Abstract
Fibronectin is a multidomain protein secreted by various cell types. It forms a network of fibers within the extracellular matrix and impacts intracellular processes by binding to various molecules, primarily integrin receptors on the cells. Both the presence of several isoforms and the ability of the various domains and isoforms to bind to a variety of integrins result in a wide range of effects. In vivo findings suggest that fibronectin isoforms produced by the osteoblasts enhance their differentiation. Here we report that the isoform characterized by the presence of extradomain A activates α4β1 integrin and augments osteoblast differentiation. In addition, the isoform containing extradomain B enhances the binding of fibronectin through the RGD sequence to β3-containing integrin, resulting in increased mineralization by and differentiation of osteoblasts. Our study thus reveals novel functions for two fibronectin isoforms and the mediating receptors in osteoblast differentiation.
Keywords: differentiation, fibronectin, integrin, osteoblast, signaling
Introduction
Osteoblasts represent a unique mammalian cell type. They lay down an extracellular matrix that they subsequently mineralize to form bone. This function is tightly regulated through various mechanisms, including interaction with a cytokine-rich environment and the systemic regulation of osteoblasts by various hormones (1). The composition of the extracellular matrix of bone may itself control functional aspects of the osteoblasts (2, 3). This is achieved by binding to cell surface receptors on the osteoblasts (4). A major type of cell surface receptors that transfer signals from the matrix into the cell are the integrins, which are heterodimeric transmembrane receptors, each consisting of an α and a β subunit. Due to the presence of 18 different α and 8 different β subunits, 24 integrin pairs with varied ligand-binding properties have been described in mice and humans. Depending on the integrin member involved or the ligand attached, diverse intracellular signals are activated, leading to a large spectrum of different intracellular effects (5).
Fibronectin is a ubiquitous and large extracellular matrix protein in bone that can bind to itself and to collagen to form a network (6). It contains several domains that can become ligated to various integrin dimers (7). In addition, several splice variants have been described, and the possibility of posttranslational modifications further increases the heterogeneity of the binding receptors. The function of two alternatively spliced extradomains A and B (EDA and EDB)3 is still being investigated. Interestingly, the EDA domain can directly bind to α4β1 or α9β1 integrin, and its presence enhances the binding of the RGD sequence to α5β1 integrin, the classical fibronectin receptor (8–10). In contrast, EDB has not been studied as extensively, but it was shown to require αvβ3 integrin to enhance phagocytosis (11).
The circulating isoform of fibronectin, which lacks the EDA and EDB domains, is produced by the liver. We had shown that it improves the material properties of bone matrix but is unable to affect osteoblast differentiation. In contrast, the osteoblasts differentiate in response to the fibronectin that they themselves produce (2). We have shown that the osteoblasts produce isoforms, in which we identified both extradomains: EDA and EDB. The aim of this study was therefore to elucidate the effects of the presence of EDA and EDB on osteoblasts and the receptors mediating these effects. The ultimate goal is the discovery of new strategies for osteoblast modulation.
Results
The presence of the extradomains A and B enhances osteoblast differentiation
The two extradomains EDA and EDB are produced by osteoblasts (2). We therefore generated expression constructs consisting of the whole fibronectin cDNA and including either the EDA (FN-EDA) or the EDB domain (FN-EDB) (Fig. 1A). As a control, we used plasma fibronectin cDNA (FN), which lacks both extradomains (12). All constructs used contain the variable region (13).
Figure 1.
The expression of FN-EDA or FN-EDB in osteoblasts enhances their differentiation. A, the three constructs used for transfection containing EDA, EDB, or neither. VR, the so-called variable region present in all constructs used. B, microscopic evaluation of cells confirms the expression of GFP, and flow cytometry shows a transfection efficiency of 36%. Primary osteoblasts were transfected with a vector expressing GFP and evaluated after 2 days in culture (n = 18/9 in 2 experiments). C, transfection of FN-EDA in wild-type osteoblasts enhances osteoblast function as shown by mineralization (quantification of von Kossa stained area), alkaline phosphatase protein activity in cell lysates and conditioned medium, and osteocalcin mRNA expression (biological replicates of von Kossa stain: n = 16/19 in 8 experiments; alkaline phosphatase in cell lysates: n = 16/19 in 8 experiments; alkaline phosphatase in medium: n = 16/7 in 5 experiments; and osteocalcin mRNA expression: n = 19/25 in 8 experiments). D, the expression of FN-EDB in differentiating primary osteoblasts (by transfection) induced mineralization, as shown by von Kossa staining, alkaline phosphatase activity, and osteocalcin mRNA expression (biological replicates of von Kossa stain: n = 18/19 in 8 experiments; alkaline phosphatase in cell lysates: n = 19/20 in 8 experiments; alkaline phosphatase in medium: n = 16/9 in 5 experiments; and osteocalcin mRNA expression: n = 17/27 in 8 experiments). E, evaluation of conditioned medium from transfected cells confirmed increases in various isoforms of fibronectin. Transfection of primary osteoblasts with a plasmid containing a fibronectin construct lacking both EDA and EDB domains (FN) increased total fibronectin in conditioned medium over the duration of differentiation compared with non-transfected cells (CT). Transfection with a plasmid containing EDA (FN-EDA) increased expression of EDA-containing fibronectin, whereas transfection with a plasmid containing EDB (FN-EDB) increased expression of EDB-containing fibronectin compared with the FN construct. Following transfection, primary calvarial osteoblasts were cultured in mineralizing conditions for 2 weeks in the presence of 10% FCS depleted of fibronectin, and differentiation was evaluated at the end of the experiments. Fibronectin concentrations were evaluated by ELISA for total fibronectin, EDA-containing fibronectin (EDA), and EDB-containing fibronectin (EDB) (biological replicates of total fibronectin ELISA: CT versus FN, n = 7–9/7–10 each time point in 4 experiments; EDA ELISA: n = 8–9 FN/8–10 EDA each time point; and EDB ELISA: n = 6–10 FN/6–8 EDB each time point, both in 3 experiments). F, deletion of fibronectin in early osteoblasts using the osterix promoter attached to Cre in homozygous fibronectin floxed mice (FN cKO) results in diminished fibronectin release in the medium (biological replicates: n = 12/12 in 3 experiments). G, transfection of FN-EDA and FN-EDB in fibronectin-depleted osteoblasts from FN cKO mice confirmed the increase in osteocalcin mRNA expression 3 days after transfection (biological replicates: n = 4/4/4 in 1 experiment). Freshly isolated newborn calvarial osteoblasts were transiently transfected with the fibronectin constructs shown in A and differentiated for 2–3 weeks (or 3 days in the case of FN cKO osteoblasts shown in F and G) in mineralizing medium, after which alkaline phosphatase enzymatic activity was assayed in medium and cell lysates. Wells were stained using von Kossa staining, and quantification of the stained area was performed with the help of ImageJ. Cell lysates were also obtained for mRNA, and osteocalcin was determined by qPCR and normalized to HPRT. Results are expressed as mean ± S.D. (error bars). Bars, 200 μm in B and 1 mm in C and D. *, p < 0.05; **, p < 0.01; ***, p < 0.005.
