Abstract
Aims
The purpose of this study was to explore clinical markers reflecting developmental changes in drug clearance by preterm infants.
Methods
Preterm infants administered aminophylline or theophylline to treat apnoea of prematurity were enrolled in this study. Trough and one of 2 h, 4 h or 6 h post‐dose blood samples and urine samples were collected during steady state, to determine the concentrations of theophylline and its targeted metabolites. CYP1A2*1C and CYP1A2*1F genotypes were analyzed. Total, renal and nonrenal clearances of theophylline were calculated, and cytochrome P450 1A2 (CYP1A2) activity was obtained from the ratio of 1‐methyluric acid and 3‐methylxanthine to theophylline in urine. Multiple linear regression analysis was performed to evaluate the relationships between theophylline clearance and the clinical characteristics of preterm infants.
Results
A total of 152 samples from 104 preterm infants were analyzed. A strong association between the serum trough and urine theophylline concentrations was found (P < 0.001). Total, renal and nonrenal clearances of theophylline were 0.50 ± 0.29 ml kg−1 min−1, 0.16 ± 0.06 ml kg−1 min−1 and 0.34 ± 0.28 ml kg−1 min−1, respectively. CYP1A2 activity correlated positively with the postnatal age and postmenstrual age. However, CYP1A2 genotype was not associated with CYP1A2 activity, which was significantly associated with nonrenal clearance. CYP1A2 activity, postnatal age , weight and 24‐h urine output were significantly associated with total theophylline clearance.
Conclusions
CYP1A2 activity can be monitored using noninvasive random urine samples, and it can be used to assess developmental changes in theophylline clearance by preterm infants.
Keywords: cytochrome P450 1A2, drug monitoring, pharmacokinetics, premature infants, theophylline
What is Already Known about this Subject
Neonatal theophylline pharmacokinetics are influenced by developmental changes in liver metabolism and urinary elimination.
Previous population pharmacokinetic studies have focused on the effect of weight and age.
Previous studies have not explored the effects of phenotypic cytochrome P450 1A2 (CYP1A2) activity and genetic polymorphism on theophylline pharmacokinetics in preterm infants.
What this Study Adds
CYP1A2 activity is a major determinant of theophylline clearance in preterm infants.
Genetic polymorphisms do not affect CYP1A2 activity during the preterm and neonatal period.
Noninvasive urinary metabolite analyses in real time can be used to assess developmental changes in theophylline clearance by preterm infants.
Tables of Links
| LIGANDS |
|---|
| Caffeine |
| Theophylline |
Introduction
The newborn population, especially preterm infants receiving intensive care, are the most vulnerable to the adverse effects of drugs because of their immaturity. Most drugs employed in the newborn population have been introduced without determining the appropriate dose, efficacy or safety because of the ethical concerns about clinical trials in these patients, the difficulties for frequent blood sampling, the limited number of patients available for study and the lack of appropriate study designs. Novel clinical trial designs are required in neonates to address these issues related to ethics, acceptability, rarity, standardization, end points, safety, dosing and feasibility 3. These days, population pharmacokinetics 4, 5, 6 and metabolomics 7, 8, 9 have been introduced as new technologies in neonatal pharmaceutical clinical trials.
Methylxanthines have been commonly used as medications in preterm infants since the 1970s and their mechanism of action and metabolic pathway are relatively well established 10, 11. The pharmacokinetics of theophylline (TP, 1,3‐dimethylxanthine) in neonates differ from those observed in adults 12, 13: (i) its oral bioavailability is about 80%; (ii) its protein binding is low in neonates; (iii) its serum half‐life is prolonged from 20 to 30 hours; (iv) adult clearance values are reached at approximately 55 weeks postmenstrual age (PMA); and (v) 50% of the dose is excreted unchanged in the urine. The pharmacokinetics of TP in preterm infants are influenced by developmental changes in its metabolism by liver enzymes and urinary elimination 14, 15; clearance of TP seems to mature and change continuously as the PMA increases, but the pattern and timing of these changes remain controversial.
Cytochrome P450 1A2 (CYP1A2) 16 is one of the major hepatic enzymes in humans and it plays important roles in the metabolism of xenobiotics (e.g., clozapine, TP, tacrine and zolmitriptan) and in the metabolism of endogenous molecules (e.g., steroids, retinols, melatonin and arachidonic acids). The estimated contribution rate of CYP1A2 to TP metabolism is 90–95% in adults (Figure 1), and it is mainly involved in N‐demethylation. Other minor hepatic enzymes that contribute to TP metabolism include CYP2E1 and CYP3A4, which are associated with 8‐hydroxylation. The drug metabolic ability of individual patients can be assessed indirectly by measuring biomarkers that reflect CYP activity.
Figure 1.
