Abstract
Aims
To describe and assess current effectiveness studies published up to 2014 using Swedish Prescribed Drug Register (SPDR) data.
Methods
Study characteristics were extracted. Each study was assessed concerning the clinical relevance of the research question, the risk of bias according to a structured checklist, and as to whether its findings contributed to new knowledge. The biases encountered and ways of handling these were retrieved.
Results
A total of 24 effectiveness studies were included in the review, the majority on cardiovascular or psychiatric disease (n = 17; 71%). The articles linked data from four (interquartile range: three to four) registers, and were published in 21 different journals with an impact factor ranging from 1.58 to 51.66. All articles had a clinically relevant research question. According to the systematic quality assessments, the overall risk of bias was low in one (4%), moderate in eight (33%) and high in 15 (62%) studies. Overall, two (8%) studies were assessed as contributing to new knowledge. Frequently occurring problems were selection bias making the comparison groups incomparable, treatment bias with suboptimal handling of drug exposure and an intention‐to‐treat approach, and assessment bias including immortal time bias. Good examples of how to handle bias problems included propensity score matching and sensitivity analyses.
Conclusion
Although this review illustrates that effectiveness studies based on dispensed drug register data can contribute to new evidence of intended effects of drug treatment in clinical practice, the expectations of such data to provide valuable information need to be tempered due to methodological issues.
Keywords: effectiveness, evidence‐based medicine, pharmacoepidemiology, systematic review
What is Already Known about this Subject
For rational use of medicines, a positive benefit/risk balance and an acceptable cost/benefit ratio is essential. A basic prerequisite for assessing this balance and ratio is relevant and adequate evidence on drug effectiveness, that is, the intended beneficial effects of a drug in clinical practice.
Register research is expected to provide evidence on drug effectiveness, but the evidence achieved, when data collected in a non‐interventional setting are used, has not been systematically assessed.
During the first decade after the establishment of the Swedish Prescribed Drug Register (SPDR), 24 out of 338 publications based on SPDR data concerned drug effectiveness, constituting a representative sample of current studies on the topic.
What this Study Adds
In this review, two out of 24 effectiveness studies based on data from SPDR contributed to new knowledge on beneficial drug effects in clinical practice, and only one study had a low risk of bias.
Researchers performing and reviewing drug effectiveness studies, as well as decision makers interpreting the results, need to pay particular attention to selection, treatment and assessment biases.
Propensity score matching, within‐individual analyses and sensitivity analyses concerning population studied, drug exposure and outcomes represent ways to handle methodological challenges associated with drug effectiveness studies.
Table of Links
This Table lists key ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 1.
Introduction
A basic prerequisite for rational use of medicines and wise decisions on drug treatment, at both societal and patient levels, is access to relevant and adequate evidence. We need to know that every drug has a positive benefit/risk balance, as assessed initially upon approval by the drug authorities and thereafter by physicians when prescribing a drug to a specific patient. As health care resources are limited, we also need to know that the costs for a drug are reasonable given its effects, as for example illustrated by the cost/benefit ratio assessed by health technology assessment (HTA) authorities. In order to make these assessments, decision makers, including physicians, want to know about the size of the intended beneficial effects of a drug. Such information can be obtained from randomized controlled trials, reflecting the efficacy of a drug. However, as patients in health care differ in many respects from study participants, evidence of the intended effects of a drug in clinical practice, that is, the effectiveness, is desirable. In this context, increased attention has been drawn to register data, representing the non‐interventional setting 2.
Prevailing expectations are that register data will provide information on drug effectiveness needed for decision making. In fact, for new drugs targeting unmet medical needs, these expectations may have contributed to the introduction of accelerated approval processes to facilitate rapid market entrance 3. Such processes imply that less extensive evidence on drug effects is required before approval, and leave much of the generation of evidence to the post‐approval stage. Indeed, the number of new drugs approved on the basis of single‐arm studies, i.e. prospective case series without a concurrent control group, is increasing 4.
Great efforts are invested in collection of register data; however, as far as we are aware, the evidence achieved, concerning drug effects, when such data are used has not been systematically evaluated. As evidence regarding intended beneficial effects needs to be stronger than evidence on safety concerns, according to the benefit of doubt and the better safe than sorry principles, effectiveness studies are of particular interest. Indeed, spontaneously reported adverse drug reactions may suffice to reinforce the re‐evaluation of drug safety issues 5, whereas beneficial effects need to be more convincingly proved. Therefore, we need to know to what extent register data can contribute to new evidence on intended beneficial effects of drug treatment, using today's methodologies.
Swedish national health registers are acknowledged worldwide as they are generally of good quality and cover the entire Swedish population. Furthermore, they hold much information, including, for example, health data and civic information, which can be linked via citizens’ unique personal identity number 6. They can also be linked to other data sources, including clinical data in electronic medical records transferred to a data warehouse or a quality register. The Swedish Prescribed Drug Register (SPDR) was expanded on 1 July 2005 to include the identity of the patient 7. As all pharmacies in Sweden are required by law to submit data on dispensed prescription drugs, the SPDR contains more than one decade of patient‐level data on all dispensed prescription drugs in the country and may constitute a valuable data source for drug effectiveness studies.
