Abstract
Objective
To examine the association of individually derived infant weight growth velocity patterns with general and abdominal adiposity measures in childhood.
Methods
In a population-based prospective cohort study among 5,126 children, we used repeated growth measurements between 0 and 3 years of age to derive peak weight velocity (PWV), age at adiposity peak (AGEAP) and body mass at adiposity peak (BMIAP). At the median age of 6.0 years (95% range 5.7, 6.8), we estimated body mass index (BMI), body fat percentage, android/gynoid fat mass ratio and pre-peritoneal abdominal fat area by using Dual-energy X-ray absorptiometry (DXA) and abdominal ultrasound.
Results
Higher infant PWV and BMIAP were associated with higher childhood BMI, body fat percentage, android/gynoid fat mass ratio and pre-peritoneal abdominal fat area (all P-values < 0.05), with the strongest effect estimates for BMI (differences in BMI: 0.37 standard deviation (SD), 95% confidence interval (CI): 0.34, 0.39) and 0.45 SD (95% CI: 0.43, 0.48) per 1-SD increase in infant PWV and BMIAP, respectively). Infant AGEAP in the highest tertile (>0.75 years) was associated with higher general and abdominal adiposity among girls at the age of 6 years (all P-values < 0.05). Similarly, a 1-SD higher infant PWV and BMIAP were associated with increased risks of childhood overweight (Odds Ratios (95% CI): 2.1 (1.9, 2.3) and 2.5 (2.2, 2.8), respectively). These associations were independent of gestational age and size at birth and tended to be stronger among girls.
Conclusion
Higher infant PWV and BMIAP are associated with adverse general and abdominal fat distribution profiles and increased risks of overweight at school-age. Whether infant growth patterns add to the prediction of later overweight should be further studied.
Keywords: pediatrics, infant weight growth, obesity, body fat distribution
Introduction
Childhood overweight is associated with cardiovascular and metabolic diseases in later life (1, 2). Early childhood seems to be a critical period for overweight (3, 4). Increased weight gain during early childhood is associated with higher risks of overweight in later life (5–7). We have previously observed that a higher peak weight velocity (PWV) and body mass index at adiposity peak (BMIAP) and that fetal and infant height and weight gain during infancy are associated with increased risks of overweight in preschool children (8, 9). Also, infant PWV was positively associated with body mass index (BMI) in adults (10).
BMI is a widely accepted outcome measure of obesity. However, more detailed body fat distribution measures may be more strongly related with adverse health outcomes and the risk of mortality in adults (11, 12). Yet, studies in children and adolescents reported inconsistent associations of specific total and abdominal fat measurements with cardiovascular and metabolic outcomes (13–17). Previously, we have found that higher total fat mass, android/gynoid fat ratio and pre-peritoneal abdominal fat area are, independent of BMI, associated with cardiovascular risk factors in children (18). For the current study, we hypothesized that infant weight growth velocity patterns not only influence BMI, but also specific general and abdominal fat distribution outcomes in school-age children.
Therefore, we examined the associations of PWV, age at adiposity peak (AGEAP) and BMIAP with general and abdominal adiposity measures at the age of 6 years. We also explored whether any association was influenced by gestational age and size at birth. This study extends our previous studies showing first associations of PWV and BMIAP with BMI in preschool children and second associations of fetal and infant height and weight gain with total body and abdominal fat in childhood (8, 9). Thus far, it is not known whether specific derived infant growth patterns, including PWV and BMIAP, also affect childhood adiposity outcomes.
Methods
Study design
This study was embedded in the Generation R Study, a prospective population-based cohort study from fetal life onwards in Rotterdam, the Netherlands, which has been described in detail previously (19, 20). The Medical Ethics Committee of the Erasmus MC approved the study. We obtained written informed consent from the mothers.
For the current study, information about infant growth measures was available in 7,455 singleton live born children with a known sex, birth weight and gestational age. From these, we excluded 612 children who had fewer than three infant growth measurements, which were necessary for infant weight growth modeling. Of the remaining 6,843 children, 5,126 children participated in childhood adiposity measurements at the age of 6 years (Supplementary Material Figure S1). Children who did not participate in the follow-up measurements had a lower birth weight, were less often breastfeed and had higher infant PWV (Results from non-response analysis is given in Supplementary Material Table S1).
Longitudinal infant weight growth velocity patterns
Gestational age and sex adjusted standard deviation scores for birth weight and length were calculated using North-European growth charts (21). Small size for gestational age at birth and large size for gestational age at birth were defined as a gestational age adjusted birth weight below the 10th percentile (-1.69 SD) and above the 90th percentile (1.61 SD) in the study cohort, respectively.
As described previously, length and weight were measured according to standardized procedures at the ages of 1, 2, 3, 4, 6, 11, 14, 18, 24 and 36 months. The median number of postnatal growth measurements was 5 (full range: 3-13) (19). Age- and sex-adjusted SDS for all growth characteristics were obtained with Dutch reference growth charts (22). These growth measures were used to construct longitudinal weight and BMI growth patterns, and derive infant PWV, AGEAP and BMIAP. PWV refers to the greatest weight growth in infancy, AGEAP refers to the infant age at which the infant BMI peak is reached, and BMIAP refers to the BMI level reached at the adiposity peak. These growth measures give more detailed information about infant weight growth patterns than catch up growth, which just reflects a difference between two growth measures. The procedures to derive these measures have previously been described and are presented in detail in the Supplementary Material (8, 10, 23).
General and abdominal fat outcomes at school-age
At the median age of 6 years (median: 6.0 years (95% range: 5.7, 6.8 years)) height and weight were measured without shoes and heavy clothing. All measurements were performed in a dedicated research center by research staff, who were trained to perform the measures according to specific research protocols. Height was measured to the nearest 0.1 cm by a stadiometer (Holtain Limited, Crosswell, Crymych, UK). Weight was measured to the nearest gram using an electronic scale (SECA 888, Almere, The Netherlands). Childhood overweight/obesity was defined by the International Obesity Task Force cut offs (24). Total body and regional fat mass was measured using a Dual-energy X-ray absorptiometry (DXA) scanner (iDXA, GE-Lunar, 2008, Madison, WI, USA), and analyzed with the enCORE software v.12.6 (25). Body fat percentage at the age of 6 years was calculated as the ratio of total body fat (kg) and total body weight (kg) measured by DXA. The ratio of android fat mass and gynoid fat mass was calculated. Abdominal ultrasound examinations were used to measure pre-peritoneal abdominal fat area as measure of visceral abdominal fat, as previously described (26, 27). Scans were made longitudinally just below the xiphoid process to the navel along the midline (linea alba). Pre-peritoneal abdominal fat mass distance was measured as distance of the linea alba to the peritoneum on top of the liver. Pre-peritoneal fat mass area was measured as area of 2 cm length along the midline starting from the maximum pre-peritoneal distance in direction of the navel.