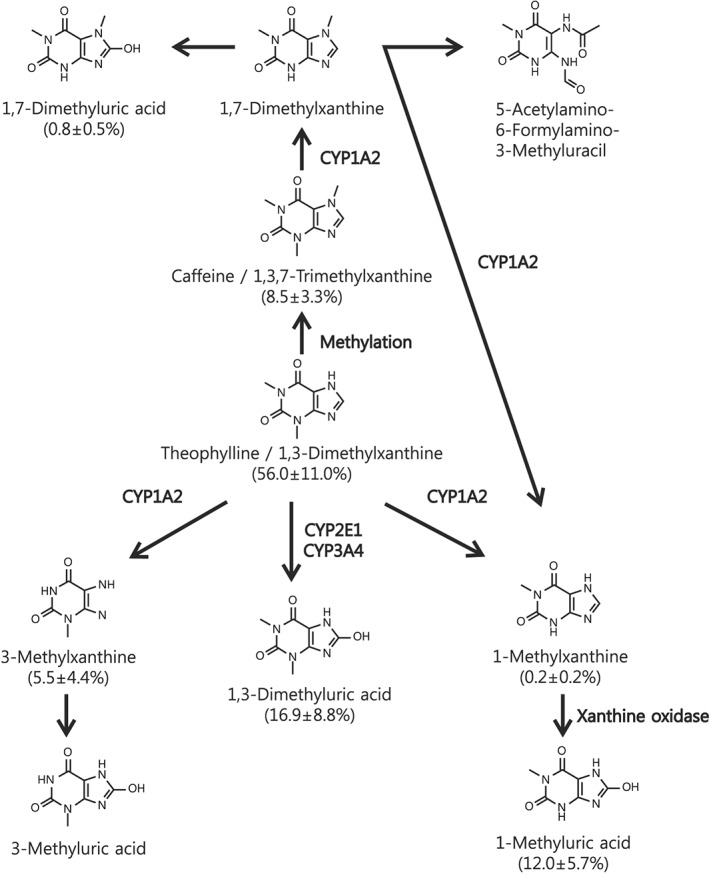
The theophylline metabolic pathway. Molar percentages of the metabolites analyzed in our preterm infant group are expressed in parentheses. CYP1A2 is a major hepatic enzyme involved in the decomposition of approximately 90–95% of theophylline in human adults. CYP1A2 mainly catalyzes the conversion of theophylline to 1‐methylxanthine and 3‐methylxanthine (N‐demethylation), while CYP2E1 and CYP3A4 catalyze the conversion of theophylline to 1,3‐dimethyluric acid (8‐hydroxylation). 1‐Methylxanthine is oxidized very rapidly to 1‐methyluric acid by xanthine oxidase
The technical difficulties and limited circulating blood volume in neonates, and especially in preterm infants, exclude standard pharmacokinetic and pharmacodynamic study designs with repeated sampling. Therefore, there have been several attempts to predict drug concentrations using noninvasive methods employing saliva 17 or urine 18 samples, and modelling 19; however, the optimal approach to this has not yet been established. To address this, we aimed to explore clinical markers reflecting the developmental change in TP pharmacokinetics in preterm infants using a noninvasive approach. This study evaluated the relationships between TP clearance (total, renal and nonrenal clearance) and clinical characteristics, including CYP1A2 activity, in preterm infants.
Methods
Subjects
Preterm infants younger than 37 weeks gestational age who had been admitted to the neonatal intensive care unit of Seoul National University (SNU) Children's Hospital, SNU Bundang Hospital and Seoul Metropolitan Government (SMG)‐SNU Boramae Medical Center were enrolled in this study from July 2010 to December 2011. Preterm infants were included in the study if they were treated for apnoea of prematurity using intravenous (IV) aminophylline or peroral (PO) TP. The exclusion criteria were: (i) any major congenital anomaly; (ii) death within 72 h after birth; and (iii) proven secondary apnoea.
The study protocol was approved by the Institutional Review Board of SNU Hospital (IRB No. H‐1005‐044‐318), SNU Bundang Hospital (IRB No. B‐1006‐103‐402) and SMG‐SNU Boramae Medical Center (IRB No. 20100616/06–2010‐83/97). This prospective observational cohort study was registered at www.ClinicalTrials.gov (ID: NCT01509248) and conducted in compliance with the current revision of the Declaration of Helsinki and Good Clinical Practice guidelines. All patients were enrolled after informed consent had been obtained from their parents or guardians.