In a recent systematic review describing the scientific output of the SPDR during the first 10 years after its establishment, all identified publications were categorized according to study type 8. The basis for that review was a broad literature search strategy, using the terms ‘drug’, ‘register’ and ‘Swedish’ in different combinations, with alternative wordings. Further, additional publications were identified by personal knowledge, reference lists, contact with active authors and a citation search of the Web of Science database (for search details, see Wallerstedt et al. 8). As Sweden has a long tradition in epidemiology 9, publications in the described review, categorized as effectiveness studies, may serve as a representative sample of effectiveness studies today. Indeed, the identified studies were published in a wide range of journals, including top‐ranked journals on general medicine, and medium‐ and low‐ranked journals with a more specific focus. Consequently, the articles can be expected to reflect both pitfalls and good examples. The aim of this study was to describe and assess effectiveness studies published up to 2014 and using data from the SPDR, with a focus on lessons for the future when it comes to design, methods and reporting.
Methods
In the present review, we have included 24 articles characterized as effectiveness studies in a previous systematic review (Figure 1) 8.
Figure 1.
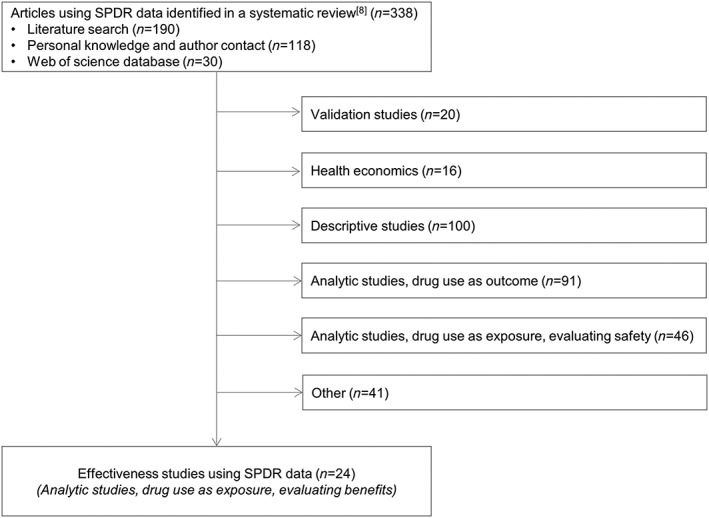
Flowchart of studies based on Swedish Prescribed Drug Register (SPDR) data and included in the present review
Data extraction
One author (S.M.W.) extracted data from the studies, and the other author (M.H.) checked these. Data extraction included the design of the study as well as the studied therapeutic area including drug(s) and outcome(s). We also recorded information regarding registers used, authors, open access publishing and impact factor of the scientific journals during the year in question, retrieved from Journal Citation Index.
Study assessments
All assessments were performed independently by both authors, after which any disparities were resolved by discussion, and consensus reached. To coordinate the assessments, we first assessed and discussed a random sample of four studies. After consensus was reached, we independently assessed and then discussed the remaining studies.
The initial assessment concerned clinical relevance. Here, we simply judged whether the research question was of interest to health care and the decision makers, based on our expertise in clinical pharmacology 10, 11, 12.
As a next step, we assessed the study quality according to a checklist on observation studies from the Swedish Council on Health Technology Assessment 13, including aspects of six biases (selection, treatment, assessment, exclusion, reporting, and conflicts of interest), weighed together in an overall assessment of study quality as having a low, moderate or high risk of bias. The biases encountered and ways of handling them were noted.
Finally, we assessed each study as to whether its results contributed to new knowledge or not. For this assessment, we used our experience as clinical pharmacologists as well as the study's introductory text reflecting the state of knowledge. We also considered the credibility of the results, given the methodology used.
No ethics approval was required as no sensitive data were handled.
Statistics
Descriptive analyses were performed using SPSS, version 20.0 (IBM SPSS Statistics for Windows, Armonk, NY). Kappa statistics were used to assess inter‐rater agreement on the study assessments. Values are presented as numbers (percentages) or medians (interquartile ranges).
Results
Characteristics of the 24 reviewed effectiveness studies are presented in Table 1. The majority were designed as cohort studies (n = 21; 88%). The therapeutic area most often focused upon was cardiovascular disease (n = 11; 46%), predominantly concerning antiplatelet agents (n = 6) and/or warfarin (n = 3). The six (25%) studies within psychiatry concerned mainly antipsychotics (n = 3) and medications for attention deficit hyperactivity disorder (ADHD) (n = 3). The five studies (21%) within the field of cancer concerned acetylsalicylic acid (n = 3) and tyrosine kinase inhibitors (n = 2).
Table 1.