Covariates
We obtained information about maternal age, pre-pregnancy weight, height and BMI, parity, marital status, educational level, smoking, alcohol consumption, and folic acid supplement use during pregnancy at enrollment (19). Information on gestational diabetes and gestational hypertensive disorders was obtained from medical records (28). Weight, height and BMI were measured in 3,681 fathers at enrollment (72% of mothers with this information) (19, 20). Child´s ethnicity (European, Non-European) was classified by the countries of birth of the parents (29). Information on duration of breastfeeding, age at introduction solid foods was assessed by questionnaires at the ages of 2, 6 and 12 months. At the age of 6 years, hours of watching television were obtained by questionnaires (19).
Statistical analysis
First, we compared characteristics between boys and girls using One-Way ANOVA and Chi-square tests. The correlations between early weight growth measures and adiposity outcomes were explored using Pearson correlation coefficients. Second, we assessed the associations of infant PWV, AGEAP and BMIAP with childhood BMI, body fat percentage, android/gynoid fat mass ratio and pre-peritoneal abdominal fat area using multivariate linear regression models. The models were first adjusted for child’s age and sex only, and additionally for maternal age, pre-pregnancy BMI, parity, marital status, educational level, smoking, alcohol consumption, folic acid supplement use, gestational diabetes and gestational hypertensive disorders, paternal BMI, gestational age adjusted birth weight, child’s ethnicity, duration of breastfeeding, age at introduction of solid foods and hours of TV watching per day. Analyses focused on fat mass outcomes were additionally adjusted for height (30). Covariates were included in these regression models based on previous literature, a strong association with the outcome or a significant change in effect estimates (>10%). Third, the associations of infant PWV, AGEAP and BMIAP with the risk of overweight/obesity were assessed using logistic regression models with similar adjustments. To explore whether these associations were modified by gestational age and size at birth, we tested the interaction of PWV, AGEAP and BMIAP with gestational age and size at birth in relation to adiposity outcomes. If any of these statistical interactions was significant (P<0.05 ), we performed the regression analyses in strata of gestational age at birth (preterm; term) and gestational age adjusted birth weight (small size for gestational age; appropriate size for gestational age; large size for gestational age). Analyses were additionally adjusted for multiple testing using a Bonferroni correction (p <0.0125 considered as significant). For the interaction analyses, if we would apply multiple testing correction only the interactions between size at birth with age at adiposity peak in relation to BMI and android/gynoid fat mass ratio would be significant, considering a p-value < 0.05/8 =0.0125. As the distribution of pre-peritoneal abdominal fat area was skewed, this variable was natural log-transformed. To handle missing values in covariates in order to reduce potential bias associated with missing data, multiple imputations by generating 5 independent datasets using the Markov Chain Monte Carlo (MCMC) was performed, a method after which the pooled effect estimates (95% Confidence Interval) are presented (31). Imputations were based on the relationships between covariates, determinants or outcomes. Statistical analyses were performed using the Statistical Package of Social Sciences version 21.0 for Windows (IBM Corp, Armonk, NY, USA).
Results
Subjects characteristics
Table 1 shows that girls had lower birth weight and lower infant PWV and BMIAP (all P values <0.05). At the age of 6 years, girls had a higher body fat percentage, android/gynoid fat mass ratio and pre-peritoneal abdominal fat area, and were more frequently overweight or obese than boys (all P values <0.05). Supplementary Material Table S2 gives the correlations between infant PWV, AGEAP and BMIAP with childhood BMI, body fat percentage, the android/gynoid fat mass ratio and pre-peritoneal abdominal fat area.
Table 1. Parental and child characteristics (N = 5,126).
| Total (N=5,126) | Boys (N= 2,570) | Girls (N=2,556) | P value | |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Age (years) | 31.5 (24.0, 36.9) | 31.4 (23.8, 37.1) | 31.5 (24.1, 36.7) | 0.91 |
| Height (cm) | 168.0 (7.4) | 168.0 (7.2) | 168.0 (7.6) | 0.64 |
| Weight (kg) | 66.4 (12.2) | 66.2 (12.0) | 66.7 (12.3) | 0.22 |
| Body mass index (kg/m2) | 22.7 (19.4, 28.9) | 22.7 (19.4, 28.9) | 22.8 (19.5, 29.0) | 0.15 |
| Parity (%) | 0.41 | |||
| 0 | 56.8 | 56.2 | 57.5 | |
| >= 1 | 43.2 | 43.8 | 42.5 | |
| Marital status (%) | 0.45 | |||
| No partner | 11.2 | 11.6 | 10.9 | |
| Married/living together | 88.8 | 88.4 | 89.1 | |
| Educational level (%) | 0.93 | |||
| Primary | 8.0 | 8.1 | 8.5 | |
| Secondary | 41.5 | 41.8 | 41.5 | |
| Higher | 50.5 | 50.1 | 50 | |
| Smoking during pregnancy (%) | <0.05 | |||
| Ever | 14.8 | 15.9 | 14.0 | |
| Never | 85.2 | 84.1 | 85.9 | |
| Alcohol consumption (%) | 0.58 | |||
| Ever | 57.7 | 57.1 | 56.7 | |
| Never | 42.3 | 42.9 | 43.3 | |
| Folic acid supplement use (%) | 0.21 | |||
| No | 22.5 | 24.8 | 23.1 | |
| Yes | 77.5 | 75.1 | 76.9 | |
| Maternal complications (%) | ||||
| Gestational diabetes, Yes | 0.7 | 0.9 | 0.5 | 0.07 |
| Gestational hypertension, Yes | 3.6 | 3.3 | 4.0 | 0.22 |
| Preeclampsia, Yes | 1.4 | 1.7 | 1.2 | 0.11 |
| Paternal characteristics | ||||
| Age (years) | 33.2 (27.0, 40.2) | 33.1 (27.0, 40.2) | 33.3 (27.3, 40.2) | 0.46 |
| Height (cm) | 182.5 (7.8) | 182.5 (7.9) | 182.5 (7.8) | 0.87 |
| Weight (kg) | 84.0 (12.8) | 83.8 (12.8) | 84.2 (12.8) | 0.31 |
| Body mass index (kg/m2) | 25.1 (21.2, 29.4) | 25.3 (21.2, 29.4) | 25.3 (21.2, 29.4) | 0.25 |
| Birth and infant characteristics | ||||