To evaluate the effect of the presence of either the EDA or the EDB domain on osteoblast differentiation, primary osteoblasts isolated from the calvariae of newborn mice were transfected with either FN-EDA, FN-EDB, or the control construct lacking both domains (FN). To determine transfection efficiency, the vector containing green fluorescent protein (GFP) was used. Expression of GFP was detected in cultured cells, and a transfection efficiency of 36% was confirmed by flow cytometry (Fig. 1B). The presence of either EDA or EDB domain augmented mineralization by the osteoblasts, as determined by the nodule formation assay, whereby osteoblasts are cultured for 2–3 weeks in mineralizing medium and stained using the von Kossa method. Enhanced osteoblast differentiation was determined by increased alkaline phosphatase protein activity, a marker of early osteoblast differentiation present in the cell lysates or released in the medium, as well as higher osteocalcin mRNA expression, a late marker of osteoblast differentiation (Fig. 1, C and D). The amount of the relevant fibronectins released in the medium was increased: total fibronectin for the FN construct in comparison with non-transfected control (CT) cells as well as EDA-containing fibronectin for the FN-EDA construct and EDB-containing fibronectin for the FN-EDB construct, both in comparison with the FN construct (Fig. 1E).
To examine this further, we deleted fibronectin in early osteoblasts. Newborn fibronectin floxed homozygous mice carrying a CRE recombinase under the control of the osterix promoter active in early osteoblasts (Osx-Cre/fibronectinfloxed/floxed mice = FN cKO) were used to obtain primary calvarial osteoblasts lacking fibronectin. These were compared with osteoblasts from littermates not carrying osterix-Cre (CT) (14–16). We verified deletion by measuring fibronectin in the conditioned medium. Although the decrease is limited, it is expected, based on the low expression of osterix in primary calvarial osteoblasts (roughly 5–10% by flow cytometry based on fluorescent protein expression in the promoter) (Fig. 1F) (17). Transfection of these cells with either the FN-EDA or FN-EDB constructs enhanced osteocalcin mRNA expression already 3 days after transfection compared with the control fibronectin construct (FN), again indicating boosted differentiation of osteoblasts in the presence of EDA- or EDB-containing fibronectin, as was the case in wild-type osteoblasts shown in Fig. 1, C and D (Fig. 1G).
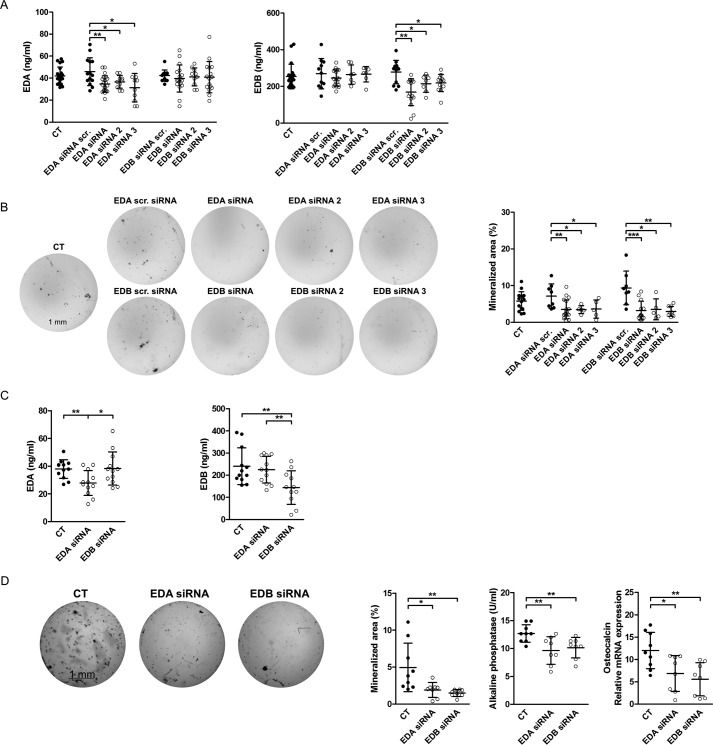
Next, we selectively silenced EDA- or EDB-containing fibronectin in wild-type osteoblasts by transfection with siRNA directed against the EDA or the EDB domain as described (18). Three constructs were first tested for each, and the decrease in the expression of the isoforms was confirmed in the conditioned medium (Fig. 2A) (measured by ELISA using specific antibodies directed against the EDA or the EDB domain). Nodule formation was diminished in all EDA siRNA and EDB siRNA constructs (Fig. 2B). Repeat experiments using the first siRNA constructs for each, EDA and EDB, were performed. After 72 h in culture, protein levels were only diminished for EDA-containing fibronectin if cells were treated with EDA siRNA and for EDB-containing fibronectin if cells were treated with EDB siRNA (Fig. 2C). Silencing either EDA- or EDB-containing fibronectin confirmed suppression of osteoblast differentiation in vitro (Fig. 2D), as evidenced by nodule formation, alkaline phosphatase protein activity, or osteocalcin mRNA expression in line with the stimulatory effect of either domain on osteoblasts.
Figure 2.