Characteristics of studies included in the review, as well as resulting assessments
| Author | Therapeutic area | Design | Drug/drug group | Outcome studied | Risk of bias | Contributes to new knowledge | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Selection | Treatment | Assessment | Exclusion | Reporting | COI | Overall risk | ||||||
| Ambring et al. 42 | Cancer | Cohort | Tyrosine kinase inhibitors | Time to death, duration of treatment | High | High | Low | Low | Moderate | High | High | No |
| Björck et al. 43 | CVD | Case/control | Warfarin | Stroke | High | High | Low | Low | Moderate | Moderate | High | No |
| Bodén et al. 26 | Psychiatry | Cohort | Antipsychotics | Rehospitalization, time to first relapse | Moderate | High | Moderate | Low | Moderate | Moderate | Moderate | No |
| Chang et al. 15 | Psychiatry | Cohort | ADHD medication | Serious transport accident | Low | High | Moderate | Low | Moderate | Low | Moderate | No |
| Chen et al. 16 | Psychiatry | Cohort | ADHD medication | Suicide‐related events | Low | High | Moderate | Low | Moderate | Low | Moderate | No |
| Ekström et al. 34 | CVD | Cohort | Metformin | CVD, mortality, acidosis/serious infection | High | High | Moderate | Low | Moderate | Low | High | No |
| Ekström, Hermansson et al. 17 | CVD | Cohort | ACEI, antiplatelets, betablockers, statins | Mortality | High | High | High | High | Moderate | Low | High | No |
| Ekström, Cederholm et al. 18 | CVD | Cohort | ASA | CVD, CHD, stroke, mortality, bleedings | High | High | Moderate | High | Moderate | Low | High | No |
| Eliasson et al. 35 | CVD | Cohort | Lipid‐lowering drugs | Lipid levels | High | High | Low | High | Moderate | Moderate | High | No |
| Fazel et al. 19 | Psychiatry | Cohort | Antipsychotics and mood stabilizers | Violent crime conviction | Low | High | Moderate | Low | Moderate | Low | Moderate | No |
| Forslund et al. 44 | CVD | Cohort | ASA, warfarin | Ischaemic stroke, bleeding, death | High | High | Moderate | Low | Moderate | Low | High | No |
| Friberg et al. 27 | CVD | Cohort | Warfarin | Net benefit (avoided stroke minus excess intracranial bleeding) | Moderate | High | Low | Low | Moderate | Moderate | Moderate | No |
| Holmes et al. 20 | Cancer | Case/control | ASA | Breast cancer death | Moderate | High | High | Low | Moderate | Low | High | No |
| Jonasson et al. 28 | Gastrointestinal disease | Case/control | PPI adherence in NSAID users | Peptic ulcer, bleeding | Moderate | Moderate | Moderate | Low | Moderate | High | Moderate | No |
| Jonsson et al. 21 | Cancer | Cohort | ASA | TNM characteristics | High | Moderate | High | Moderate | Moderate | Low | High | No |
| Landfeldt et al. 22 | Osteoporosis | Cohort | Bone‐active drugs | Osteoporotic fractures related to persistence | High | Moderate | Moderate | Low | Moderate | High | High | No |
| Lichtenstein et al. 23 | Psychiatry | Cohort | ADHD medication | Crime conviction | Low | High | Moderate | Low | Moderate | Low | Moderate | Yes |
| Ljung et al. 45 | Cancer | Cohort | Antiplatelets | Lymph node metastasis | High | High | High | Low | Moderate | Low | High | No |
| Lund et al. 14 | CVD | Cohort | Betablockers | All‐cause mortality, HF hospitalisation | Low | Low | Low | Low | Moderate | Low | Low | Yes |
| Ringback Weitoft et al. 46 | Psychiatry | Cohort and case/control | Antipsychotics | Death, suicide, suicide attempt, rehospitalization, prescription refill | High | High | Moderate | Low | Moderate | Low | High | No |
| Själander et al. 24 | CVD | Cohort | ASA | Ischaemic stroke, thromboembolic events, intracranial haemorrhage, major bleeding | High | High | Moderate | Low | Moderate | Low | High | No |
| Wahlgren et al. 47 | Cancer | Cohort | Tyrosine kinase inhibitors | Overall survival | High | High | High | Low | Moderate | High | High | No |
| Wang et al. 25 | CVD | Cohort | Clopidogrel and PPI | Recurrent AMI, stroke, angina, all‐cause mortality | High | Moderate | Moderate | Moderate | Moderate | Low | High | No |
| Welin et al. 29 | CVD | Cohort | ASA | Mortality, bleeding | High | Moderate | Moderate | Low | Moderate | Low | Moderate | No |
ACEI, angiotensin converting enzyme inhibitor; ADHD, attention deficit hyperactivity disorder; AMI, acute myocardial infarction; ASA, acetylsalicylic acid; COI, conflict of interest; CHD, coronary heart disease; CVD, cardiovascular disease; HF, heart failure; NSAID, non‐steroidal anti‐inflammatory drug; PPI, proton pump inhibitor; TNM, tumour extent, nodal involvement, metastatic status.