| Gestational age (weeks) | 40.1 (38.0, 41.7) | 40.1 (38.0, 41.9) | 40.1 (38.0, 41.6) | 0.15 |
| Birth weight (g) | 3447 (543) | 3552 (552) | 3400 (525) | <0.01 |
| Ethnicity (%) | 0.18 | |||
| Dutch or European | 67.9 | 66.9 | 68.8 | |
| Non- European | 32.1 | 33.0 | 31.2 | |
| Duration of breastfeeding | 3.6 (0.5, 10.8) | 3.6 (0.5, 10.5) | 3.8 (0.5, 11.8) | 0.52 |
| Age at introduction of solid foods (%) | ||||
| < 4 months | 9.0 | 9.5 | 8.6 | 0.57 |
| 4 – 5 months | 61.6 | 61.4 | 61.8 | 0.73 |
| 5 – 6 months | 25.5 | 25.3 | 25.5 | 0.81 |
| > 6 months | 3.9 | 3.8 | 4.1 | 0.62 |
| Peak weight velocity (kg/year) | 12.2 (2.1) | 13.1 (2.1) | 11.3 (1.8) | <0.01 |
| AGEAP (years) | 0.7 (0.7, 0.8) | 0.7 (0.7, 0.8) | 0.7 (0.7, 0.8) | 0.89 |
| BMIAP (kg/m2) | 17.6 (16.6, 18.7) | 17.8 (16.8, 18.8) | 17.4 (16.4, 18.4) | <0.01 |
| Childhood characterstics | ||||
| Age at visit (years) | 6.1 (0.4) | 6.1 (0.4) | 6.1 (0.4) | <0.05 |
| Height (cm) | 118.9 (5.7) | 119.4 (5.7) | 118.5 (5.7) | <0.01 |
| Weight (kg) | 22.9 (3.9) | 23.1 (3.8) | 22.8 (4.0) | <0.01 |
| BMI (kg/m2) | 16.1 (1.8) | 16.1 (1.7) | 16.1 (1.9) | 0.88 |
| Body fat percentage (%) | 24.6 (5.5) | 22.5 (4.9) | 26.8 (5.1) | <0.01 |
| Android/gynoid fat mass ratio | 0.3 (0.1) | 0.2 (0.1) | 0.3 (0.1) | <0.05 |
| Pre-peritoneal abdominal fat area (mm2) | 0.4 (0.2, 0.7) | 0.4 (0.2, 0.6) | 0.5 (0.3, 0.8) | <0.01 |
| Overweight/Obesity (%) | 15.9 | 13.3 | 18.4 | <0.01 |
| Watching television (%) | 0.17 | |||
| < 2 hours a day | 80.3 | 79.6 | 81.0 | |
| ≥ 2 hours a day | 19.7 | 20.4 | 19.0 |
Abbreviations: N: number. BMI: body mass index, PWV: peak weight velocity, AGEAP: age at adiposity peak, BMIAP: body mass index at adiposity peak. Values are means (standard deviation), percentages or medians (90% range) for variables with skewed distribution. Imputed data. P-value (included in analysis: yes versus no) was estimated by using One-Way Anova test and Chi-square tests.
Infant weight growth velocity patterns and body fat outcomes in childhood
Table 2 shows that infant PWV and BMIAP were positively associated with all general and abdominal fat mass measures in childhood (all P values <0.05), with the strongest associations present for BMI. An increase of one standard deviation score (SDS) infant PWV (2.1 kg/year) was associated with a higher childhood BMI, body fat percentage, android/gynoid fat mass ratio and pre-peritoneal abdominal fat area. Also, a 1 SDS higher infant BMIAP (0.8 kg/m2) was associated with a higher BMI, body fat percentage, android/gynoid fat mass ratio and pre-peritoneal abdominal fat area in childhood (P-value <0.05). Although the direction of association tended to be similar in boys and girls, there seems to be stronger associations of PWV with general and abdominal fat mass measures in childhood in girls. Infant AGEAP was positively associated with all childhood general and abdominal fat measures among girls, but only with BMI among boys (P-values for interaction <0.05). After correction for multiple testing the associations of AGEAP with pre-peritoneal abdominal fat area attenuated into non-significant. Supplementary Material Table S3 gives the associations of early weight growth measures with general and abdominal fat mass in absolute values instead of SDS. Models adjusted for age and sex only showed similar associations as the fully adjusted model (Supplementary Material Table S4).
Table 2. Associations of infant weight growth measures with childhood adiposity outcomes (N=5,126).
| SDS difference in childhood body fat outcomes (95% Confidence Interval) | ||||
|---|---|---|---|---|
|
| ||||
| BMI | Body fat percentage | Android/gynoid fat mass ratio | Pre-peritoneal abdominal fat area | |
| Total group | ||||
|
| ||||
| PWV (1 SDS = 2.1 kg/year) | 0.37 (0.34, 0.40)**,*** | 0.21 (0.18, 0.24) **,*** | 0.14 (0.10, 0.17) **,*** | 0.13 (0.10, 0.17) **,*** |
| AGEAP (1 SDS = 0.04 years) | 0.08 (0.06, 0.11) **,*** | 0.05 (0.03, 0.08) **,*** | 0.03 (-0.01, 0.06)*** | 0.02 (-0.01, 0.05) |
| BMIAP (1 SDS = 0.8 kg/m2) | 0.45 (0.43, 0.48)** | 0.24 (0.21, 0.27)** | 0.17 (0.10, 0.17) **,*** | 0.11 (0.08, 0.15)** |
|
| ||||
| Boys | ||||
|
| ||||
| PWV (1 SDS = 2.0 kg/year) | 0.33 (0.30, 0.37)**,**** | 0.19 (0.15, 0.23)**,**** | 0.08 (0.04, 0.13)**,**** | 0.11 (0.06, 0.16)** |
| AGEAP (1 SDS = 0.04 years) | 0.05 (0.02, 0.09)**,**** | 0.01 (-0.02, 0.05)**** | -0.02 (-0.06, 0.02)**** | -0.01 (-0.05, 0.03)**** |
| BMIAP (1 SDS = 0.8 kg/m2) | 0.46 (0.42, 0.49)** | 0.23 (0.19, 0.24)** | 0.12 (0.06, 0.14)**,**** | 0.11 (0.06, 0.16)** |
|
| ||||
| Girls | ||||
|
| ||||
| PWV (1 SDS = 1.8 kg/year) | 0.42 (0.38, 0.46)**,**** | 0.25 (0.20, 0.30)**,**** | 0.22 (0.16, 0.27)**,**** | 0.17 (0.11, 0.23)** |
| AGEAP (1 SDS = 0.04 years) | 0.11 (0.08, 0.15)**,**** | 0.09 (0.05, 0.13)**,**** | 0.07 (0.02, 0.12)**,**** | 0.05 (0.01, 010)*,**** |
| BMIAP (1 SDS = 0.8 kg/m2) | 0.45 (0.042, 0.49)** | 0.24 (0.20, 0.29)** | 0.21 (0.16, 0.26)**,**** | 0.12 (0.07, 0.17)** |
Abbreviations: N: number, SDS: standard deviation scores, BMI: body mass index, PWV: peak weight velocity, AGEAP: age at adiposity peak, BMIAP: body mass index at adiposity peak. Values are linear regression coefficients (95% CI) based on multiple linear regression models and reflect the change in outcome per SD increase in each infant weight growth characteristics. Model adjusted for age, sex (total group), age mother, body mass index before pregnancy, parity, marital status, education mother, smoking during pregnancy, use of alcohol during pregnancy, folic acid supplement use, gestational diabetes and gestational hypertensive disorders, paternal body mass index, standard deviation score birth weight, child’s ethnicity, number of postnatal measurements, breast feeding, age at introduction of solid foods and watching television. Analyses with fat mass were additionally adjusted for height of the child. Models adjusted for age and sex only are given in Supplementary Materials Table S3.