Knockdown of EDA- or EDB-containing fibronectin diminishes osteoblast differentiation. A, a decrease in secreted EDA-containing fibronectin is detected in EDA siRNA-transfected osteoblasts or EDB-containing fibronectin protein in EDB siRNA-transfected osteoblasts, respectively (biological replicates of EDA: n = 21/14/22/10/10/13/18/10/16; EDB: n = 20/11/19/7/7/12/15/7/12, both in 4 experiments). B, suppressed differentiation of primary osteoblasts transfected with EDA or EDB siRNAs (biological replicates of von Kossa stain: n = 15/8/18/5/5/8/13/5/9 in 4 experiments). Freshly isolated newborn calvarial osteoblasts were transiently transfected with siRNAs directed against the EDA or EDB domain and differentiated for 2–3 weeks in mineralizing medium. At the end of the experiments, wells were stained using von Kossa stain, and quantification of the stained area was performed with the help of ImageJ. C, a decrease in secreted EDA- or EDB-containing fibronectin protein is detected in EDA or EDB siRNA-transfected osteoblasts, respectively (biological replicates: n = 12/12/11–12 in 3 experiments). D, suppressed differentiation of primary osteoblasts transfected with EDA or EDB siRNA (biological replicates of von Kossa stain, alkaline phosphatase activity, and osteocalcin mRNA: n = 9/8/8 in 2 experiments). Freshly isolated newborn calvarial osteoblasts were transiently transfected with siRNAs against the EDA or EDB domain and differentiated for 2–3 weeks in mineralizing medium, after which alkaline phosphatase enzymatic activity was assayed in medium. Wells were stained using von Kossa staining, and quantification of the stained area was performed with the help of ImageJ. Cell lysates were also obtained for mRNA, and osteocalcin was determined by qPCR and normalized to HPRT. Results are expressed as mean ± S.D. (error bars). Bars, 1 mm. *, p < 0.05; **, p < 0.01.
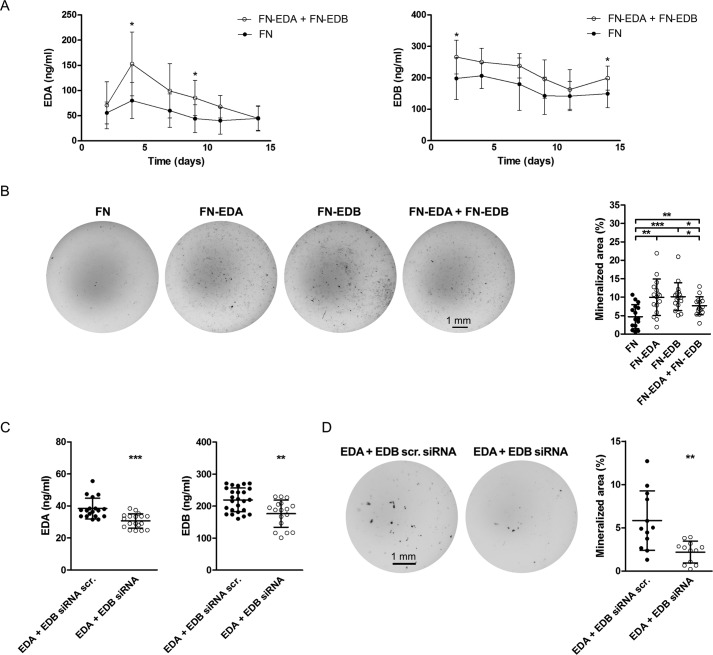
To determine whether the expression of both the EDA- and the EDB-containing constructs at the same time will lead to further enhancement of differentiation, we co-transfected both and compared the nodules formed with FN-, EDA-, and EDB-transfected cells. Evaluation of EDA- and EDB-containing fibronectin in the medium showed an increase in their production (significant at day 4 for EDA and at day 2 for EDB) (Fig. 3A). The combination of both, however, failed to further enhance nodule formation beyond the increase achieved with the expression of either EDA- or EDB-containing fibronectin alone (Fig. 3B). In line with these findings, silencing both simultaneously using EDA siRNA together with EDB siRNA led to a decrease in nodule formation, but the decrease was not more pronounced than silencing each alone (Fig. 3, C and D). A summary of changes in the relevant fibronectin isoforms and the consequences for mineralization is shown for overexpression and knockdown (Tables 1 and 2). The failure of overexpression of both or depletion of both isoforms in exerting additive effects could be due to the limited overexpression and depletion obtained when both isoforms were modulated simultaneously, as shown in Fig. 3 (A–D) and summarized in Tables 1 and 2. Thus, the presence of either domain, EDA or EDB, seems to enhance osteoblast differentiation.
Figure 3.
The expression of FN-EDA + FN-EDB together in osteoblasts enhances their differentiation. A, transfection with a plasmid containing the EDA domain and a plasmid containing the EDB domain increased expression of EDA- and EDB-containing fibronectin. Fibronectin concentrations were evaluated by ELISA for EDA-containing fibronectin (EDA) and EDB-containing fibronectin (EDB) (biological replicates of EDA: n = 8–9 FN/4–6 EDA; EDB: n = 6–10 FN/5–6 EDB, each time point in 3 experiments). B, the expression of both FN-EDA and FN-EDB simultaneously increased nodule formation (biological replicates: n = 16/16/16/16 in 4 experiments). Following transfection, primary calvarial osteoblasts were cultured in mineralizing conditions with 10% FCS depleted of fibronectin for 2 weeks, and differentiation was evaluated at the end of the experiments. Wells were stained using von Kossa staining, and quantification of the stained area was performed with the help of ImageJ. Knockdown of both EDA- and EDB-containing fibronectin simultaneously diminishes osteoblast differentiation. C, a decrease in secreted EDA- or EDB-containing fibronectin protein is detected in EDA + EDB siRNA-transfected osteoblasts (biological replicates for EDA: n = 18/18; EDB: n = 25/17 in 4 experiments). D, suppressed differentiation of primary osteoblasts transfected with EDA + EDB siRNA (von Kossa: n = 12/12 in 2 experiments). Freshly isolated newborn calvarial osteoblasts were transiently transfected with siRNAs against the EDA and/or EDB domain and differentiated for 2–3 weeks in mineralizing medium. Wells were stained using von Kossa staining, and quantification of the stained area was performed with the help of ImageJ. Results are expressed as mean ± S.D. (error bars). Bars, 1 mm. *, p < 0.05; **, p < 0.01; ***, p < 0.005.
Table 1.
Summary of overexpression data for the various isoforms and the consequences for in vitro mineralization
Changes in the levels of the relevant fibronectin isoforms are summarized numerically on day 4 of overexpression together with the associated changes in mineralization at the end of in vitro differentiation. (Summary of data presented in Figs. 1 (C–E) and 3 (A and B).) Note that only EDB protein concentration in the conditioned medium in the double transfection is not significantly increased at day 4, whereas all other values are.
Table 2.
Summary of knockdown data for the various isoforms and the consequences for in vitro mineralization
Changes in the levels of the relevant fibronectin isoforms are summarized numerically on day 3 of depletion using siRNA, together with the associated changes in mineralization at the end of in vitro differentiation. (Summary of data presented in Figs. 2 (C and D) and 3 (C and D)). All changes are statistically significant.