The articles were authored by a median of five (four to seven) persons. In all, 103 authors were involved in the studies, 39 (38%) of whom acted as main authors, that is, appeared as first or last names. The articles were published in 21 different journals with an impact factor ranging from 1.58 to 51.66, median 4.78 (2.74–14.40). Ten studies (42%) were published under Open Access conditions.
Each publication used data from two registers or more, linked by patients’ personal identity number. The studies linked data from a median of four (three to four) registers. In addition to SPDR data, the studies most often used health data from either the National Board of Health and Welfare (Patient Register: n = 19; Cause of Death Register: n = 16) or quality registers (n = 5) such as the National Diabetes Register, and civic information from Statistics Sweden (n = 8).
Regarding the study assessments, the inter‐rater agreement was good (kappa = 0.79). We assessed all articles to have had a clinically relevant research question. According to the systematic quality assessments, the risk of bias was low in one (4%), moderate in eight (33%) and high in 15 (62%) articles (Table 1). Moderate and high risk of bias related to selection (n = 19; 79%), treatment (n = 23; 96%), assessment (n = 19; 79%), exclusion (n = 5; 21%), reporting (n = 24; 100%) and conflicts of interest (n = 8; 33%).
Issues related to different biases identified in the publications – in other words, problems and ways to handle them – are presented in Table 2, with corresponding reference to the publications illustrating these to various extents. An evident problem concerned the comparison groups. In ten (42%) studies, characteristics of these groups were not reported, and in nine (38%) studies, the groups differed importantly. Further, we identified two frequent problems related to the assessments of drug exposure. Firstly, in eleven (46%) studies, the exposure was only assessed at baseline mimicking intention‐to‐treat analyses in randomized controlled trials. Secondly, in six (25%) studies, the exposure was suboptimally estimated given the dispensed drug data, for example by defining exposure as the time between drug dispensings rather than as a specified time after dispensings.
Table 2.
Issues related to different biases identified in the publications, either a problem (P) or a way to handle a problem (H). Values are presented as number of articles (percent) in which the issue was encountered
| Bias | Issue | Type | n (%) | Reference number |
|---|---|---|---|---|
| Selection | Characteristics of comparison groups not reported | P | 10 (42) | 20, 21, 22, 25, 26, 28, 29, 35, 43, 47 |
| Important differences between comparison groups | P | 9 (38) | 17, 18, 24, 27, 34, 42, 44, 45, 46 | |
| Within‐individual comparison | H | 4 (17) | 15, 16, 19, 23 | |
| Propensity score matching | H | 2 (8) | 14, 24 | |
| Sensitivity analysis concerning studied population | H | 8 (35) | 14, 15, 16, 18, 19, 20, 23, 24 | |
| Treatment | ‘Intention to treat’ analysis | P | 11 (46) | 18, 24, 25, 26, 27, 34, 35, 43, 44, 46, 47 |
| Estimation of drug exposure suboptimally handled | P | 6 (25) | 15, 16, 19, 23, 29, 47 | |
| Exposure not satisfactorily covered by SPDR data | P | 1 (4) | 47 | |
| Sensitivity analysis concerning treatment exposure | H | 8 (33) | 14, 15, 16, 19, 21, 22, 23, 25 | |
| Assessment | Order of causality, confounding by indication | P | 9 (38) | 17, 19, 20, 21, 23, 24, 25, 44, 46 |
| Immortal time bias | P | 4 (17) | 22, 25, 27, 47 | |
| Design does not answer the research question | P | 3 (13) | 21, 26, 45 | |
| Sensitivity analysis concerning outcome | H | 5 (21) | 15, 16, 19, 20, 23 | |
| Exclusion | Missing register data | P | 3 (13) | 18, 34, 35 |
| Sensitivity analysis, imputation | H | 1 (4) | 17 | |
| Reporting | Unclear whether the protocol was defined beforehand | P | 24 (100) | 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 34, 35, 42, 43, 44, 45, 46, 47 |
| Conflict of interest | Funding from the industry | P | 6 (25) | 22, 26, 28, 35, 42, 47 |
| Author reported involvement in industry activities | P | 7 (29) | 14, 22, 27, 28, 34, 43, 47 | |
| Only public funding reported | H | 13 (54) | 14, 15, 16, 17, 19, 21, 23, 24, 25, 43, 44, 45, 46 |
A good example of how to handle selection bias was the use of propensity score matching. This performance was particularly successful in a publication that was based on extensive data from a quality register and allowed control patients to differ by ≤0.01 14. Another good example, for handling selection, treatment, assessment, and exclusion biases, was the use of sensitivity analyses. Twelve (50%) studies performed at least one such analysis 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25. The analyses concerned population studied, drug exposure, outcomes, as well as missing data. In six (25%) studies, sensitivity analyses were performed within more than one of these domains. No studies provided a predefined protocol to minimize the reporting bias. Thirteen (54%) studies were entirely publicly funded, avoiding conflicts of interest bias.
