Tables of Links
| LIGANDS |
|---|
| Midazolam |
| Tacrolimus |
These Tables list key protein targets and ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 1, and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 2.
We recently published the results from a study performed in 147 stable renal recipients treated with tacrolimus, in which multivariable models based on the cytochrome P450 (CYP) 3A metrics weight‐corrected 4β‐hydroxycholesterol/cholesterol (4β‐OHC/C/W) and weight‐corrected midazolam (MDZ) apparent oral clearance (Cl/F/W) performed similarly in explaining interindividual differences in tacrolimus Cl/F/W 3. In a letter to the editor, Storset et al. argued that weight correction of the CYP3A4 metrics and tacrolimus Cl/F may have led to inflation of the correlation coefficients between these parameters 4.
While we agree that bodyweight correction of two variables will generally increase their correlation, the degree to which this occurs is complex and variable as it depends on the exact relationship between the variables and bodyweight. Table S1 shows the correlation coefficients between nonweight‐corrected tacrolimus Cl/F and the different CYP3A metrics (analogous to Table 2 in the original publication). The correlation coefficient between 4β‐OHC/C/W and tacrolimus Cl/F/W was 30% higher than that between 4β‐OHC/C and tacrolimus Cl/F (r = 0.408 vs. 0.314), in contrast to the 142% increase observed in the 43 renal recipients that Storset et al. described in their letter (r = 0.46 vs. 0.19). We do not agree, however, with the statement regarding ‘the potential pitfall of falsely detecting significant associations when transforming both axes … using a joint third variable’. The correlation coefficient between weight‐corrected 4β‐OHC/C and tacrolimus Cl/F is as statistically valid as the one between the uncorrected variables, regardless of whether 4β‐OHC/C and weight are negatively or positively related. The key question is whether the weight‐corrected variables are parameters that are biologically meaningful and interpretable as such. We admit that this is debatable. It might have been more intuitive to report partial correlations, which answer the question: ‘What would be the correlation between 4β‐OHC/C and tacrolimus Cl/F if all patients had the same weight?’ The partial r between 4β‐OHC/C and tacrolimus Cl/F was 0.422 (P < 0.001), virtually identical to the correlation between the weight‐corrected variables (r = 0.408).
Secondly, we disagree with Storset and colleagues' objection to performing a weight correction as such. Figure 1 shows that tacrolimus Cl/F, MDZ Cl/F and 4β‐OHC/C were all significantly related to weight. Storset and colleagues' statement that ‘there is no evidence in the literature for a linear relationship between weight and tacrolimus Cl/F in renal transplanted adults’ is based on a rather selective interpretation of the available data as, on the whole (considering the entire body of evidence relating to all transplant recipients), the comprehensive review they refer to states in the abstract that ‘variability in tacrolimus whole blood apparent clearance among transplant recipients was most commonly related to CYP3A5 genotype (rs776746), patient haematocrit, patient weight, postoperative day and hepatic function...’ 5. It is also noteworthy that our study included more patients who had undergone tacrolimus 8‐h area under the curve (AUC) analysis than any of the individual studies in that review. If Storset et al. do not consider bodyweight to be a relevant determinant of tacrolimus Cl/F, one wonders why they included it as one of the predictors of tacrolimus dose requirements in their recently published, outstanding, computerized dosing study in renal recipients 6.
Figure 1.