* P value <0.05
** P value <0.01
*** P values for interaction with sex <0.05
**** P values for heterogeneity between sex<0.05.
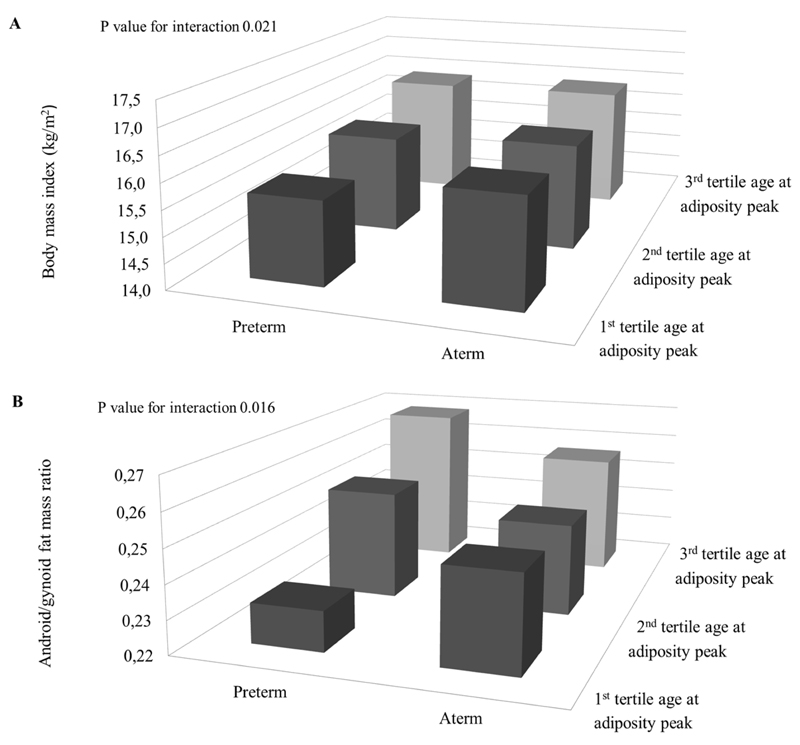
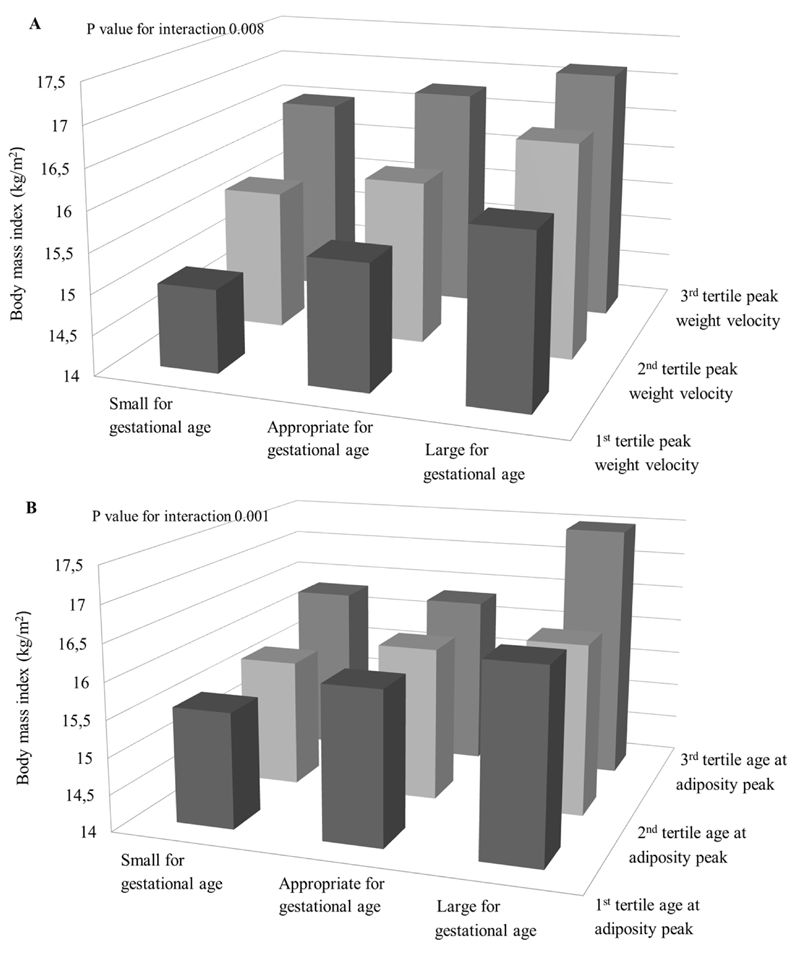
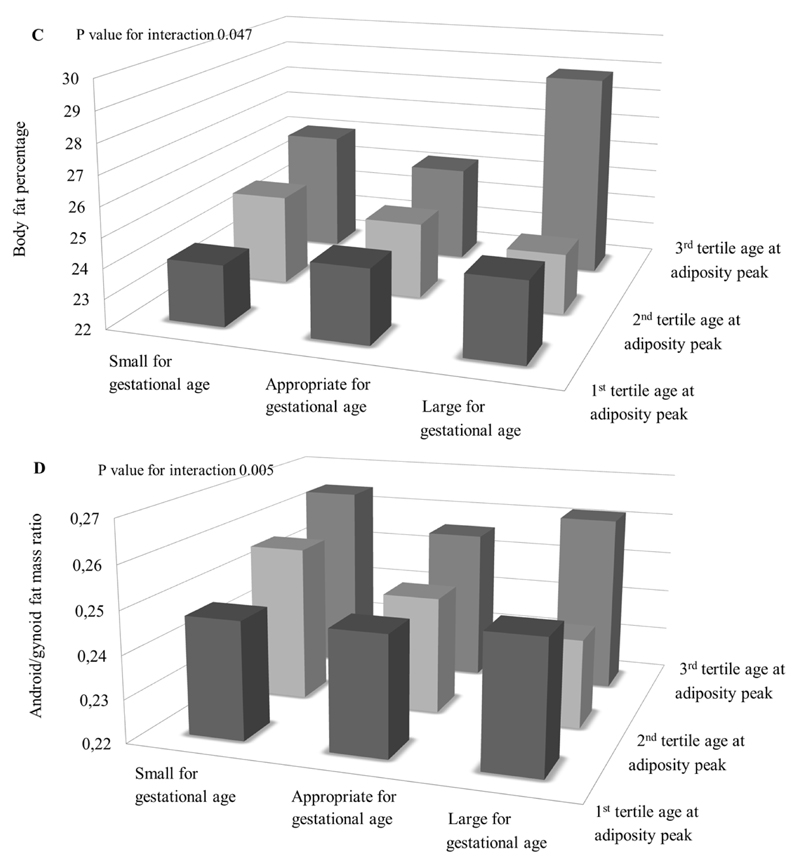
Figures 1 and 2 show the associations of infant weight growth patterns with childhood adiposity outcomes stratified by gestational age and size at birth, respectively, when the statistical test for interaction was significant (p-value for interaction < 0.05; all tested p-values are given in Supplementary Material Table S5). The effect estimates for each stratum are given in Supplementary Table S6. Figure 1 shows that AGEAP in the highest tertile (>0.75 years) was positively associated with childhood BMI and android/gynoid fat ratio among children born preterm. Figure 2A shows the highest BMI in children both born with a large size for gestational age and in the highest tertile of infant PWV. However, the strongest effect estimate for the association of infant PWV with childhood BMI was observed among children born small for gestational age. Figure 2B and 2C show that the highest childhood BMI and body fat percentage was observed among children born large size for gestational age within the highest tertile of infant AGEAP. The strongest effect estimates for the associations between AGEAP with childhood BMI and body fat percentage were observed among children born small size for gestational age. Figure 2D shows the highest android/gynoid fat ratio among children born with a small size for gestational age within the highest tertile of infant AGEAP.