Among nine studies with low or moderate risk of bias, two were assessed to contribute to new evidence for intended beneficial effects of drugs (Table 1) 14, 23. The others either confirmed prevailing evidence (n = 4) 26, 27, 28, 29 or had methodological problems, hampering the credibility of the results (n = 3) 15, 16, 19. The six studies explicitly funded by the pharmaceutical industry focused on therapeutic areas with new treatment alternatives, and had either a high risk of bias (n = 4) or supported prevailing evidence (n = 2).
Discussion
In this review, we illustrate that effectiveness studies, using register data on dispensed prescription drugs, focus on relevant research questions. However, few of the published studies passed the overall assessment of evidence, as only two out of 24 publications contributed to new knowledge on intended beneficial effects of drug treatment in clinical practice, and only one publication had a low risk of bias. Moreover, our review illustrates that drug effectiveness studies can be funded by public means and published in high‐impact journals. Therefore, our results are encouraging, on the one hand, as they illustrate the scientific endeavour for methodological advancements within a challenging field, as well as the willingness of public funders to support this kind of research and of high‐impact journals to publish the results. On the other hand, our results indicate that the expectations need to be tempered concerning the ability of research based on data from a non‐interventional setting to give decision makers, including physicians, relevant information for benefit/risk and cost/benefit assessments, given the status of drug effectiveness studies today. The same applies to the expectation that post‐approval register‐based studies will provide missing evidence for drugs approved by single‐arm studies.
Interestingly, this review probably reflects the better subset of evaluations of drug effectiveness from register data. Indeed, for every published study, there may be numerous negative studies that are not publicly available. Further, effectiveness evaluations performed under circumstances other than scientific publishing, for example by the pharmaceutical industry in applications for reimbursement, do not undergo formal peer review. Although not evaluated in this review, it may be speculated that use of register data in that context may be highly biased as it lies in the interests of the applicant to show large drug effect size.
As, owing to ethical and regulatory issues 30, 31, 32, randomization has not yet been implemented in drug effectiveness studies, our review highlights the importance of taking advantage of methodology developed to minimize differences between comparison groups. Indeed, in only one study in this review, the control group did not differ considerably from the intervention group. In all other studies making between‐groups comparisons, the groups differed in important aspects, or information on characteristics of comparison groups was missing and important differences could be anticipated.
Propensity score matching represents one technique to reduce the risk of confounding. Our review illustrates that taking advantage of data from quality registers, focusing on certain conditions or procedures 33, in a narrow matching procedure, may be a successful approach. Indeed, administrative health databases including dispensed prescription drugs and diagnoses may not be sufficient to retrieve essential clinical patient data. On the other hand, quality registers may suffer from incomplete data, which is illustrated by the fact that, in this review, some studies exclude a large proportion of the patients for this reason 18, 34, 35. Indeed, to provide adequate evidence, justifying the efforts in health care to collect the data, registers need to have both acceptable coverage and a set‐up including the relevant variables.
Interestingly, the two studies using propensity score matching focused on cardiovascular disease, illustrating that researchers within this therapeutic field are often pioneers when it comes to methodological advancements, as was the case when randomized controlled design was introduced. Indeed, although propensity score matching was introduced several years ago 36, this technique seems to have undergone limited divergence within the academic world. Some articles using propensity scores for adjustments were also identified 18, 34, although such applications are controversial 36.
Another approach to minimize confounding is to make intra‐individual comparisons, as in the studies on the effects of antipsychotics 19 and ADHD medications 15, 16, 23. However, a patient's health condition, including their psychiatric status, may vary over time and be associated with drug treatment adherence, and this is consequently a limitation of this technique.
In randomized controlled studies, intention‐to‐treat analyses are often preferred as they reflect the clinical situation to a greater extent, and give a more realistic picture on what to expect upon prescribing a drug. In fact, this is an accepted approach as it introduces a systemic error which makes it harder to reach a specified end point. Our review shows that this technique has been adopted in almost half of the effectiveness studies included. However, in clinical practice the compliance is much lower than in well‐monitored clinical trials with selected patients and thorough follow‐up. Therefore, misclassification due to lack of compliance or change of treatment can be expected to be considerably greater in an epidemiological study. Consequently, the intention‐to‐treat approach may be less appropriate in this setting. Further, methodologically suboptimal assumptions concerning drug exposure were identified, an acknowledged challenge in pharmacoepidemiology 37. Therefore, involving researchers with pharmacoepidemiological skills may be valuable in the authoring and reviewing processes of effectiveness studies.
Performing sensitivity analyses, as done to various extents in half of the publications, is an illustrative way of handling uncertainties when making assumptions and evaluations in effectiveness studies based on register data. These analyses can reflect several aspects of uncertainty, including population studied, exposure estimations, outcome measured and missing data.