Study protocol for collecting serum and urine samples
Infants diagnosed with apnoea of prematurity were prescribed a maintenance dose of 1.5–3.0 mg kg−1 every 8 or 12 h after the administration of a loading dose of 8.0 mg kg−1 IV aminophylline or PO TP. The salt factor of aminophylline (TP with ethylenediamine at a 2:1 ratio) is 0.8, and the bioavailability of oral TP is approximately 80% in neonates 12. Therefore, the same dose was administered when changing from IV aminophylline to PO TP. We obtained a trough blood sample and a 2 h, 4 h or 6 h postadministration blood sample at steady state (at least 5 days after starting aminophylline or TP). These were used to determine plasma TP concentrations and CYP1A2 genotypes. The blood sample volume was minimized to 500 μl using BD Microtainer Tubes (BD, NJ, USA). The amount of urine in the bladder before drug administration was checked using a Vivid 7 Dimension device (GE Vingmed Ultrasound AS N‐1390; GE, Horten, Norway) and emptied by gentle manual compression. Urine samples were collected with an adhesive urine bag directly after drug administration to determine the concentration of TP and its targeted metabolites. Serum TP concentrations were measured within 3 hours in the Department of Laboratory Medicine, and the Department of Clinical Pharmacology and Therapeutics of each hospital was consulted for therapeutic drug monitoring. Centrifuged plasma, haemocytes and urine samples were kept at −70°C for CYP1A2 genotyping and urine TP metabolite determination.
Measurement of TP and its metabolites
Serum concentrations of TP were measured using a fluorescence polarization immunoassay, the AxSYM Theophylline II assay (AxSYM; Abbott Laboratories, Diagnostic Division, Abbott Park, IL, USA), in SNU Children's Hospital and SMG‐SNU Boramae Medical Center or using a chemiluminescent microparticle immunoassay, the ARCHITECT i Theophylline assay (ARCHITECT; Abbott Laboratories) in SNU Bundang Hospital. There is no significant difference between the TP concentrations measured by these two different analytical methods 20.
High‐performance liquid chromatography (Agilent 1100 HPLC system; Agilent Technologies, CA, USA)–tandem mass spectrometry (API 4000LC/MS/MS system, AB Sciex, MA, USA) was used to quantify TP and its metabolites: 3‐methylxanthine (3X), 1‐methylxanthine (1X), 1‐methyluric acid (1U), 1,3‐dimethyluric acid (13U), caffeine (C, 1,3,7‐trimethylxanthine) and 1,7‐dimethyluric acid (17U). Protein was removed from the urine samples using methanol. Phenacetin was used as an internal standard for quantification of the above compounds and was separated on a Luna C18 column (100 × 2.0 mm, 5 μm; Phenomenex, Torrance, CA, USA). Validation of this analytical procedure for the determination of each compound was performed according to FDA guidance. The calibration curves were linear over the range of 5–1000 ng ml−1 for TP, C, 1X and 17U, and of 100–2000 ng ml−1 for 3X, 1U and 13U (R2 < 0.990). The intra‐ and interday precisions of the urine quality control samples were <12.52% for each compound. The accuracies were within 86.03–111.4% for each compound.
Analysis of CYP1A2 genotypes
Genomic DNA was isolated and purified from the peripheral leucocytes of each subject using DNA extractor kits (QIAamp DNA blood mini kit; QIAGEN GmbH, Germany). CYP1A2*1C (−3860 G > A, rs2069514) and CYP1A2*1F (−163 C > A, rs762551) genotypes were detected by real‐time polymerase chain reaction (PCR), which was performed by incubation for 10 min at 95°C, followed by 50 cycles of denaturation for 15 s at 92°C, annealing for 1 min at 60°C, and extension for 1 min at 60°C using TaqMan SNP Genotyping Assays (Applied Biosystems, CA, USA) methods. The obtained data were analyzed using a 7500 Real‐Time PCR System (Applied Biosystems, CA, USA).
Calculation of TP clearance and CYP1A2 activity
The area under the serum concentration–time curve (AUC) during the dosing interval at steady state was calculated for TP using the trapezoidal rule. The amount of drug excreted in urine (Ae) was calculated by multiplying the TP concentration in urine, the urine volume per hour and the dosing interval. The clearances of TP were calculated using the equations below:
Total clearance (CLt) = Dose / AUC: serum clearance
Renal clearance (CLr) = Ae / AUC: urinary excretion
Non‐renal clearance (CLnr) = CLt – CLr: enzyme activity, etc.
CYP1A2 activity was calculated using the ratio of metabolites to TP. We assessed CYP1A2 activity as the ratio: (1U + 3X) / TP, using urinary metabolites of TP with reference to a previous study 21.
Statistical analysis
Descriptive data are appropriately presented as numbers or as the arithmetic mean ± standard deviation (range, median). A linear mixed effects model was used to compare the concentrations of TP in serially collected urine samples in each subject. The urine concentrations were used as dependent variables in the model, the subject was used as a random effect and sampling points were used as a fixed effect. The association of serum and urine TP concentrations was assessed using simple linear regression analysis. The relationship between CYP1A2 activity, the clearance of TP and other clinical characteristics was performed by simple and multiple stepwise linear regression analyses. A P‐value <0.05 was considered statistically significant, and statistical analyses were conducted by IBM SPSS Statistics 19.0 (IBM, Somers, NY, USA) and SAS 9.3 (SAS Institute Inc., Cary NC, USA).