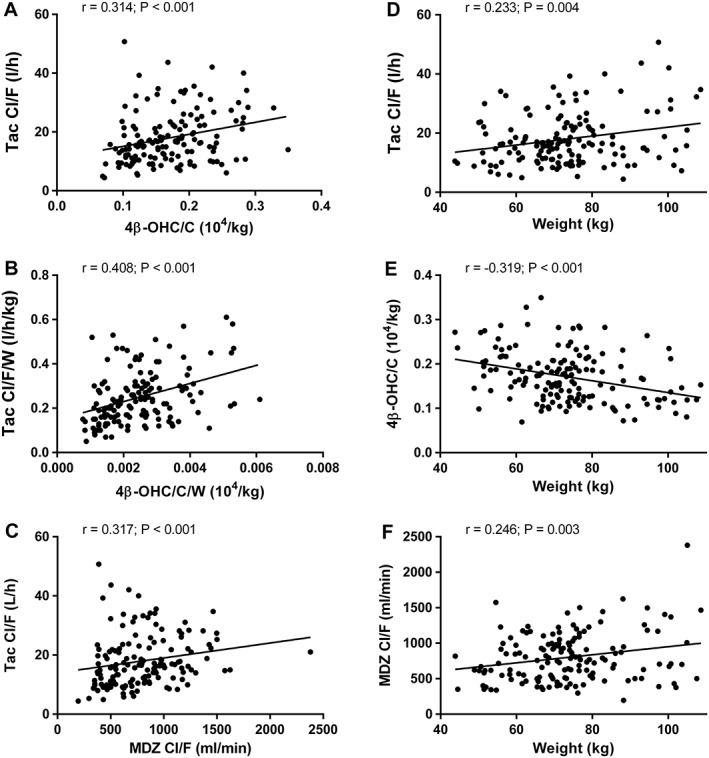
Correlations between nonbodyweight‐corrected tacrolimus (Tac) apparent oral clearance (Cl/F) and 4β‐hydroxycholesterol/cholesterol (4β‐OHC/C) (A), weight‐corrected Tac Cl/F/W and 4β‐OHC/C/W (B), Tac Cl/F and midazolam (MDZ) Cl/F (C) as well as between bodyweight and Tac Cl/F (D), 4β‐OHC/C (E) and MDZ Cl/F (F)
Thirdly, and most importantly, correlation coefficients are not the point. The main issue is how models based on the different CYP3A metrics compare with regard to the prediction of tacrolimus Cl/F(/W). As requested by Storset et al., we repeated the multivariable regression models for tacrolimus Cl/F using nonweight‐corrected 4β‐OHC/C and MDZ Cl/F, which are shown in Table 1 (analogous to Table 3 in the original paper). Bodyweight was included as a separate predictor variable. Overall model fit was very similar in uncorrected and weight‐corrected analyses in all subgroups. For the whole group (n = 147), an interaction was identified between 4β‐OHC/C and weight, whereby the effect of 4β‐OHC/C was greater with increasing weight (B = 0.004; P = 0.001). One could point out that the R 2 values of 4β‐OHC/C are lower than they were for 4β‐OHC/C/W but, again, overall model fit is what matters. Any CYP3A metric used to predict tacrolimus disposition is only as good as what it adds to other well‐established determinants of tacrolimus Cl/F (e.g. CYP3A5 genotype and haematocrit).
Table 1.
Multivariable determinants of tacrolimus apparent oral clearance
| MDZ model | 4β‐OHC model | ||||||
|---|---|---|---|---|---|---|---|
| Determinants | B value | P | R 2 | Determinants | B value | P | R 2 |
| All patients (n = 147) | 0.569 | 0.525 | |||||
| CYP3A5 expresser | 0.671 | <0.001 | 0.292 | CYP3A5 expresser | 0.604 | <0.001 | 0.292 |
| MDZ Cl/F | 0.337 | <0.001 | 0.103 | 4β‐OHC/C | 0.304 | 0.002 | 0.032 |
| Haematocrit | –3.358 | <0.001 | 0.060 | Haematocrit | –2.661 | <0.001 | 0.074 |
| Weight (kg) | 0.008 | <0.001 | 0.046 | Weight (kg) | 0.012 | <0.001 | 0.050 |
| Age (years) | –0.008 | 0.001 | 0.040 | Age (years) | –0.008 | 0.001 | 0.061 |
| TAC QD | 0.182 | 0.003 | 0.029 | TAC QD | 0.139 | 0.026 | 0.017 |
| CYP3A5 non‐expressers (n = 118) | 0.437 | 0.319 | |||||
| MDZ Cl/F | 0.423 | <0.001 | 0.220 | 4β‐OHC/C | 0.357 | 0.002 | 0.063 |
| Haematocrit | –3.398 | <0.001 | 0.097 | Haematocrit | –2.556 | <0.001 | 0.074 |
| Weight (kg) | 0.005 | 0.029 | 0.024 | Weight (kg) | 0.012 | <0.001 | 0.083 |
| Age (years) | –0.008 | 0.002 | 0.049 | Age (years) | –0.008 | 0.005 | 0.100 |
| TAC QD | 0.190 | 0.005 | 0.047 | – | |||
| CYP3A5 expressers (n = 29) | 0.342 | ||||||
| Weight (kg) | 0.014 | 0.001 | 0.247 | ||||
| Age (years) | –0.008 | 0.045 | 0.095 | ||||
Neither MDZ Cl/F nor 4β‐OHC/C explained TAC Cl/F variability in CYP3A5 expressers
4β‐OHC/C, 4β‐hydroxycholesterol/cholesterol; CYP, cytochrome P450; MDZ Cl/F, midazolam apparent oral clearance; QD, once‐daily formulation; TAC, tacrolimus
