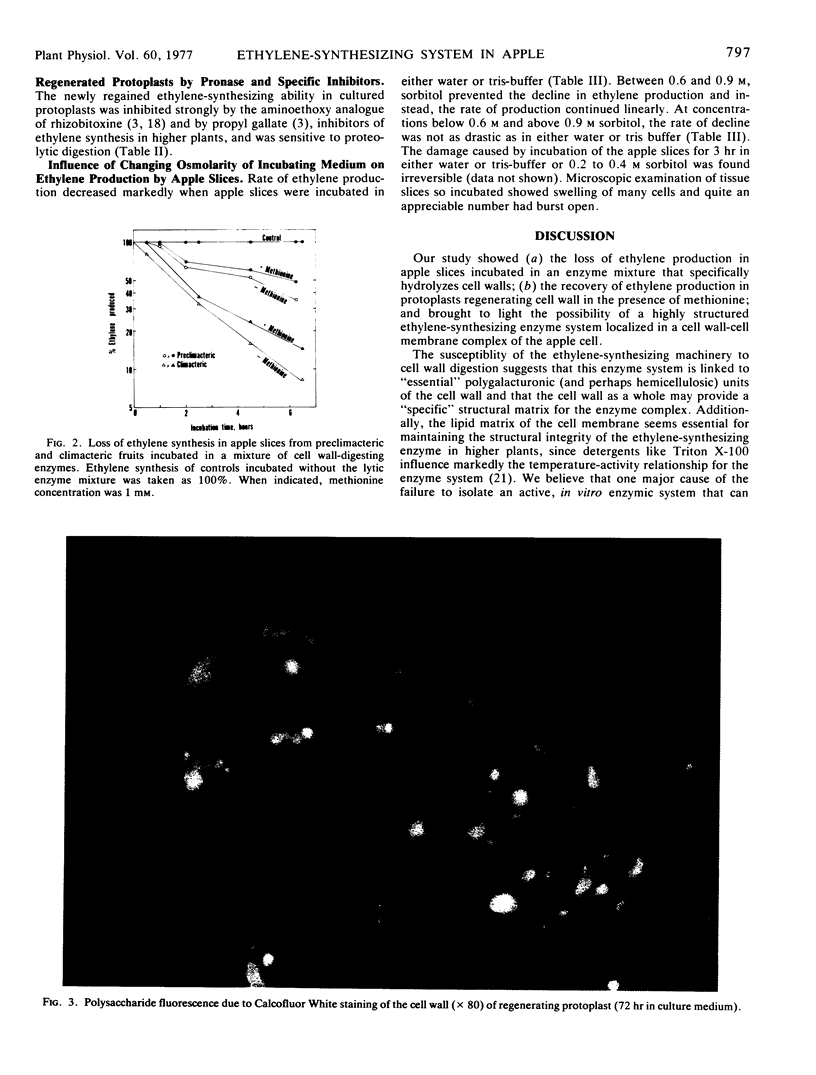
Apple (Malus sp.) slices gradually lost the ability to synthesize ethylene when incubated with a mixture of enzymes that digest cell walls. The released protoplasts did not produce ethylene. The release of protoplasts was faster from climacteric fruit slices than from preclimacteric tissue. In protoplast suspension culture, as new cell wall was deposited (as judged by the intensity of fluorescence of regenerating protoplasts stained with Calcofluor White and the incorporation of labeled myo-inositol into their ethanol-insoluble residue), ethylene synthesis was gradually regained. Restored ethylene synthesis reached a maximum after 80 hours in protoplasts from preclimacteric fruit and in 120 hours in those from climacteric tissue. Addition of methionine (1 mm) to the culture medium was essential for appreciable synthesis of ethylene; and this synthesis was inhibited by the aminoethoxy analogue of rhizobitoxine and by propyl gallate, inhibitors of ethylene synthesis in higher plants. We suggest that the ethylene-synthesizing enzyme system is highly structured in the apple cell and is localized in a cell wall-cell membrane complex.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BYERRUM R. U., FLOKSTRA J. H., DEWEY L. J., BALL C. D. Incorporation of formate and the methyl group of methionine into methoxyl groups of lignin. J Biol Chem. 1954 Oct;210(2):633–643. [PubMed] [Google Scholar]
- Burg S. P., Thimann K. V. Studies on the Ethylene Production of Apple Tissue. Plant Physiol. 1960 Jan;35(1):24–35. doi: 10.1104/pp.35.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhlen P., Stein S., Dairman W., Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973 Mar;155(1):213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- HAMILL R. L., BYERRUM R. U., BALL C. D. A study of the biosynthesis of the methoxyl groups of lignin in tobacco plants. J Biol Chem. 1957 Feb;224(2):713–716. [PubMed] [Google Scholar]
- Lieberman M., Kunishi A. Stimulation of ethylene production in apple tissue slices by methionine. Plant Physiol. 1966 Mar;41(3):376–382. doi: 10.1104/pp.41.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewus F. Inositol metabolism and cell wall formation in plants. Fed Proc. 1965 Jul-Aug;24(4):855–862. [PubMed] [Google Scholar]
- Meudt W. J., Stecher K. J. Promotion of peroxidase activity in the cell wall of Nicotiana. Plant Physiol. 1972 Jul;50(1):157–160. doi: 10.1104/pp.50.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M., Dohrmann U. Characterization of naphthaleneacetic Acid binding to receptor sites on cellular membranes of maize coleoptile tissue. Plant Physiol. 1977 Mar;59(3):357–364. doi: 10.1104/pp.59.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge I., Osborne D. J. Role of peroxidase when hydroxyproline-rich protein in plant cell walls is increased by ethylene. Nat New Biol. 1971 Feb 17;229(7):205–208. doi: 10.1038/newbio229205a0. [DOI] [PubMed] [Google Scholar]
- Roberts R. M., Deshusses J., Loewus F. Inositol Metabolism in Plants. V. Conversion of Myo-inositol to Uronic Acid and Pentose Units of Acidic Polysaccharides in Root-tips of Zea mays. Plant Physiol. 1968 Jun;43(6):979–989. doi: 10.1104/pp.43.6.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. M., Shah R. H., Golebiewski A., Loewus F. Incorporation of methanol into pectic substance. Plant Physiol. 1967 Dec;42(12):1737–1742. doi: 10.1104/pp.42.12.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATO C. S., BYERRUM R. U., BALL C. D. The biosynthesis of pectinic acid methylesters through transmethylation from methionine. J Biol Chem. 1957 Feb;224(2):717–723. [PubMed] [Google Scholar]
- Strand L. L., Rechtoris C., Mussell H. Polygalacturonases Release Cell-Wall-bound Proteins. Plant Physiol. 1976 Dec;58(6):722–725. doi: 10.1104/pp.58.6.722. [DOI] [PMC free article] [PubMed] [Google Scholar]