Figure 1. Preterm birth, infant weight growth and childhood adiposity outcomes (N=5,126).
Bars represent mean body mass index (BMI) (A) and android/gynoid fat mass ratio (B) by term and age at adiposity peak (A, B) (tertiles). P<0.05 for interaction between age at adiposity peak and gestational age at birth for the association with BMI and android/gynoid fat mass ratio. Preterm, AGEAP: 1st tertile N=85; 2nd tertile N=102, 3rd tertile N=33. Aterm, AGEAP: 1st tertile N=2,854, 2nd tertile N=1,281, 3rd tertile N=340.
Figure 2. Size at birth, infant weight growth and childhood adiposity outcomes (N=5,126).
Bars represent mean body mass index (BMI) (A, B), body fat percentage (C) and android/gynoid fat mass ratio (D) stratified by size at gestational age and peak weight velocity (A) and age at adiposity peak (B, C, D) (tertiles). P<0.01 for interaction between peak weight velocity and standard deviation score birth weight for the association with BMI, P<0.01 for interaction between age at adiposity peak and standard deviation score birth weight for the association with BMI and android/gynoid fat mass ratio, P<0.05 for interaction between age at adiposity peak and standard deviation score birth weight for the association with body fat percentage. SGA, PWV: 1st tertile N=172; 2nd tertile N=181, 3rd tertile N=166. AGA, PWV: 1st tertile N=1,309, 2nd tertile N=1,373, 3rd tertile N=1,400. LGA, PWV: 1st tertile N=225, 2nd tertile N=152, 3rd tertile N=140. SGA, AGEAP: 1st tertile N=254; 2nd tertile N=158, 3rd tertile N=64. AGA, AGEAP: 1st tertile N=2,324, 2nd tertile N=1,128, 3rd tertile N=291. LGA, AGEAP: 1st tertile N=363, 2nd tertile N=97, 3rd tertile N=17.
Infant weight growth velocity patterns and risk of overweight/obesity in childhood
Table 3 shows that in the full group both higher infant PWV, AGEAP and BMIAP were associated with an increased risk of childhood overweight/obesity, with the strongest associations present for BMIAP. Sex stratified analyses showed that the associations of PWV and AGEAP with the risk of overweight/obesity were stronger among girls. The association of BMIAP with the risk of overweight/obesity was not statistically significant different between boys and girls.
Table 3. Associations of infant weight growth measures with risk of overweight/obesity (N=5,126).
| Odds ratio for Overweight/Obesity (95% Confidence Interval) | |
|---|---|
| Total group | Overweight/Obesity (N=812) |
|
| |
| PWV (1 SDS = 2.1) | 2.1 (1.9, 2.3)**,*** |
| AGEAP (1 SDS = 0.04) | 1.2 (1.1, 1.3)**,*** |
| BMIAP (1 SDS = 0.8) | 2.8 (2.2, 2.8)**,*** |
|
| |
| Boys | Overweight/Obesity (N=342) |
|
| |
| PWV (1 SDS = 2.0) | 2.0 (1.7, 2.2)**,**** |
| AGEAP (1 SDS = 0.04) | 1.0 (0.9, 1.2)**** |
| BMIAP (1 SDS = 0.8) | 2.8 (2.3, 3.3)** |
|
| |
| Girls | Overweight/Obesity (N=470) |
|
| |
| PWV (1 SDS = 1.8) | 2.4 (2.1, 2.8)**,**** |
| AGEAP (1 SDS = 0.04) | 1.3 (1.1, 1.4)**,**** |
| BMIAP (1 SDS = 0.8) | 2.3 (2.0, 2.7)** |
Abbreviations: N: number, SDS: standard deviation scores, PWV: peak weight velocity, AGEAP: age at adiposity peak, BMIAP: body mass index at adiposity peak. Overweight includes overweight and obesity cases based on standard definitions established by Cole et al (24). Values represent odds ratios (95% CI) based on multivariate logistic regression. Model adjusted for age, sex (total group), age mother, body mass index before pregnancy, parity, maternal complications during pregnancy, education mother, marital status, smoking during pregnancy, use of alcohol during pregnancy, folic acid supplement use, paternal body mass index, standard deviation score birth weight, child’s ethnicity, number of postnatal measurements, duration of breastfeeding, age at introduction of solid foods and watching television.
* P value <0.05
** P value <0.01
*** P values for interaction with sex <0.05
**** P values for heterogeneity between sex<0.05.
Discussion
We observed in a large population-based prospective cohort study that infant weight growth velocity patterns are associated with both general and abdominal adiposity measures in childhood. Both higher PWV and BMIAP during infancy were associated with increased risks of overweight/obesity and adverse general and abdominal fat distribution profiles. The observations tended to be stronger among girls, and partly modified by gestational age and weight at birth.