Results
Study population
A total of 110 patients were enrolled and 169 samples were collected during this study. We analyzed a total of 152 samples from 104 preterm infants, after excluding six patients with inadequate urine collection. The basic demographic data and clinical characteristics at the point of sampling in the study group are shown in Table 1.
Table 1.
Clinical characteristics of the study group
| Number or mean ± SD (range, median) | ||
|---|---|---|
| Subjects (n = 104) | Gestational age (week) | 29+1 (24+2–35+5, 29+4) |
| Birth weight (g) | 1210.5 ± 422.1 (520–2500, 1140) | |
| Birth height (cm) | 37.5 ± 4.6 (24.5–48.0, 37.5) | |
| Sex (male:female) | 58:46 | |
| CYP1A2*1C genotype (AA:AG:GG) | 11:39:54 | |
| CYP1A2*1F genotype (AA:AC:CC) | 47:45:12 | |
| Samples (n = 152) | Postmenstrual age (week) | 31+4 (25+3–37+1, 31+6) |
| Postnatal age (day) | 21.1 ± 17.7 (5–74, 12.5) | |
| Weight (g) | 1348.3 ± 443.0 (540–2900, 1335) | |
| Height (cm) | 38.6 ± 3.9 (24.5–48.0, 38.5) | |
| Route of administration (IV:PO) | 90:62 | |
| Dose of drug (mg kg−1 d−1) | 5.6 ± 1.5 (1.9–9.6, 5.4) | |
| Serum theophylline trough concentration (μg ml−1) | 7.96 ± 4.18 (0.90–22.33, 7.78) | |
| 24‐h urine output (ml kg−1 h−1) | 4.2 ± 1.1 (1.6–7.0, 4.1) | |
| Serum BUN (mg dl−1) | 11.1 ± 9.1 (1–47, 8) | |
| Serum Cr (mg dl−1) | 0.55 ± 0.22 (0.16–1.40, 0.53) | |
| Serum AST (IU/l) | 27.6 ± 21.1 (13–168, 22.5) | |
| Serum ALT (IU/l) | 9.1 ± 9.8 (0–83, 7) | |
| Serum protein (g dl−1) | 4.8 ± 0.6 (3.7–6.2, 4.7) | |
| Serum albumin (g dl−1) | 3.3 ± 0.4 (2.5–4.3, 3.3) | |
| Clearance of theophylline | Total clearance (ml kg−1 min−1) | 0.50 ± 0.29 (0.10–1.76, 0.36) |
| Renal clearance (ml kg−1 min−1) | 0.16 ± 0.06 (0.04–0.36, 0.15) | |
| Nonrenal clearance (ml kg−1 min−1) | 0.34 ± 0.28 (0.02–1.64, 0.22) |
IV, intravenous; PO, peroral; BUN, blood urea nitrogen; Cr, creatinine; AST, aspartate aminotransferase; ALT, alanine aminotransferase
TP concentrations in serum and urine
There were no significant differences in the TP concentrations detected in the urine samples collected serially from each subject (P = 0.983). We found a good association between serum trough concentrations and urine concentrations of TP (Figure 2). Serum trough concentrations of TP were correlated with the concentration of TP in spot urine (R = 0.863, P < 0.001), the molar percentage of TP to all metabolites (R = 0.477, P < 0.001) and the excreted amount of TP adjusted by urine volume (R = 0.760, P < 0.001).
Figure 2.
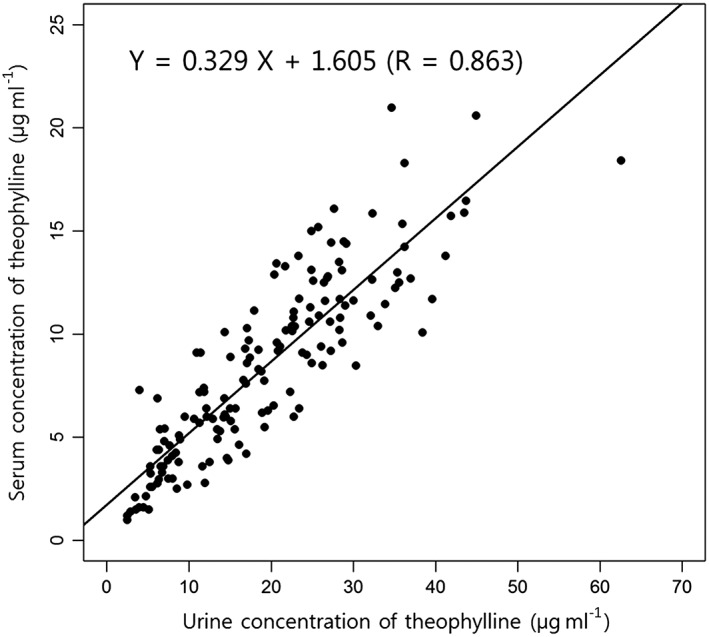
Correlation between the serum and urine theophylline concentrations. There was a good correlation between the serum trough theophylline concentration and the theophylline concentration in spot urine. The association between serum and urine theophylline concentrations was assessed using simple linear regression analysis
CYP1A2 activity
CYP1A2 activity was positively correlated with PMA, postnatal age (PNA), weight, height, body mass index, serum aspartate aminotransferase and alanine aminotransferase, but was inversely related to serum blood urea nitrogen, creatinine and albumin levels (Table 2). Multiple linear regression analysis (Table 3) found that PNA and PMA were factors affecting CYP1A2 activity. CYP1A2*1C and CYP1A2*1F genotypes did not affect CYP1A2 activity (Tables 2 and 3).