EDA- and EDB-containing fibronectin activate intracellular molecules associated with integrin signaling
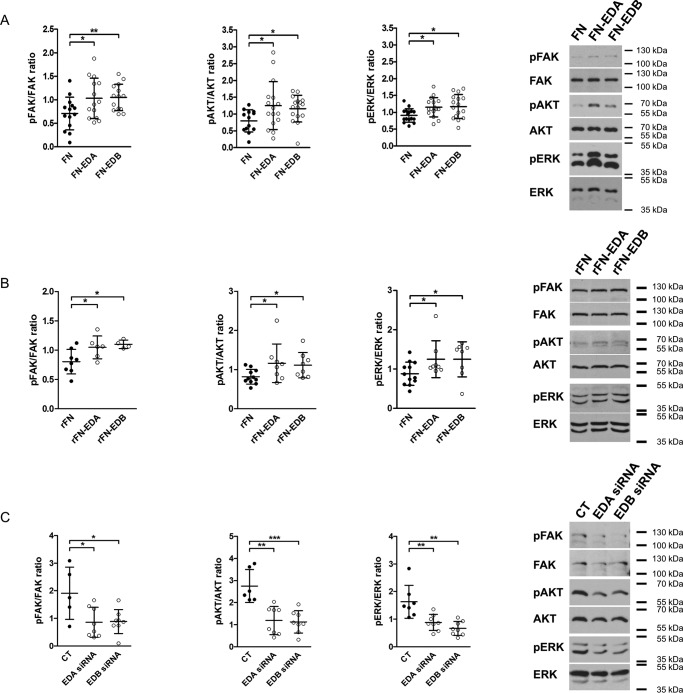
Because the presence of EDA in fibronectin can lead to the activation of distinct integrins (8, 19–21), we asked whether the stimulatory effect of the presence of EDA on osteoblast differentiation might be mediated through integrin signaling. Indeed, transfection of osteoblasts with FN-EDA, FN-EDB, and FN constructs followed by culture in fibronectin-depleted fetal calf serum (FCS) for 2 days was associated with increased phosphorylation of FAK, AKT, and ERK, suggesting enhanced integrin-mediated signaling (Fig. 4A) (5). Acute stimulation with recombinant FN-EDA (rFN-EDA) or rFN-EDB also increased phosphorylation of FAK, AKT, and ERK to a similar degree compared with the control construct lacking both domains (rFN) (Fig. 4B) (55). Finally, silencing EDA- or EDB-containing fibronectin (by transfecting primary osteoblasts with siRNA directed against the EDA or EDB domain) reduced phosphorylated FAK, AKT, and ERK (Fig. 4C) evaluated after 2 days of culture in medium containing fibronectin-depleted FCS. Thus, the presence of FN-EDA or FN-EDB results in activation of integrin signaling. Taken together, these data suggest that both EDA- and EDB-containing fibronectin trigger integrin signaling
Figure 4.
EDA- or EDB-containing fibronectin increase integrin signaling, whereas silencing them diminishes signaling. A, expression of FN-EDA or FN-EDB in osteoblasts enhances FAK, AKT, and ERK phosphorylation compared with cells transfected with the control construct lacking the EDA and the EDB domains (FN) (biological replicates for FAK: n = 14/14/14 in 3 experiments; AKT: n = 13/16/16 in 6 experiments; and ERK: n = 16/16/16 in 5 experiments). B, exposing osteoblasts to rFN-EDA or rFN-EDB similarly enhances FAK, AKT, and ERK phosphorylation compared with the control construct (rFN) lacking both EDA and EDB domains (biological replicates for FAK: n = 8/6/4; AKT: n = 11/8/8; and ERK: n = 12/8/8 in 4 experiments). Cells were exposed to 200 ng/ml recombinant proteins produced as outlined under “Experimental procedures” for 15 min after culture in serum-free medium for 8 h. C, silencing EDA- or EDB-containing fibronectin using siRNA diminished integrin-mediated signaling (biological replicates for FAK: n = 5/8/8; AKT: n = 6/8/8; and ERK: n = 7/8/8 each in 2 experiments). Osteoblasts were transfected and cultured for 2 days in medium containing fibronectin-depleted FCS, and cell lysates were collected in A and C. Results are expressed as mean ± S.D. (error bars). *, p < 0.05; **, p < 0.01; ***, p < 0.005.
Expression of integrins relevant for EDA-FN and EDB-FN effects on osteoblasts
Because both FN-EDA and FN-EDB enhanced osteoblast differentiation and activated molecules classically associated with integrin-mediated signaling, we sought to examine the expression of integrin subunits on osteoblasts that have been associated with the presence of these two domains in fibronectin both before and after differentiation. Our aim was to narrow our search for the mediating receptor. The EDA domain can bind directly to either α4β1, α9β1, or possibly α4β7 integrins, whereas EDB-containing fibronectin binds to αvβ3 integrin (8, 11, 20, 22, 23). Through the RGD sequence, all fibronectin isoforms can bind to several other integrins, including α5β1 (19), but the presence of EDA in fibronectin enhances the binding of RGD to α5β1 (9, 21). We therefore evaluated the mRNA and protein expression of the relevant integrin subunits in freshly isolated wild-type (CT) osteoblasts and compared these with differentiated osteoblasts cultured in mineralizing medium for 2 weeks.