The methodological problem of immortal time bias 38 was identified in a few of the reviewed effectiveness studies. Another pitfall, which is relatively easy to handle, was that no publication in this review stated that it was guided by a predefined protocol describing the statistical analyses and defining the size of a clinically meaningful difference between the comparison groups. As register data can be obtained for a large number of individuals, more attention needs to be drawn to clinical relevance, rather than power calculations being done. Encouragingly, protocols for effectiveness studies seem to emerge in the scientific literature 39, illustrating a positive trend in this area.
As the results of effectiveness studies are largely dependent on the methodology used, it is reassuring that public funding exists within the field. Indeed, although associations between study conflicts of interest and effect sizes have not been found in randomized controlled trials 40, 41, effectiveness studies may be more sensitive to this type of bias, as the methodological alternatives are numerous and harbour biases to various extents. Thus, knowledge and awareness of methodological issues is essential when interpreting results of effectiveness studies. In the present review, although the publications funded by the pharmaceutical industry focused on therapeutic areas with new treatment alternatives, none of these studies contributed new knowledge on intended drug effects.
Strengths and limitations
An important strength of the present study is that it starts off from a comprehensive review of the output of the SPDR, using several strategies to identify relevant publications. Indeed, drug effectiveness studies may be identified only to a limited extent in literature searches. Further, the assessments were performed independently by two clinical pharmacologists with experience in evidence‐based decision making, both at the patient and at the societal level, with good inter‐rater agreement.
The fact that we have focused on Swedish studies using SPDR may be regarded as a limitation affecting the generalizability of the results. However, this country may be considered a gold mine for drug effectiveness studies because of its numerous linkable, nationwide and individual‐based registers of high quality. Further, the methodologies can be expected to be advanced because Sweden has a long tradition of epidemiological studies 9, which is illustrated by the fact that several articles were published in the highest ranked scientific journals. Nevertheless, the SPDR is a relatively new data source, and methodology can be expected to evolve over time. Because of the limited material in the present study, the time factor has not been assessed.
Conclusions and implications
In the present study, we show that drug effectiveness studies, based on register data collected in a non‐interventional setting, can contribute, but seldom do, to new knowledge on intended beneficial drug effects. Indeed, the problems of published studies regarding scientific quality ought to moderate the prevailing expectations that registers will provide the drug effectiveness data needed for decision making. To take advantage of the opportunities offered by the increasing availability of data, researchers and decision makers need to be aware of both pitfalls and good practice, bearing in mind the importance of having access to relevant data, using sound designs and methods, as well as reporting the results in an adequate way.
Competing Interests
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The study was funded by the Swedish Research Council, the Health & Medical Care Committee of the Region Västra Götaland, and the Network for Pharmacoepidemiology (NEPI) Trust. The funding sources did not influence the design, methods, subject recruitment, data collections, analysis, the preparation of the paper, or the decision to submit the paper for publication.
Contributors
S.M.W. conceived the study. S.M.W. and M.H. designed the study and performed the assessments. S.M.W. performed the statistical analyses and drafted the manuscript. M.H. revised the manuscript for intellectual content.
Wallerstedt, S. M. , and Hoffmann, M. (2017) Evaluating beneficial drug effects in a non‐interventional setting: a review of effectiveness studies based on Swedish Prescribed Drug Register data. Br J Clin Pharmacol, 83: 1309–1318. doi: 10.1111/bcp.13206.
References
- 1. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016; 44: D1054–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mahajan R. Real world data: Additional source for making clinical decisions. Int J Appl Basic Med Res 2015; 5: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martinalbo J, Bowen D, Camarero J, Chapelin M, Demolis P, Foggi P, et al. Early market access of cancer drugs in the EU. Ann Oncol 2016; 27: 96–105. [DOI] [PubMed] [Google Scholar]
- 4. Wallerstedt SM. Medical and scientific assessments as the basis for prioritization and resource allocation for new drug treatment – experiences from five years of systematic work [Swedish]. Lakartidningen 2016; 113: D43D. [PubMed] [Google Scholar]
- 5. Wysowski DK, Swartz L. Adverse drug event surveillance and drug withdrawals in the United States, 1969–2002: the importance of reporting suspected reactions. Arch Intern Med 2005; 165: 1363–1369. [DOI] [PubMed] [Google Scholar]
- 6. Ludvigsson JF, Otterblad‐Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol 2009; 24: 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wettermark B, Hammar N, Fored CM, Leimanis A, Otterblad‐Olausson P, Bergman U, et al. The new Swedish Prescribed Drug Register – opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf 2007; 16: 726–735. [DOI] [PubMed] [Google Scholar]
- 8. Wallerstedt SM, Wettermark B, Hoffmann M. The first decade with the Swedish Prescribed Drug Register – a systematic review of the output in the scientific literature. Basic Clin Pharmacol Toxicol 2016; 119: 464–469. [DOI] [PubMed] [Google Scholar]
- 9. Rosén M. National Health Data Registers: a Nordic heritage to public health. Scand J Public Health 2002; 30: 81–85. [DOI] [PubMed] [Google Scholar]
- 10. Aronson JK. A manifesto for clinical pharmacology from principles to practice. Br J Clin Pharmacol 2010; 70: 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Birkett D, Brösen K, Cascorbi I, Gustafsson LL, Maxwell S, Rago L, et al. Clinical pharmacology in research, teaching and health care: Considerations by IUPHAR, the International Union of Basic and Clinical Pharmacology. Basic Clin Pharmacol Toxicol 2010; 107: 531–559. [DOI] [PubMed] [Google Scholar]
- 12. Wallerstedt SM, Rosenborg S. Characteristics and apprehensions of clinical pharmacologists in Swedish healthcare – a questionnaire study. Eur J Clin Pharmacol 2013; 69 (Suppl 1): 95–99. [DOI] [PubMed] [Google Scholar]
- 13. Swedish Council on Health Technology Assessment . Checklist for quality assessment of observation studies [Mall för kvalitetsgranskning av observationsstudier]. In: Utvärdering av metoder i hälso‐ och sjukvården: en handbok, ed. SBU. Stockholm: Statens beredning för medicinsk utvärdering (SBU), 2014; 3:1–3:13.