Finally, it is unfortunate that Storset et al. chose to supply only limited information regarding their unpublished cohort of 43 renal recipients, as this makes it difficult to examine why their results differed from ours. The figures they provide raise a number of questions. It seems that 4β‐OHC was not corrected for cholesterol, even though this is preferred 7. The correlation between tacrolimus Cl/F and 4β‐OHC is quite possibly distorted by two outliers with very high 4β‐OHC values. Additionally, bodyweights are significantly higher than in our cohort (11/43 patients >100 kg). Were tacrolimus Cl/F values calculated from full AUCs, partial AUCs or trough concentrations? Is CYP3A4/5 genotype information available? This is relevant as, in our hands, 4β‐OHC/C/W was only related to tacrolimus Cl/F in CYP3A5 non‐expressers 3.
In conclusion, there is good evidence that bodyweight is a relevant (but not a dominant) determinant of tacrolimus Cl/F as well as the CYP3A metrics 4β‐OHC/C and MDZ Cl/F. The fact that bodyweight adjustment alters their correlations mainly reflects the importance of correcting for weight in a (CYP3A4 metric‐based) model for tacrolimus Cl/F. The dramatic difference in correlation coefficients that Storset et al. observe between bodyweight‐corrected and uncorrected 4β‐OHC and tacrolimus Cl/F is likely to have biological meaning; we would urge them not to dismiss it out of hand as ‘false detection of significant associations’ but to explore it further in detail.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no competing interests.
Supporting information
Table S1 Correlations between MDZ Cl/F, tacrolimus Cl/F, 4β‐OHC/C and EBT
Vanhove, T. , Annaert, P. , and Kuypers, D. R. J. (2017) Response to: ‘Bodyweight‐adjustments introduce significant correlations between CYP3A metrics and tacrolimus clearance’. Br J Clin Pharmacol, 83: 1353–1356. doi: 10.1111/bcp.13249.
References
- 1. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016 44 (Database Issue): D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al. The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 2015; 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vanhove T, de Jonge H, de Loor H, Annaert P, Diczfalusy U, Kuypers DRJ. Comparative performance of oral midazolam clearance and plasma 4beta‐hydroxycholesterol to explain interindividual variability in tacrolimus clearance. Br J Clin Pharmacol 2016; 82: 1539–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Størset E, Hole K, Midtvedt K, Bergan S, Molden E, Åsberg A. Bodyweight‐adjustments introduce significant correlations between CYP3A metrics and tacrolimus clearance. Br J Clin Pharmacol 2017; 83: 1350–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brooks E, Tett SE, Isbel NM, Staatz CE. Population pharmacokinetic modelling and Bayesian estimation of tacrolimus exposure: is this clinically useful for dosage prediction yet? Clin Pharmacokinet 2016; 55: 1295–1335. [DOI] [PubMed] [Google Scholar]
- 6. Størset E, Asberg A, Skauby M, Neely M, Bergan S, Bremer S, et al. Improved tacrolimus target concentration achievement using computerized dosing in renal transplant recipients – a prospective, randomized study. Transplantation 2015; 99: 2158–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Björkhem‐Bergman L, Nylén H, Eriksson M, Parini P, Diczfalusy U. Effect of statin treatment on plasma 4β‐hydroxycholesterol concentrations. Basic Clin Pharmacol Toxicol 2016; 118: 499–502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Correlations between MDZ Cl/F, tacrolimus Cl/F, 4β‐OHC/C and EBT