Table 2.
Correlation between subject factors, CYP1A2 activity and theophylline clearance
| CYP1A2 activitya | Total clearance | Renal clearance | Nonrenal clearance | |||||
|---|---|---|---|---|---|---|---|---|
| β | P | β | P | β | P | β | P | |
| Gestational age | –0.039 | 0.635 | –0.046 | 0.572 | –0.151 | 0.063 | –0.024 | 0.771 |
| Postmenstrual age | 0.307 | <0.001 | 0.482 | <0.001 | 0.143 | 0.079 | 0.460 | <0.001 |
| Postnatal age | 0.377 | <0.001 | 0.575 | <0.001 | 0.321 | <0.001 | 0.522 | <0.001 |
| Weight | 0.228 | 0.005 | 0.488 | <0.001 | 0.201 | 0.013 | 0.456 | <0.001 |
| Height | 0.178 | 0.028 | 0.352 | <0.001 | 0.100 | 0.222 | 0.335 | <0.001 |
| Body mass index | 0.216 | 0.008 | 0.488 | <0.001 | 0.257 | 0.001 | 0.446 | <0.001 |
| Sex (1: male, 2: female) | –0.065 | 0.429 | –0.048 | 0.554 | –0.138 | 0.091 | –0.011 | 0.889 |
| CYP1A2 a 1C genotype (1: AA, 3: GG) | –0.024 | 0.770 | 0.045 | 0.586 | 0.007 | 0.935 | 0.040 | 0.623 |
| CYP1A2 a 1F genotype (1: AA, 3: CC) | –0.104 | 0.200 | –0.098 | 0.230 | –0.055 | 0.500 | –0.085 | 0.297 |
| 24‐h urine output | –0.003 | 0.969 | 0.209 | 0.010 | 0.085 | 0.302 | ||
| Serum BUN | –0.304 | <0.001 | –0.492 | <0.001 | –0.338 | <0.001 | –0.427 | <0.001 |
| Serum Cr | –0.306 | <0.001 | –0.548 | <0.001 | –0.303 | <0.001 | –0.400 | <0.001 |
| Serum AST | 0.139 | 0.087 | 0.150 | 0.065 | 0.134 | 0.099 | 0.137 | 0.094 |
| Serum ALT | 0.243 | 0.003 | 0.218 | 0.007 | 0.135 | 0.096 | 0.192 | 0.018 |
| Serum protein | –0.129 | 0.113 | –0.212 | 0.009 | –0.221 | 0.006 | –0.170 | 0.037 |
| Serum albumin | –0.174 | 0.032 | –0.142 | 0.080 | –0.178 | 0.028 | –0.098 | 0.233 |
| Urine caffeine/theophylline ratio | 0.238 | 0.003 | –0.315 | <0.001 | 0.304 | <0.001 | ||
| CYP1A2 activity a | 0.696 | <0.001 | –0.014 | 0.867 | 0.720 | <0.001 | ||
BUN, blood urea nitrogen; Cr, creatinine; AST, aspartate aminotransferase; ALT, alanine aminotransferase
Simple linear regression was used for data analysis.
CYP1A2 activity = ratio of (1‐methyluric acid +3‐methylxanthine) / theophylline
Table 3.
Factors affecting CYP1A2 activity and theophylline clearance
| CYP1A2 activitya | Total clearance | Renal clearance | Nonrenal clearance | |
|---|---|---|---|---|
| Model power | R2 = 0.165 | R2 = 0.661 | R2 = 0.289 | R2 = 0.655 |
| Associated factors | PNA (β = 0.299, P = 0.001) | CYP1A2 activitya (β = 0.548, P < 0.001) | BUN (β = −0.322, P < 0.001) | CYP1A2 activitya (β = 0.556, P < 0.001) |
| PMA (β = 0.172, P = 0.043) | PNA (β = 0.261, P < 0.001) | C/TP ratio (β = −0.391, P < 0.001) | Weight (β = 0.234, P < 0.001) | |
| Weight (β = 0.221, P < 0.001) | PNA (β = 0.176, P = 0.033) | PNA (β = 0.210, P < 0.001) | ||
| 24 h UO (β = 0.101, P = 0.048) | C/TP ratio (β = 0.128, P = 0.012) |
PNA, postnatal age; PMA, postmenstrual age; UO, urine output; BUN, blood urea nitrogen; C/TP, caffeine/theophylline
Multiple stepwise linear regression analysis was used for data analysis.