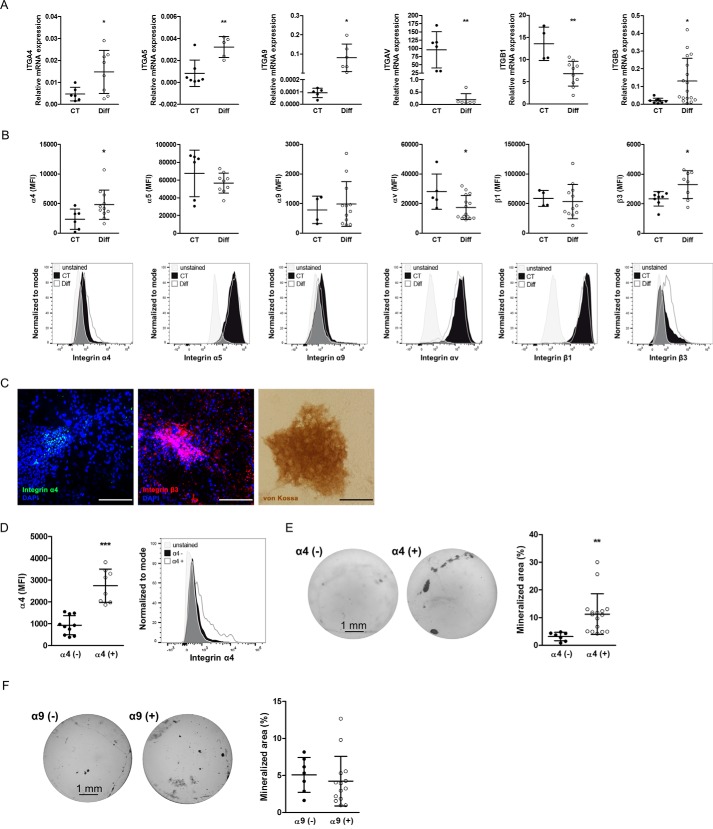
We could not detect any protein expression of β7 on the surface of osteoblasts by flow cytometry or immunoblotting (24). Thus, we present in Fig. 5 (A and B) the integrin expression profile for the other relevant integrin subunits affected by the presence of EDA or EDB. In the top panel, we show mRNA expression for α4, α5, α9, αv, β1, and β3 in osteoblasts at baseline and after differentiation in mineralizing conditions. Differentiation was associated with an increase of α4, α5, α9, and β3 mRNA and a decrease in αv and β1 mRNA expression (Fig. 5A). By flow cytometry, we found an increase in the mean fluorescence intensity (MFI) of α4 or β3 after differentiation, a decrease in αv, and no change in α5, α9, and β1 expression (Fig. 5B, top row, examples are shown in the bottom row). To determine the localization of α4 and β3 integrin in differentiated osteoblast cultures, we cultured wild-type osteoblasts for 2 weeks in mineralizing medium and stained the wells at the end of the experiments for α4 and β3 integrin as well as using von Kossa stain. α4 integrin was detected within the nodules, whereas β3 was mostly within but was also expressed outside the nodules (Fig. 5C). Taken together, these data suggest that α4- as well as β3-containing integrins on the osteoblasts increase with osteoblast differentiation.
Figure 5.
Expression profile of integrin subunits relevant for EDA- and EDB-containing fibronectin on osteoblasts at baseline and after differentiation. A, mRNA expression of integrins α4, α5, α9, αv, β1, and β3 before and after differentiation. Primary newborn calvarial osteoblasts were cultured for 2–3 weeks in mineralizing medium and compared with cells cultured in medium without additives (biological replicates for α4: n = 6/8 in 2 experiments; α5: n = 7/5 in 1 experiment; α9: n = 5/6 in 2 experiments; αv: n = 6/6 in 1 experiment; β1: n = 4/10 in 3 experiments; and β3: n = 9/17 in 2 experiments). B, flow cytometry of osteoblasts stained for the mentioned integrin subunits showing the MFI in the first row and examples of the measurements in the second row (corrected to show equivalent numbers of cells), with the gray peaks representing the autofluorescence of the cells (biological replicates for α4: n = 6/11; α5: n = 6/9; α9: n = 4/12; αv: n = 5/15; β1: n = 4/12; β3: n = 8/8 in 3 experiments). C, mineralized nodules stained against α4 integrin (green), β3 integrin (red), and von Kossa confirm the presence of α4 within the nodules and β3 mostly within, but also around, the nodules. A sister well was stained against von Kossa to confirm mineralization. Osteoblasts mineralized for 2–3 weeks were stained against α4 and β3 and with von Kossa. Nuclei were stained with DAPI. Bars, 100 μm. D, osteoblasts were separated based on their α4 integrin subunit expression. Following isolation of α4-enriched and α4-depleted cells and culture for 7 days, flow cytometry was repeated and revealed persistent low expression of α4 in the depleted fraction and higher expression in the α4-enriched fraction (biological replicates: n = 10/8 in 4 experiments). E, α4-enriched osteoblasts (α4(+)) show enhanced differentiation compared with α4-depleted cells (α4(−)). Shown are examples of von Kossa staining and quantification of the stained area (biological replicates: n = 7/16 in 3 experiments). F, no differences in mineralization are found between osteoblasts that express α9 (α9(+)) and those that do not (α9(−)) (biological replicates: n = 7/14 in 2 experiments). Primary osteoblasts were stained for α4 or α9 and separated into two populations based on the expression of α4 or α9 and then differentiated for 2–3 weeks in mineralizing medium, stained by von Kossa, and evaluated. Results are expressed as mean ± S.D. (error bars). *, p < 0.05; **, p < 0.01; ***, p < 0.005.
EDA-containing fibronectin stimulates osteoblast differentiation by activating integrin α4β1
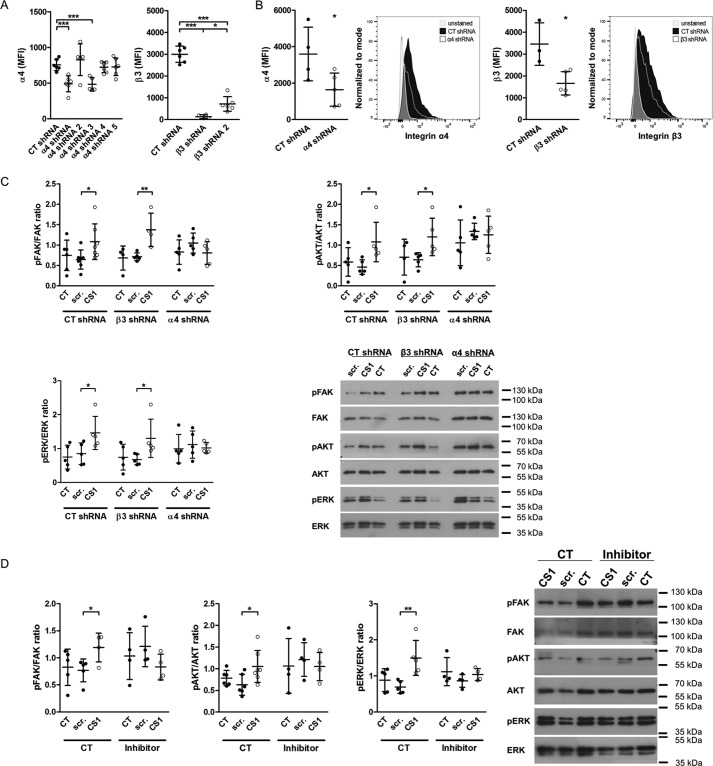
Based on enhanced α4 integrin subunit expression in differentiated osteoblasts and the ability of EDA to bind to α4-containing integrins, we sought to determine whether α4-containing integrin mediates EDA-fibronectin effects. However, because expression of α4 integrin was low, we decided to first evaluate whether α4 indeed played any role during osteoblast differentiation. For this, we performed two sets of experiments. We first separated freshly isolated wild-type osteoblasts based on α4 surface expression into a population enriched with α4 expression (α4(+)) and a population depleted of α4-expressing cells (α4(−)). Roughly 10% of osteoblasts were enriched for α4 expression (α4(+)). Both cell populations were then cultured in mineralizing medium. Expression remained different between both populations after 7 days in culture, and the α4(+) cells showed increased mineralization compared with the α4(−) population (Fig. 5, D and E). Because EDA can bind directly to α9β1, we repeated the experiment with α9(+) and α9(−) cells. In contrast to cells expressing α4, no difference in mineralization could be detected between α9(+) and α9(−) osteoblasts (Fig. 5F). These data thus suggest that the presence of α4 on osteoblasts is associated with enhanced osteoblast differentiation. We next aimed to use a peptide of 25 amino acids called CS1, representing a fragment of the variable region of fibronectin and containing a sequence (LDV) that binds to α4-containing integrins (19, 25), but needed to confirm that it indeed acts through α4 integrin (56). We therefore tested various shRNA constructs to delete both α4 and β3 integrin in the murine osteoblastic cell line MC3T3 and evaluated the success of deletion by flow cytometry of the expression of the two integrin subunits (Fig. 6A). Cells were transduced with the best constructs to deplete α4 or β3 integrins. This resulted in decreased expression by 56% for α4 and 42% for β3 (Fig. 6B). Stimulation with CS1 or the scrambled peptide for 15 min failed to enhance the phosphorylation of FAK, AKT, and ERK only in the α4-depleted cells. This confirms the need for α4 to mediate CS1 effects on the cells (Fig. 6C). Adding this peptide to osteoblasts in vitro enhances osteoblast differentiation (Fig. 7A).