Interpretation of main findings
Growth in fetal life and infancy is related to BMI in later life. Ong et al. demonstrated among 848 full-term born children that infant rapid weight growth in early life was associated with a higher BMI and total fat mass at the age of 5 years (32). Stettler et al. showed in a prospective cohort study among 27,899 full term children that, independent of birth weight, rapid weight gain in infancy was associated with overweight at the age of 7 years (7). In line with these findings, we have previously reported that growth in weight and length during fetal life and infancy are also associated with higher BMI and total and abdominal fat mass measures (9, 33).
More detailed infant growth patterns can be derived from longitudinal collected growth measures. For a typical individual, BMI increases from birth until a maximum (BMIAP) around the age of 9 months of age, after which it decreases until a rebound around the age of 6 years (34, 35). Previously, we have shown that higher PWV and BMIAP are associated with an increased risk of overweight and obesity at 4 years of age (8). In the same study, we observed a negative association of third trimester fetal weight and length with PHV and PWV. Other studies showed persistent differences in length and weight after being born small or large for gestational age until the age of 8-18 years (36–39). Additionally, children born small or large for their gestational age and followed by infant catch-up growth have a higher BMI across the full range and are at higher risks for developing obesity and in later life (9, 33).
In line with these previous studies, we observed in the current study that PWV, AGEAP and BMIAP affect BMI at school-age. Also, we observed that infant PWV and BMIAP were associated with the risk of overweight/obesity. The associations of PWV and AGEAP with childhood BMI were stronger among girls than among boys. The stronger associations of early weight growth with adiposity outcomes among girls were also observed in another study among 1,162 subjects between the age of 5 to 13 years (34). The sex differences may be explained by differences in pattern and timing of body fat development and puberty between boys and girls (40). Because we adjusted our analysis for several potential confounders related to physical activity, we do not expect that the observed sex differences in body fat are explained by physical activity. Also, the results were in line with a previous study from the same cohort, which reported associations of early life exposures with body fat distribution among girls only (41). It might be that the differences can be detected at younger ages among girls than among boys. We observed that the associations of infant PWV and AGEAP with childhood outcomes tended to be stronger among children born preterm or with a small size for gestational age. These findings are in line with previous studies suggesting that children with a small size at birth followed by high infant weight growth rates are especially at risk for an adverse body fat distribution (10).
Childhood BMI may not accurately reflect fat mass. Body fat distribution is stronger related with cardio-metabolic risk factors in childhood and adulthood than BMI (11, 12). Therefore, for the current study we measured both total and abdominal fat mass using DXA and ultrasound. These specific adiposity measures are associated with higher cardiovascular risk factors in childhood, independent of BMI (7). We observed that both infant PWV and BMIAP were associated with higher childhood body fat percentage, android/gynoid fat mass, a proxy for waist to hip ratio, and pre-peritoneal abdominal fat area, a proxy for visceral fat mass. Our findings are in line with a recent study among 311 Danish children, which reported that BMIAP was associated with higher fat mass at the age of 3 years (42). However, this study had no information on detailed abdominal fat mass measures. Also, a prospective cohort study in the UK among 561 children showed that rapid weight gain in infancy was associated with higher skinfold thicknesses at the age of 7 years (43). Another study among 4,121 individuals in Finland showed that AGEAP and BMIAP were associated with higher waist circumference in adults (44). An UK study among 121 obese individuals ranged 5 to 22 years, suggested that the variability in central adiposity was more strongly influenced by infant growth than by birth weight (45). We have also shown that children born small for gestational age followed by rapid weight growth, have the highest levels for adverse body fat distribution (9). Thus, rapid infant weight growth might influence development of adverse body fat distribution later in life. Whether these specific fat patterns influence the risk of complications in later life, next to BMI, should be further studied. Also, the optimal infant weight growth patterns are not known yet. Physiological catch up growth refers to compensation of previous losses, for example in children born with fetal growth restriction. Rapid weight growth refers to weight growth overcompensation as pathological condition that often leads to increased risks in later life health outcomes. The later life health consequences of physiological weight catch up growth and pathological rapid weight growth should be further studied.
Strengths and limitations
We used a population-based, prospective cohort design with a large number of subjects whom we studied from early fetal life onwards. The repeated infant growth measurements enabled us to study the effect of infant weight growth velocity patterns on the development of an adverse body fat distribution. Of the total group of singleton live born children with information on growth, follow-up measurements were available in 69%. This loss to follow-up would have led to a selection bias if the associations of infant PWV, AGEAP and BMIAP with childhood adiposity outcomes would be different between those included and not included in the final analysis. Non-response analysis showed a higher PWV among the children that were lost to follow up. This could have led to bias estimates for the associations of infant PWV with the body fat outcomes in childhood. We performed detailed measurements of childhood fat distribution outcomes. Both DXA and abdominal ultrasound have been validated against Computed Tomography (CT) (46). Ultrasound is a reliable method to differentiate between abdominal visceral and subcutaneous fat compartments by using an area measurement as a proxy for these fat compartments (46). Finally, although we performed adjustments for a large number of potential maternal and childhood confounders, residual confounding still might have occurred, as in any observational study. As example, we had insufficient information about childhood diet and physical activity, as this information was available in only a small subgroup of the study population. Previously we reported that infant breastfeeding patterns did not affect childhood body fat distribution (47).
Conclusion
Our study showed that higher infant PWV and BMIAP are associated with an adverse general and abdominal fat distribution and increased risks of overweight in childhood. The effect of infant weight growth patterns on childhood adiposity outcomes may be influenced by gestational age and size at birth. More studies are needed focused on weight growth patterns in children born extremely preterm or with a low birth weight. Even though the observed associations were small, these results are important for a better understanding of early development of obesity and adverse fat distribution. Whether infant weight growth patterns add to the prediction of later obesity should be further studied.
Supplementary Material
Supplementary information is available at the journal’s website.
Acknowledgments
The Generation R Study is conducted by the Erasmus Medical Center in close collaboration with the School of Law and Faculty of Social Sciences of the Erasmus University Rotterdam, the Municipal Health Service Rotterdam area, Rotterdam, the Rotterdam Homecare Foundation, Rotterdam and the Stichting Trombosedienst and Artsenlaboratorium Rijnmond (STAR), Rotterdam. We gratefully acknowledge the contribution of participating mothers, general practitioners, hospitals, midwives and pharmacies in Rotterdam.
Funding: The Generation R Study is financially supported by the Erasmus Medical Center, Rotterdam, the Erasmus University Rotterdam and the Netherlands Organization for Health Research and Development. Vincent Jaddoe received an additional grant from the Netherlands Organization for Health Research and Development (NWO, ZonMw-VIDI 016.136.361) and an European Research Council Consolidator Grant (ERC-2014-CoG-648916). The research leading to these results has received funding from the European Union’s Seventh Framework Programme (FP7/2007-2013), project EarlyNutrition under grant n°289346. Abdominal ultrasound assessments were partially funded by an unrestricted grant from Danone Early Nutrition. CJK, OG, RG and VWVJ designed and conducted the research and wrote the paper. CJK, OG analysed the data. AH provided comments and consultation regarding the analyses and manuscript. CJK had primary responsibility for final content. All authors gave final approval of the version to be published.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
Disclosure statement: The authors have nothing to disclosure.