CYP1A2 activity = ratio of (1‐methyluric acid + 3‐methylxanthine ) / theophylline
Clearance of TP
The total, renal and nonrenal clearances of TP in our preterm infants' group were 0.50 ± 0.29 ml kg−1 min−1, 0.16 ± 0.06 ml kg−1 min−1, and 0.34 ± 0.28 ml kg−1 min−1, respectively (Table 1). The proportion of renal clearance was 32% and that of nonrenal clearance was 68%.
Table 2 shows the associations between each subject factor and TP clearance. Multiple linear regression analysis adjusting for compounding factors (Table 3) found that the factors associated with total clearance of TP were CYP1A2 activity, PNA, weight, and 24‐h urine output. Similarly, nonrenal clearance was determined by CYP1A2 activity, weight, PNA and urine caffeine/TP ratio. Renal clearance was associated with the serum blood urea nitrogen level, urine caffeine/TP ratio and PNA.
Discussion
We explored clinical markers reflecting TP clearance in preterm infants, including CYP1A2 activity, by analyzing urine metabolites using noninvasive methods. To our knowledge, this is the first published study to investigate the relationship between phenotypic CYP1A2 activity and pharmacokinetic characteristics in preterm infants. This study shows that developmental changes in CYP1A2 activity and in TP clearance were affected by PNA, rather than PMA, and that CYP1A2 activity had a greater influence on TP clearance than urinary excretion did during the preterm period. Previous population pharmacokinetics studies of TP in preterm infants reported the importance of body weight and age 22, 23, 24, which was consistent with our results. However, we also found that CYP1A2 activity was a major determinant of TP clearance in preterm infants.
Several studies have reported developmental changes in the pharmacokinetics of TP in preterm infants, but the pattern and timing of these have not been fully elucidated 14, 15, 25. TP clearance and urine metabolite patterns reach adult values from the age of 3 months to 3 years, depending on the study. Many studies have emphasized that TP elimination occurs primarily by renal excretion of unchanged drug because the available CYP1A2 would be easily saturated in preterm infants, and that PMA rather than PNA should be used to assess maturity in infancy. However, the present study found that nonrenal clearance, representing CYP1A2 activity, predominated over renal clearance, and that PNA was more crucial than PMA in the maturation of this process. These differences could occur due to the investigation of study populations with relatively early PMA (25+3–37+1) and may reflect the fact that CYP1A2 is highly inducible at both the mRNA and protein levels by a variety of chemicals, by smoking and by several dietary factors experienced after birth 16. In addition, neither the CYP1A2*1C nor CYP1A2*1F genotype affected TP clearance or CYP1A2 activity; this contrasted with the results in adults, where the CYP1A2*1C and CYP1A2*1F genotypes were associated with low and high CYP1A2 inducibility, respectively 26, 27. These data suggest that genetic polymorphism‐associated differences might not yet be expressed in this study population, where developmental changes in the phenotype may be more crucial determinants of activity. However, the relatively low coefficient of determination (R2 = 0.165, Table 3) for CYP1A2 activity, PNA and PMA suggests the possibility that other unknown factors affect CYP1A2 activity in preterm infants. Therefore, the ability to evaluate phenotypic CYP1A2 activity directly using noninvasive urine metabolite analyses is very useful.
Urine is the most suitable biological sample for metabolite analysis even in very young preterm infants, because of the simple and noninvasive collection methods involved. In addition, modelling approaches can facilitate the application of data acquired from a small number of patients to the entire patient population. In our study, the urinary excretion rates of TP were constant over time. This reflected the special environmental and physiological characteristics of these neonates, who were provided continuous IV fluid infusion or regular and frequent milk feeding at 2‐ or 3‐h intervals and who could not control voiding intentionally in the neonatal intensive care unit. In our study, the concentration of TP in spot urine as well as the molar percentage or excreted amount of TP showed a good correlation with the serum trough concentration of TP. The use of spot urine to analyze TP concentrations could provide a more convenient and useful method in view of the difficulties associated with 24‐h urine collection in neonates.
Recently, caffeine has been replacing TP for the treatment of apnoea of prematurity because its wider therapeutic window and longer half‐life means that it does not require routine therapeutic drug monitoring 10. Although the clinical importance of TP in preterm infants is diminishing, it is still used now and importantly, extensive data relating to the perinatal and neonatal maturation of the relevant metabolic pathways have been gathered over the past 40 years 14, 15, 25. Therefore, we chose TP as a model drug to explore noninvasive clinical urinary metabolite markers that reflect developmental changes in preterm infants. Besides TP 22, 23, 24, other population pharmacokinetic studies of various drugs in preterm infants have reported developmental changes that were associated with body weight and age 28, 29, 30, 31, 32, 33. In preterm and newborn infants, developmental metabolic state may affect drug metabolism directly and can be expressed as the phenotypic enzyme activity by analyzing urinary metabolites. Our study design using noninvasive urine metabolite analyses can also be applied to other drug studies in preterm infants.