Figure 6.
CS1 requires α4 integrin to result in phosphorylation of integrin signaling molecules. A, various constructs for shRNA directed against α4 and β3 integrin were tested to evaluate which one best depletes the expression of the relevant integrin subunit. Depletion was evaluated by flow cytometry of cells transduced with the respective shRNA construct and stained against the integrin subunits (biological replicates: n = 6/6/5/5/6/6 and 6/4/6, both in 2 experiments). B, the best shRNAs were used to deplete α4 and β3 integrin. Cells were transduced, and depletion was confirmed by flow cytometry for the stained subunits the next day (n = 4/5 and 3/5 in 2 experiments). Representative histograms are shown. C, CS1 fails to enhance phosphorylation of FAK, AKT, and ERK in α4-depleted cells but increases pFAK, pAKT, and pERK in β3-depleted osteoblasts or CT cells (biological replicates for FAK: 6/7/7/4/5/4/5/5/5 in 3 experiments; AKT: n = 5/5/5/4/5/5/5/5/5 in 2 experiments; and ERK: n = 5/5/5/5/5/5/5/5/5 in 2 experiments). D, the inhibitor for α4β1 prevents CS1 from increasing pFAK, pAKT, and pERK. This confirms its ability to inhibit α4-containing integrins (biological replicates for FAK: n = 5/5/4/4/4/4 in 2 experiments; AKT: n = 6/6/7/4/4/4 in 3 experiments; and ERK: n = 5/5/5/4/4/4 in 2 experiments). Osteoblasts were cultured overnight without serum. The inhibitor was added at 1.8 nm together with CS1 at 25 μg/ml for 15 min. Results are expressed as mean ± S.D. (error bars). *, p < 0.05; **, p < 0.01; ***, p < 0.005.
Figure 7.
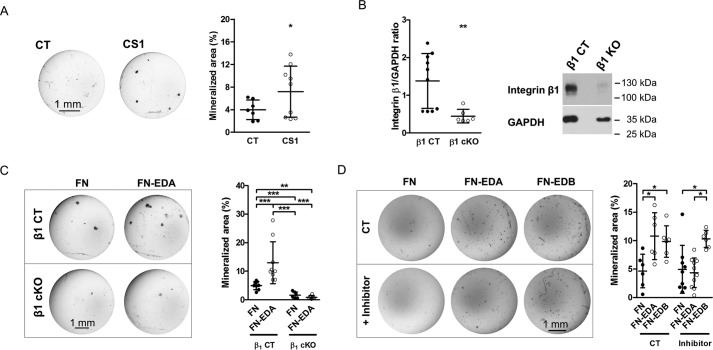
Enhanced osteoblast mineralization by EDA-containing fibronectin is mediated by α4β1 integrin. A, the CS1 peptide, which binds α4β1, enhances osteoblast mineralization after 2–3 weeks in mineralizing medium. CS1 and scrambled peptide were added at 25 μg/ml with every medium change (biological replicates: n = 7/9 in 3 experiments). B, deletion of β1 integrin in osteoblasts using the osterix promoter attached to Cre in mice homozygous for floxed β1 integrin decreases β1 expression as shown by immunoblotting (biological replicates: n = 10/6 in 3 experiments). Primary osteoblasts were evaluated 3 days after isolation. C, whereas transfection of wild-type osteoblasts with FN-EDA enhances differentiation, FN-EDA fails to increase mineralization in the absence of β1 integrin. However, deletion of β1 integrin itself suppresses mineralization considerably (biological replicates: n = 8/9/6/6 in 3 experiments). Osteoblasts were transfected and cultured in mineralizing medium for 2–3 weeks. D, chemical inhibition of α4β1 prevents enhancement of mineralization by FN-EDA but not by FN-EDB (biological replicates: n = 6/6/6/9/10/6 in 3 experiments). Osteoblasts were cultured in mineralizing medium for 2–3 weeks, and the inhibitor was added with each medium change (3 times/week) at 1.8 nm. Results are expressed as mean ± S.D. (error bars). Bars, 1 mm. *, p < 0.05; **, p < 0.01; ***, p < 0.005.
Functional integrin dimers are required to affect cellular functions. Because α4 can team up with the β1-subunit, which is expressed on osteoblasts, we embarked on evaluating the role of β1 (26, 27). To this end, we isolated osteoblasts from mice that are homozygous for floxed β1 integrin and carry the CRE recombinase under the control of the osterix promoter (Osx-Cre β1fl/fl mice = β1 cKO) (15, 28). A decrease of β1 protein expression by 58% was detected by Western blotting (Fig. 7B). Transfecting these cells with FN-EDA showed that FN-EDA was no longer able to enhance osteoblast differentiation in comparison with the control FN construct (Fig. 7C). This suggests that β1 integrin might be a mediator of EDA effects. Because of the marked suppression of differentiation in the absence of β1 integrin, however, this effect might be only secondary to failure of differentiation in the absence of β1 integrin (26).