References
- 1.Han JC, Lawlor DA, Kimm SYS. Childhood obesity. Lancet. 2010;375:1737–1748. doi: 10.1016/S0140-6736(10)60171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Looker HC. Childhood Obesity, Other Cardiovascular Risk Factors, and Premature Death. New Engl J Med. 2010;362:485–493. doi: 10.1056/NEJMoa0904130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kemp MW, Kallapur SG, Jobe AH, Newnham JP. Obesity and the developmental origins of health and disease. J Paediatr Child H. 2012;48:86–90. doi: 10.1111/j.1440-1754.2010.01940.x. [DOI] [PubMed] [Google Scholar]
- 4.Godfrey KM, Inskip HM, Hanson MA. The long-term effects of prenatal development on growth and metabolism. Semin Reprod Med. 2011;29:257–265. doi: 10.1055/s-0031-1275518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eriksson J, Forsen T, Tuomilehto J, Osmond C, Barker D. Size at birth, childhood growth and obesity in adult life. Int J Obes Relat Metab Disord. 2001;25:735–740. doi: 10.1038/sj.ijo.0801602. [DOI] [PubMed] [Google Scholar]
- 6.Ong KK, Loos RJ. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr. 2006;95:904–908. doi: 10.1080/08035250600719754. [DOI] [PubMed] [Google Scholar]
- 7.Stettler N, Zemel BS, Kumanyika S, Stallings VA. Infant weight gain and childhood overweight status in a multicenter, cohort study. Pediatrics. 2002;109:194–199. doi: 10.1542/peds.109.2.194. [DOI] [PubMed] [Google Scholar]
- 8.Mook-Kanamori DO, Durmus B, Sovio U, Hofman A, Raat H, Steegers EA, et al. Fetal and infant growth and the risk of obesity during early childhood: the Generation R Study. Eur J Endocrinol. 2011;165:623–630. doi: 10.1530/EJE-11-0067. [DOI] [PubMed] [Google Scholar]
- 9.Gishti O, Gaillard R, Manniesing R, Abrahamse-Berkeveld M, van der Beek EM, Heppe DH, et al. Fetal and infant growth patterns associated with total and abdominal fat distribution in school-age children. J Clin Endocrinol Metab. 2014;99:2557–2566. doi: 10.1210/jc.2013-4345. [DOI] [PubMed] [Google Scholar]
- 10.Tzoulaki I, Sovio U, Pillas D, Hartikainen AL, Pouta A, Laitinen J, et al. Relation of immediate postnatal growth with obesity and related metabolic risk factors in adulthood: the northern Finland birth cohort 1966 study. Am J Epidemiol. 2010;171:989–998. doi: 10.1093/aje/kwq027. [DOI] [PubMed] [Google Scholar]
- 11.Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, et al. General and Abdominal Adiposity and Risk of Death in Europe. New Engl J Med. 2008;359:2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 12.Srinivasan SR, Wang R, Chen W, Wei CY, Xu JH, Berenson GS. Utility of Waist-To-Height Ratio in Detecting Central Obesity and Related Adverse Cardiovascular Risk Profile Among Normal Weight Younger Adults (from the Bogalusa Heart Study) American Journal of Cardiology. 2009;104:721–724. doi: 10.1016/j.amjcard.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 13.Maffeis C, Corciulo N, Livieri C, Rabbone I, Trifiro G, Falorni A, et al. Waist circumference as a predictor of cardiovascular and metabolic risk factors in obese girls. European Journal of Clinical Nutrition. 2003;57:566–572. doi: 10.1038/sj.ejcn.1601573. [DOI] [PubMed] [Google Scholar]
- 14.Garnett SP, Baur LA, Srinivasan S, Lee JW, Cowell CT. Body mass index and waist circumference in midchildhood and adverse cardiovascular disease risk clustering in adolescence. American Journal of Clinical Nutrition. 2007;86:549–555. doi: 10.1093/ajcn/86.3.549. [DOI] [PubMed] [Google Scholar]
- 15.Falaschetti E, Hingorani AD, Jones A, Charakida M, Finer N, Whincup P, et al. Adiposity and cardiovascular risk factors in a large contemporary population of pre-pubertal children. European Heart Journal. 2010;31:3063–3072. doi: 10.1093/eurheartj/ehq355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savva SC, Tornaritis M, Savva ME, Kourides Y, Panagi A, Silikiotou N, et al. Waist circumference and waist-to-height ratio are better predictors of cardiovascular disease risk factors in children than body mass index. Int J Obesity. 2000;24:1453–1458. doi: 10.1038/sj.ijo.0801401. [DOI] [PubMed] [Google Scholar]
- 17.Lawlor DA, Benfield L, Logue J, Tilling K, Howe LD, Fraser A, et al. Association between general and central adiposity in childhood, and change in these, with cardiovascular risk factors in adolescence: prospective cohort study. BMJ. 2010;341 doi: 10.1136/bmj.c6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gishti O, Gaillard R, Durmus B, Abrahamse M, van der Beek EM, Hofman A, et al. BMI, total and abdominal fat distribution, and cardiovascular risk factors in school-age children. Pediatr Res. 2015;77:710–718. doi: 10.1038/pr.2015.29. [DOI] [PubMed] [Google Scholar]
- 19.Jaddoe VW, van Duijn CM, Franco OH, van der Heijden AJ, van IJzendoorn MH, de Jongste JC, et al. The Generation R Study: design and cohort update 2012. Eur J Epidemiol. 2012;27:739–756. doi: 10.1007/s10654-012-9735-1. [DOI] [PubMed] [Google Scholar]
- 20.Kruithof CJ, Kooijman MN, van Duijn CM, Franco OH, de Jongste JC, Klaver CC, et al. The Generation R Study: Biobank update 2015. Eur J Epidemiol. 2014;29:911–927. doi: 10.1007/s10654-014-9980-6. [DOI] [PubMed] [Google Scholar]
- 21.Niklasson A, Ericson A, Fryer JG, Karlberg J, Lawrence C, Karlberg P. An Update of the Swedish Reference-Standards for Weight, Length and Head Circumference at Birth for Given Gestational-Age (1977-1981) Acta Paediatrica Scandinavica. 1991;80:756–762. doi: 10.1111/j.1651-2227.1991.tb11945.x. [DOI] [PubMed] [Google Scholar]