In conclusion, we confirmed that there was a significant association between the CYP1A2 activity assessed using urinary metabolites and TP clearance in preterm infants. CYP1A2 activity increased with age in preterm infants, and was independent of the CYP1A2 genotype. CYP1A2 activity, which can be monitored in real time using random urine samples collected using noninvasive methods, can thus be used to assess developmental changes in TP pharmacokinetics in preterm infants. We expect that the approach used in this study will be applicable to studies of the pharmacokinetic characteristics of other drugs in preterm infants.
Competing Interests
The authors report that there are no competing interests.
We thank Hyungmi Ahn for advising on the statistical analyses. We thank the nurses and medical staff of the Seoul National University Children's Hospital, Seoul National University Bundang Hospital, and Seoul Metropolitan Government – Seoul National University Boramae Medical Center neonatal intensive care units for their cooperation.
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A070001, Korea National Enterprise for Clinical Trials).
Contributors
H.K., J.C., K.Y. and J.S. conceptualized and designed the study. J.S., J.L., S.S., J.L., C.C., E.K., H.K. and B.K. carried out sample and data collection. J.C. and K.Y. supervised the analysis of the samples. J.S., J.O., H.K., K.Y. and E.P. analyzed data. J.S. drafted the article. All authors participated in revising the article critically for important intellectual content. All authors approved the final submitted version and agreed to be accountable for all aspects of the work.
Sohn, J. A. , Kim, H.‐S. , Oh, J. , Cho, J.‐Y. , Yu, K.‐S. , Lee, J. , Shin, S. H. , Lee, J. A. , Choi, C. W. , Kim, E.‐K. , Kim, B. I. , and Park, E. A. (2017) Prediction of serum theophylline concentrations and cytochrome P450 1A2 activity by analyzing urinary metabolites in preterm infants. Br J Clin Pharmacol, 83: 1279–1286. doi: 10.1111/bcp.13211.
References
- 1. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016; 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al. The Concise Guide to PHARMACOLOGY 2015/16: enzymes. Br J Pharmacol 2015; 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rieder M, Hawcutt D. Design and conduct of early phase drug studies in children: challenges and opportunities. Br J Clin Pharmacol 2016; 82: 1308–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marsot A, Boulamery A, Bruguerolle B, Simon N. Population pharmacokinetic analysis during the first 2 years of life: an overview. Clin Pharmacokinet 2012; 51: 787–798. [DOI] [PubMed] [Google Scholar]
- 5. Ku LC, Smith PB. Dosing in neonates: special considerations in physiology and trial design. Pediatr Res 2015; 77: 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O'Hara K, Wright IM, Schneider JJ, Jones AL, Martin JH. Pharmacokinetics in neonatal prescribing: evidence base, paradigms and the future. Br J Clin Pharmacol 2015; 80: 1281–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Atzori L, Antonucci R, Barberini L, Griffin JL, Fanos V. Metabolomics: a new tool for the neonatologist. J Matern Fetal Neonatal Med 2009; 22: 50–53. [DOI] [PubMed] [Google Scholar]
- 8. Antonucci R, Pilloni MD, Atzori L, Fanos V. Pharmaceutical research and metabolomics in the newborn. J Matern Fetal Neonatal Med 2012; 25: 22–26. [DOI] [PubMed] [Google Scholar]
- 9. Fanos V, Barberini L, Antonucci R, Atzori L. Pharma‐metabolomics in neonatology: is it a dream or a fact? Curr Pharm Des 2012; 18: 2996–3006. [DOI] [PubMed] [Google Scholar]
- 10. Abdel‐Hady H, Nasef N, Shabaan AE, Nour I. Caffeine therapy in preterm infants. World J Clin Pediatr 2015; 4: 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pacifici GM. Clinical pharmacology of theophylline in preterm infants: effects, metabolism and pharmacokinetics. Curr Pediatr Rev 2014; 10: 297–303. [DOI] [PubMed] [Google Scholar]
- 12. Neofax, 24th edn. Montvale, NJ: Thomson Reuters, 2011; 294–295. [Google Scholar]
- 13. Taketomo CK, Hodding JH, Kraus DM. Pediatric and neonatal dosage handbook, 18th edn. Hudson, OH: Lexicomp, 2011; 94–96, 1461‐3. [Google Scholar]
- 14. Kraus DM, Fischer JH, Reitz SJ, Kecskes SA, Yeh TF, McCulloch KM, et al. Alterations in theophylline metabolism during the first year of life. Clin Pharmacol Ther 1993; 54: 351–359. [DOI] [PubMed] [Google Scholar]
- 15. Tateishi T, Asoh M, Yamaguchi A, Yoda T, Okano YJ, Koitabashi Y, et al. Developmental changes in urinary elimination of theophylline and its metabolites in pediatric patients. Pediatr Res 1999; 45: 66–70. [DOI] [PubMed] [Google Scholar]
- 16. Zhou SF, Yang LP, Zhou ZW, Liu YH, Chan E. Insights into the substrate specificity, inhibitors, regulation, and polymorphisms and the clinical impact of human cytochrome P450 1A2. AAPS J 2009; 11: 481–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chereches‐Panta P, Nanulescu MV, Culea M, Palibroda N. Reliability of salivary theophylline in monitoring the treatment for apnoea of prematurity. J Perinatol 2007; 27: 709–712. [DOI] [PubMed] [Google Scholar]
- 18. Cattarossi L, Violino M, Macagno F, Logreco P, Savoia M. Correlation between plasma and urinary caffeine levels in preterm infants. J Perinat Med 2006; 34: 344–346. [DOI] [PubMed] [Google Scholar]
- 19. Björkman S. Prediction of drug disposition in infants and children by means of physiologically based pharmacokinetic (PBPK) modelling: theophylline and midazolam as model drugs. Br J Clin Pharmacol 2005; 59: 691–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. ARCHITECT i Theophylline assay [package inserts].