To confirm that, indeed, the α4β1 dimer mediates enhanced osteoblast differentiation in response to FN-EDA, we used a selective chemical inhibitor for α4β1 (29). We confirmed its specificity for α4 by taking advantage of the role of α4 in mediating CS1 cellular effects (Fig. 6D). Applying this chemical inhibitor with each medium change beginning on the day after FN-EDA transfection to primary osteoblasts cultured in mineralizing medium prevents enhanced mineralization and differentiation induction by FN-EDA, but not by FN-EDB (Fig. 7D). Thus, EDA fibronectin stimulates the differentiation of osteoblasts through activation of α4β1 integrin.
EDB-containing fibronectin enhances differentiation by binding through RGD to β3 integrin
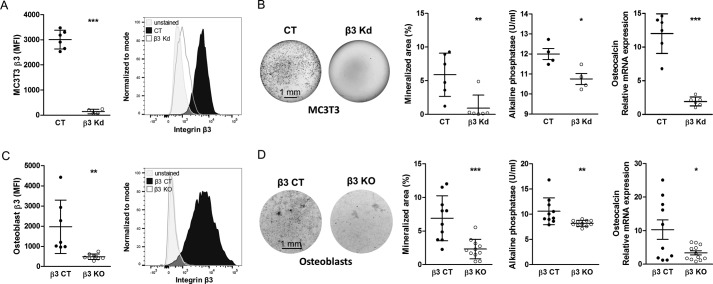
EDB-containing fibronectin exerts biological effects separate from fibronectin lacking EDB by activating integrin αvβ3 (11). In addition, β3 integrin expression on osteoblasts increased upon differentiation. We therefore asked whether β3-containing integrins influence osteoblast differentiation. For this, we induced knockdown (KD) of β3 integrin using shRNA directed against β3 integrin in the osteoblastic MC3T3-E1 clone 4 cell line (Fig. 8A). Culturing these β3 KD cells in mineralizing medium was associated with a decrease in differentiation (as evidenced by diminished mineralization, alkaline phosphatase activity, and osteocalcin mRNA expression) in comparison with CT cells containing the empty vector (Fig. 8B). To confirm the relevance of the β3 subunit in primary osteoblast differentiation, we isolated calvarial osteoblasts from β3−/− knock-out mice (β3 KO) and cultured them in mineralizing medium (30). Flow cytometry of the osteoblasts confirmed deletion of integrin β3 expression in isolated osteoblasts from β3 KO mice compared with littermate β3+/+ controls (β3 CT) (Fig. 8C). Isolated osteoblasts from β3 KO mice showed decreased differentiation compared with control osteoblasts (β3 CT) (Fig. 8D).
Figure 8.
The role of β3 integrin in osteoblast differentiation. A, shRNA-mediated knockdown of β3 in an osteoblastic cell line called MC3T3 (β3 KD) reveals successful reduction of β3 integrin expression on MC3T3 cells by flow cytometry (biological replicates: n = 6/4 in 2 experiments). The gray peak represents the autofluorescence of the cells. B, differentiation of β3 KD MC3T3 cells is reduced compared with control MC3T3 (CT) (biological replicates of von Kossa: n = 6/6; alkaline phosphatase activity in medium: n = 4/4; and osteocalcin mRNA: n = 6/6 in 2 experiments). Cells were cultured in mineralizing medium for 2–3 weeks. C, successful deletion of β3 integrin in primary osteoblasts freshly isolated from β3−/− knock-out mice (β3 KO) compared with β3+/+ littermate controls (β3 CT) (biological replicates: n = 7/9 in 2 experiments). The gray peak shows the autofluorescence of the cells. D, osteoblasts isolated from β3 KO mice show diminished differentiation compared with β3 CT (biological replicates of von Kossa and alkaline phosphatase activity in medium: n = 10/11; osteocalcin mRNA: n = 10/12 in 3 experiments) when cultured in mineralization medium for 2–3 weeks. Results are expressed as mean ± S.D. (error bars). Bars, 1 mm. *, p < 0.05; **, p < 0.01; ***, p < 0.005.
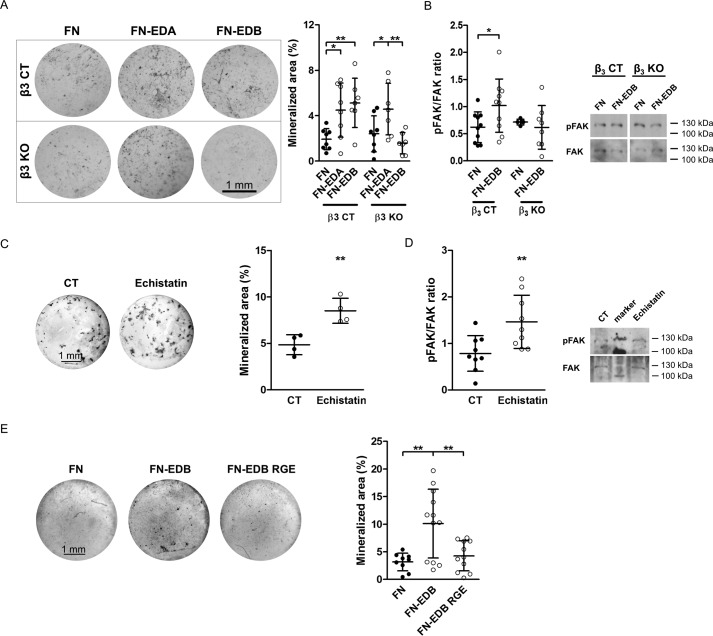
We have shown that β3 integrin mediates the enhancement of phagocytosis by fibronectin-containing EDB (11). We therefore asked whether FN-EDB acts through β3 to stimulate osteoblast differentiation. Control (β3 CT) and knock-out (β3 KO) primary newborn osteoblasts were transfected with the FN control construct and FN-EDB. Culturing these cells in mineralizing medium showed that the FN-EDA and FN-EDB construct augmented mineralization of CT osteoblasts, but in β3 KO osteoblasts, only FN-EDB was no longer able to affect differentiation (Fig. 9A). Furthermore, phosphorylation of FAK, one of the first molecules in mediating the intracellular effects of integrin signaling, increased in FN-EDB-transfected CT cells but not in FN-EDB-transfected cells from β3 KO mice (Fig. 9B). This indicates that β3 integrin mediates increased osteoblast differentiation in response to FN-EDB.
Figure 9.