- 14. Lund LH, Benson L, Dahlström U, Edner M, Friberg L. Association between use of beta‐blockers and outcomes in patients with heart failure and preserved ejection fraction. JAMA 2014; 312: 2008–2018. [DOI] [PubMed] [Google Scholar]
- 15. Chang Z, Lichtenstein P, D'Onofrio BM, Sjölander A, Larsson H. Serious transport accidents in adults with attention‐deficit/hyperactivity disorder and the effect of medication: a population‐based study. JAMA Psychiat 2014; 71: 319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen Q, Sjölander A, Runeson B, D'Onofrio BM, Lichtenstein P, Larsson H. Drug treatment for attention‐deficit/hyperactivity disorder and suicidal behaviour: register based study. BMJ 2014; 348: g3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ekström MP, Hermansson AB, Ström KE. Effects of cardiovascular drugs on mortality in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 187: 715–720. [DOI] [PubMed] [Google Scholar]
- 18. Ekström N, Cederholm J, Zethelius B, Eliasson B, Fhärm E, Rolandsson O, et al. Aspirin treatment and risk of first incident cardiovascular diseases in patients with type 2 diabetes: an observational study from the Swedish National Diabetes Register. BMJ Open 2013; 3: e002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fazel S, Zetterqvist J, Larsson H, Långstrom N, Lichtenstein P. Antipsychotics, mood stabilisers, and risk of violent crime. Lancet 2014; 384: 1206–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Holmes MD, Olsson H, Pawitan Y, Holm J, Lundholm C, Andersson TM, et al. Aspirin intake and breast cancer survival – a nation‐wide study using prospectively recorded data in Sweden. BMC Cancer 2014; 14: 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jonsson F, Yin L, Lundholm C, Smedby KE, Czene K, Pawitan Y. Low‐dose aspirin use and cancer characteristics: a population‐based cohort study. Br J Cancer 2013; 109: 1921–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Landfeldt E, Ström O, Robbins S, Borgström F. Adherence to treatment of primary osteoporosis and its association to fractures – the Swedish Adherence Register Analysis (SARA). Osteoporos Int 2012; 23: 433–443. [DOI] [PubMed] [Google Scholar]
- 23. Lichtenstein P, Halldner L, Zetterqvist J, Sjölander A, Serlachius E, Fazel S, et al. Medication for attention deficit‐hyperactivity disorder and criminality. N Engl J Med 2012; 367: 2006–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Själander S, Själander A, Svensson PJ, Friberg L. Atrial fibrillation patients do not benefit from acetylsalicylic acid. Europace 2014; 16: 631–638. [DOI] [PubMed] [Google Scholar]
- 25. Wang Q, Ljung R, Lagergren J, Lu Y. Prognosis of concomitant users of clopidogrel and proton‐pump inhibitors in a high‐risk population for upper gastrointestinal bleeding. BMC Pharmacol Toxicol 2014; 15: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bodén R, Brandt L, Kieler H, Andersen M, Reutfors J. Early non‐adherence to medication and other risk factors for rehospitalization in schizophrenia and schizoaffective disorder. Schizophr Res 2011; 133: 36–41. [DOI] [PubMed] [Google Scholar]
- 27. Friberg L, Rosenqvist M, Lip GY. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation 2012; 125: 2298–2307. [DOI] [PubMed] [Google Scholar]
- 28. Jonasson C, Hatlebakk JG, Lundell L, Kouri JP, Andersen M, Granath F. Association between adherence to concomitant proton pump inhibitor therapy in current NSAID users and upper gastrointestinal complications. Eur J Gastroenterol Hepatol 2013; 25: 531–538. [DOI] [PubMed] [Google Scholar]
- 29. Welin L, Wilhelmsen L, Björnberg A, Odén A. Aspirin increases mortality in diabetic patients without cardiovascular disease: a Swedish record linkage study. Pharmacoepidemiol Drug Saf 2009; 18: 1143–1149. [DOI] [PubMed] [Google Scholar]
- 30. Califf RM, Sugarman J. Exploring the ethical and regulatory issues in pragmatic clinical trials. Clin Trials 2015; 12: 436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kalkman S, van Thiel GJ, Grobbee DE, van Delden JJ. Pragmatic randomized trials in drug development pose new ethical questions: a systematic review. Drug Discov Today 2015; 20: 856–862. [DOI] [PubMed] [Google Scholar]