- 21. Takata K, Saruwatari J, Nakada N, Nakagawa M, Fukuda K, Tanaka F, et al. Phenotype–genotype analysis of CYP1A2 in Japanese patients receiving oral theophylline therapy. Eur J Clin Pharmacol 2006; 62: 23–28. [DOI] [PubMed] [Google Scholar]
- 22. Lee TC, Charles BG, Steer PA, Flenady VJ, Grant TC. Theophylline population pharmacokinetics from routine monitoring data in very premature infants with apnoea. Br J Clin Pharmacol 1996; 41: 191–200. [DOI] [PubMed] [Google Scholar]
- 23. Fukuda T, Yukawa E, Kondo G, Maeda T, Shin‐o T, Kondo Y, et al. Population pharmacokinetics of theophylline in very premature Japanese infants with apnoea. J Clin Pharm Ther 2005; 30: 591–596. [DOI] [PubMed] [Google Scholar]
- 24. Kim SE, Kim BH, Lee S, Sohn JA, Kim HS, Cho JY, et al. Population pharmacokinetics of theophylline in premature Korean infants. Ther Drug Monit 2013; 35: 338–344. [DOI] [PubMed] [Google Scholar]
- 25. Lowry JA, Jarrett RV, Wasserman G, Pettett G, Kauffman RE. Theophylline toxicokinetics in premature newborns. Arch Pediatr Adolesc Med 2001; 155: 934–939. [DOI] [PubMed] [Google Scholar]
- 26. Nakajima M, Yokoi T, Mizutani M, Kinoshita M, Funayama M, Kamataki T. Genetic polymorphism in the 5′‐flanking region of human CYP1A2 gene: effect on the CYP1A2 inducibility in humans. J Biochem 1999; 125: 803–808. [DOI] [PubMed] [Google Scholar]
- 27. Sachse C, Brockmöller J, Bauer S, Roots I. Functional significance of a C‐‐ > A polymorphism in intron 1 of the cytochrome P450 CYP1A2 gene tested with caffeine. Br J Clin Pharmacol 1999; 47: 445–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Al Za'abi M, Donovan T, Tudehope D, Woodgate P, Collie LA, Charles B. Orogastric and intravenous indomethacin administration to very premature neonates with patent ductus arteriosus: population pharmacokinetics, absolute bioavailability, and treatment outcome. Ther Drug Monit 2007; 29: 807–814. [DOI] [PubMed] [Google Scholar]
- 29. Wade KC, Wu D, Kaufman DA, Ward RM, Benjamin DK Jr, Sullivan JE, et al. Population pharmacokinetics of fluconazole in young infants. Antimicrob Agents Chemother 2008; 52: 4043–4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cohen‐Wolkowiez M, Sampson M, Bloom BT, Arrieta A, Wynn JL, Martz K, et al. Determining population and developmental pharmacokinetics of metronidazole using plasma and dried blood spot samples from premature infants. Pediatr Infect Dis J 2013; 32: 956–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sampson MR, Bloom BT, Lenfestey RW, Harper B, Kashuba AD, Anand R, et al. Population pharmacokinetics of intravenous acyclovir in preterm and term infants. Pediatr Infect Dis J 2014; 33: 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cohen‐Wolkowiez M, Watt KM, Zhou C, Bloom BT, Poindexter B, Castro L, et al. Developmental pharmacokinetics of piperacillin and tazobactam using plasma and dried blood spots from infants. Antimicrob Agents Chemother 2014; 58: 2856–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fuchs A, Guidi M, Giannoni E, Werner D, Buclin T, Widmer N, et al. Population pharmacokinetic study of gentamicin in a large cohort of premature and term neonates. Br J Clin Pharmacol 2014; 78: 1090–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]