- 22.Fredriks AM, van Buuren S, Burgmeijer RJ, Meulmeester JF, Beuker RJ, Brugman E, et al. Continuing positive secular growth change in The Netherlands 1955-1997. Pediatr Res. 2000;47:316–323. doi: 10.1203/00006450-200003000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Sovio U, Bennett AJ, Millwood IY, Molitor J, O'Reilly PF, Timpson NJ, et al. Genetic Determinants of Height Growth Assessed Longitudinally from Infancy to Adulthood in the Northern Finland Birth Cohort 1966. Plos Genetics. 2009;5 doi: 10.1371/journal.pgen.1000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaul S, Rothney MP, Peters DM, Wacker WK, Davis CE, Shapiro MD, et al. Dual-energy X-ray absorptiometry for quantification of visceral fat. Obesity (Silver Spring) 2012;20:1313–1318. doi: 10.1038/oby.2011.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki R, Watanabe S, Hirai Y, Akiyama K, Nishide T, Matsushima Y, et al. Abdominal wall fat index, estimated by ultrasonography, for assessment of the ratio of visceral fat to subcutaneous fat in the abdomen. Am J Med. 1993;95:309–314. doi: 10.1016/0002-9343(93)90284-v. [DOI] [PubMed] [Google Scholar]
- 27.Mook-Kanamori DO, Holzhauer S, Hollestein LM, Durmus B, Manniesing R, Koek M, et al. Abdominal fat in children measured by ultrasound and computed tomography. Ultrasound Med Biol. 2009;35:1938–1946. doi: 10.1016/j.ultrasmedbio.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Coolman M, de Groot CJ, Jaddoe VW, Hofman A, Raat H, Steegers EA. Medical record validation of maternally reported history of preeclampsia. J Clin Epidemiol. 2010;63:932–937. doi: 10.1016/j.jclinepi.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Migrants in the Netherlands, 2003: Statistics Netherlands. 2003. [Google Scholar]
- 30.Wells JCK, Cole TJ, team As Adjustment of fat-free mass and fat mass for height in children aged 8 y. Int J Obesity. 2002;26:947–952. doi: 10.1038/sj.ijo.0802027. [DOI] [PubMed] [Google Scholar]
- 31.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ. 2000;320:967–971. doi: 10.1136/bmj.320.7240.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parsons TJ, Power C, Manor O. Fetal and early life growth and body mass index from birth to early adulthood in 1958 British cohort: longitudinal study. BMJ. 2001;323:1331–1335. doi: 10.1136/bmj.323.7325.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silverwood RJ, De Stavola BL, Cole TJ, Leon DA. BMI peak in infancy as a predictor for later BMI in the Uppsala Family Study. Int J Obesity. 2009;33:929–937. doi: 10.1038/ijo.2009.108. [DOI] [PubMed] [Google Scholar]
- 35.Sovio U, Mook-Kanamori DO, Warrington NM, Lawrence R, Briollais L, Palmer CN, et al. Association between common variation at the FTO locus and changes in body mass index from infancy to late childhood: the complex nature of genetic association through growth and development. PLoS Genet. 2011;7:e1001307. doi: 10.1371/journal.pgen.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karlberg J, Albertsson-Wikland K. Growth in full-term small-for-gestational-age infants: from birth to final height. Pediatr Res. 1995;38:733–739. doi: 10.1203/00006450-199511000-00017. [DOI] [PubMed] [Google Scholar]
- 37.Hediger ML, Overpeck MD, Maurer KR, Kuczmarski RJ, McGlynn A, Davis WW. Growth of infants and young children born small or large for gestational age: findings from the Third National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med. 1998;152:1225–1231. doi: 10.1001/archpedi.152.12.1225. [DOI] [PubMed] [Google Scholar]
- 38.Ounsted MK, Moar VA, Scott A. Children of deviant birthweight at the age of seven years: health, handicap, size and developmental status. Early Hum Dev. 1984;9:323–340. doi: 10.1016/0378-3782(84)90077-x. [DOI] [PubMed] [Google Scholar]
- 39.Scott A, Moar V, Ounsted M. Growth in the first four years: I. The relative effects of gender and weight for gestational age at birth. Early Hum Dev. 1982;7:17–28. doi: 10.1016/0378-3782(82)90004-4. [DOI] [PubMed] [Google Scholar]
- 40.Taylor RW, Jones IE, Williams SM, Goulding A. Body fat percentages measured by dual-energy X-ray absorptiometry corresponding to recently recommended body mass index cutoffs for overweight and obesity in children and adolescents aged 3-18 y. Am J Clin Nutr. 2002;76:1416–1421. doi: 10.1093/ajcn/76.6.1416. [DOI] [PubMed] [Google Scholar]
- 41.Gaillard R, Steegers EA, Tiemeier H, Hofman A, Jaddoe VW. Placental vascular dysfunction, fetal and childhood growth, and cardiovascular development: The generation R study. Circulation. 2013;128:2202–2210. doi: 10.1161/CIRCULATIONAHA.113.003881. [DOI] [PubMed] [Google Scholar]
- 42.Jensen SM, Ritz C, Ejlerskov KT, Molgaard C, Michaelsen KF. Infant BMI peak, breastfeeding, and body composition at age 3 y. Am J Clin Nutr. 2015;101:319–325. doi: 10.3945/ajcn.114.092957. [DOI] [PubMed] [Google Scholar]
- 43.Wright CM, Cox KM, Sherriff A, Franco-Villoria M, Pearce MS, Adamson AJ, et al. To what extent do weight gain and eating avidity during infancy predict later adiposity? Public Health Nutr. 2012;15:656–662. doi: 10.1017/S1368980011002096. [DOI] [PubMed] [Google Scholar]
- 44.Sovio U, Kaakinen M, Tzoulaki I, Das S, Ruokonen A, Pouta A, et al. How do changes in body mass index in infancy and childhood associate with cardiometabolic profile in adulthood? Findings from the Northern Finland Birth Cohort 1966 Study. Int J Obesity. 2014;38:53–59. doi: 10.1038/ijo.2013.165. [DOI] [PubMed] [Google Scholar]
- 45.Wells JC, Haroun D, Levene D, Darch T, Williams JE, Fewtrell MS. Prenatal and postnatal programming of body composition in obese children and adolescents: evidence from anthropometry, DXA and the 4-component model. Int J Obes (Lond) 2011;35:534–540. doi: 10.1038/ijo.2011.7. [DOI] [PubMed] [Google Scholar]
- 46.Bazzocchi A, Filonzi G, Ponti F, Sassi C, Salizzoni E, Battista G, et al. Accuracy, Reproducibility and Repeatability of Ultrasonography in the Assessment of Abdominal Adiposity. Acad Radiol. 2011;18:1133–1143. doi: 10.1016/j.acra.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 47.Durmus B, Heppe DH, Gishti O, Manniesing R, Abrahamse-Berkeveld M, van der Beek EM, et al. General and abdominal fat outcomes in school-age children associated with infant breastfeeding patterns. Am J Clin Nutr. 2014;99:1351–1358. doi: 10.3945/ajcn.113.075937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.