- 32. Staa TP, Goldacre B, Gulliford M, Cassell J, Pirmohamed M, Taweel A, et al. Pragmatic randomised trials using routine electronic health records: putting them to the test. BMJ 2012; 344: e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Emilsson L, Lindahl B, Koster M, Lambe M, Ludvigsson JF. Review of 103 Swedish Healthcare Quality Registries. J Intern Med 2015; 277: 94–136. [DOI] [PubMed] [Google Scholar]
- 34. Ekström N, Schiöler L, Svensson AM, Eeg‐Olofsson K, Miao Jonasson J, Zethelius B, et al. Effectiveness and safety of metformin in 51 675 patients with type 2 diabetes and different levels of renal function: a cohort study from the Swedish National Diabetes Register. BMJ Open 2012; 2: e001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Eliasson B, Svensson AM, Miftaraj M, Jonasson JM, Eeg‐Olofsson K, Sundell KA, et al. Clinical use and effectiveness of lipid lowering therapies in diabetes mellitus – an observational study from the Swedish National Diabetes Register. PLoS One 2011; 6: e18744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sturmer T, Joshi M, Glynn RJ, Avorn J, Rothman KJ, Schneeweiss S. A review of the application of propensity score methods yielded increasing use, advantages in specific settings, but not substantially different estimates compared with conventional multivariable methods. J Clin Epidemiol 2006; 59: 437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hallas J. Drug utilization statistics for individual‐level pharmacy dispensing data. Pharmacoepidemiol Drug Saf 2005; 14: 455–463. [DOI] [PubMed] [Google Scholar]
- 38. Suissa S. Immortal time bias in pharmaco‐epidemiology. Am J Epidemiol 2008; 167: 492–499. [DOI] [PubMed] [Google Scholar]
- 39. Coupland C, Hill T, Morriss R, Moore M, Arthur A, Hippisley‐Cox J. Antidepressant use and risk of cardiovascular outcomes in people aged 20 to 64: cohort study using primary care database. BMJ 2016; 352: i1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Froud R, Bjorkli T, Bright P, Rajendran D, Buchbinder R, Underwood M, et al. The effect of journal impact factor, reporting conflicts, and reporting funding sources, on standardized effect sizes in back pain trials: a systematic review and meta‐regression. BMC Musculoskelet Disord 2015; 16: 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pang WK, Yeter KC, Torralba KD, Spencer HJ, Khan NA. Financial conflicts of interest and their association with outcome and quality of fibromyalgia drug therapy randomized controlled trials. Int J Rheum Dis 2015; 18: 606–615. [DOI] [PubMed] [Google Scholar]
- 42. Ambring A, Björholt I, Lesén E, Stierner U, Odén A. Treatment with sorafenib and sunitinib in renal cell cancer: a Swedish register‐based study. Med Oncol 2013; 30: 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Björck S, Palaszewski B, Friberg L, Bergfeldt L. Atrial fibrillation, stroke risk, and warfarin therapy revisited: a population‐based study. Stroke 2013; 44: 3103–3108. [DOI] [PubMed] [Google Scholar]
- 44. Forslund T, Wettermark B, Wändell P, von Euler M, Hasselström J, Hjemdahl P. Risks for stroke and bleeding with warfarin or aspirin treatment in patients with atrial fibrillation at different CHA(2)DS(2)VASc scores: experience from the Stockholm region. Eur J Clin Pharmacol 2014; 70: 1477–1485. [DOI] [PubMed] [Google Scholar]
- 45. Ljung R, Sennerstam R, Mattsson F, Auer G, Lagergren J. Anticoagulant medication at time of needle biopsy for breast cancer in relation to risk of lymph node metastasis. Int J Cancer 2014; 135: 238–241. [DOI] [PubMed] [Google Scholar]
- 46. Ringbäck Weitoft G, Berglund M, Lindström EA, Nilsson M, Salmi P, Rosén M. Mortality, attempted suicide, re‐hospitalisation and prescription refill for clozapine and other antipsychotics in Sweden – a register‐based study. Pharmacoepidemiol Drug Saf 2014; 23: 290–298. [DOI] [PubMed] [Google Scholar]
- 47. Wahlgren T, Harmenberg U, Sandström P, Lundstam S, Kowalski J, Jakobsson M, et al. Treatment and overall survival in renal cell carcinoma: a Swedish population‐based study (2000–2008). Br J Cancer 2013; 108: 1541–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]