EDB-containing fibronectin requires a functional RGD sequence to enhance osteoblast differentiation. A, in control cells (β3 CT), transfection with either FN-EDA or FN-EDB increased mineralization, whereas transfection with FN-EDB in β3 knock-out (β3 KO) osteoblasts no longer increased mineralization. FN-EDA transfected in β3 knock-out cells still enhanced mineralization. Osteoblasts were isolated from β3 CT and β3 KO mice; transfected with the FN, FN-EDA, or FN-EDB constructs; cultured for 2–3 weeks in mineralizing medium; and evaluated (biological replicates: n = 8/8/7/8/7/7 in 2 experiments). B, in the absence of β3 integrin, FN-EDB transfection fails to increase FAK phosphorylation (biological replicates: n = 10/10/5/8 in 4 experiments). Osteoblasts were transfected and cultured for 2 days in medium containing fibronectin-depleted FCS, and cell lysates were collected. C, echistatin, which binds to αvβ3, enhances osteoblast differentiation in vitro (biological replicates: n = 4/4 in 2 experiments). Echistatin was added at 0.27 nm with every medium change to osteoblasts cultured in mineralization medium. Wells were evaluated after 2–3 weeks. D, echistatin stimulates integrin signaling, as shown for FAK phosphorylation (biological replicates: n = 9/9 in 3 experiments). Echistatin was added at 0.27 nm to osteoblasts exposed overnight to FCS-free medium, and cell lysates were collected 15 min later. E, transfection of FN-EDB containing the mutated, non-functional RGD sequence (FN-EDB RGE) fails to enhance osteoblast differentiation compared with cells expressing the non-mutated FN-EDB construct containing functional RGD (biological replicates evaluated by von Kossa staining: n = 9/12/11 in 4 experiments). Osteoblasts were transfected with FN, FN-EDB, or FN-EDB RGE. Cells were cultured for 2–3 weeks in mineralizing medium, and wells were evaluated after von Kossa staining. Results are expressed as mean ± S.D. (error bars). Bars, 1 mm. *, p < 0.05; **, p < 0.01; ***, p < 0.005.
Unlike EDA, which itself can bind to α4-containing integrins, no particular integrin has been reported to bind directly to the EDB domain. Studies in another model system showed that EDB-containing fibronectin acts through αvβ3 integrin (11), and other data suggest that, in the presence of EDB, αvβ3 can accommodate the RGD sequence, the main integrin binding site on fibronectin (23). To test whether binding to αvβ3 through the RGD sequence affects osteoblasts, we added echistatin, which contains an RGD domain and engages αvβ3 integrin at the RGD binding site, to wild-type osteoblasts and found that culturing osteoblasts in mineralizing medium in the presence of echistatin increased osteoblast differentiation (Fig. 9C). Echistatin also increased FAK phosphorylation (Fig. 9D) (31). Consequently, binding of RGD to β3 integrin can lead to enhancement of osteoblast differentiation.
To evaluate the role of the RGD sequence in the presence of EDB, we mutated this sequence from RGD to RGE in the EDB-containing construct (FN-EDB). Interestingly, transfecting wild-type osteoblasts with the EDB construct that contained the non-functional RGE sequence instead of RGD (FN-EDB RGE) failed to enhance osteoblast differentiation, whereas the construct containing EDB and a functional RGD domain (FN-EDB) did so (Fig. 9E). Taken together, these data suggest that EDB effects on the osteoblasts are mediated by enhancing RGD binding to β3 integrin. We therefore conclude that engagement of RGD in the presence of EDB by αvβ3 integrin increases osteoblast differentiation.
Discussion
The main findings of this work are that 1) two isoforms of fibronectin, one containing EDA and one containing EDB, stimulate osteoblast differentiation, and 2) EDA-containing fibronectin activates integrin α4β1, and EDB-containing fibronectin binds through RGD to β3-containing integrin, activating it. This results in increased osteoblast differentiation.
This study was initiated because the circulating isoform of fibronectin lacking the EDA and the EDB domains was unable to support osteoblast differentiation in transgenic mice lacking fibronectin production by the osteoblast (2). We focused on the role of two isoforms of fibronectin, the one that contains EDA and the one that contains EDB, because both are produced by osteoblasts (2). Fibronectin containing EDA had some effects specifically attributed to it, including but not limited to stimulation of proliferation, cell adhesion, and modulation of lung fibrogenesis (9, 32, 33). Our in vitro studies establish that FN-EDA stimulates osteoblast differentiation (Figs. 1C and 2D). The EDA domain itself can bind to α4β1, α4β7, or even α9β1 integrin through the EDGIHEL sequence (8, 10, 20), and its presence enhances binding to the classical fibronectin receptor α5β1 integrin through the RGD sequence (32). Because other parts of fibronectin, such as LDV in the CS1 part of the variable region, can also bind to α4β1 or α4β7 (19), and the RGD sequence present in all fibronectin isoforms can itself bind to various integrins, the study of fibronectin and its isoforms has been fraught with difficulties. Nevertheless, we were able to narrow our search for the receptor mediating EDA effects on the osteoblasts by excluding the role of β7, which in our hands is not expressed on osteoblasts, and then evaluating the role of the two remaining integrins specifically binding to the EDA domain, which were α4β1 and α9β1. Only α4 changed during osteoblast differentiation, suggesting that this integrin is a possible candidate for mediating EDA effects. We therefore isolated both the high and the low expressing cells to determine whether the expression of α4 played any role in osteoblast differentiation. This was important because the expression of α4 integrin on osteoblasts has been the subject of discussions in the bone field, and contradictory reports have been published (4, 34–38). Our data show that expression of α4 integrin on osteoblasts defines a subpopulation that is more likely to differentiate into osteoblasts (Fig. 5E). Indeed, α4β1 expression is detected on mesenchymal cells, and its stimulation enhances bone repair (39). It should be noted, however, that stimulation of α4β1 integrin is problematic, because this integrin is expressed on immune cells, and several antibodies directed against this integrin have been used as immune modulatory medications in multiple sclerosis and Crohn's disease (40, 41). Therefore, although engaging α4β1 is beneficial for osteoblast differentiation, offering a novel possible therapeutic approach to the treatment of fractures, the sorting of the differences between the activation of this integrin in lymphocytes and osteoblasts will need to be undertaken before performing large scale therapeutic studies.
EDB-containing fibronectin has been less extensively studied, presumably because of the difficulty in isolating this isoform and its fast degradation.4 On one hand, its expression in cancer tissue and inflammation, in particular on newly formed blood vessels, have led to the use of antibodies against EDB-containing fibronectin to direct substances that disrupt the new blood vessels (42, 43). On the other hand, embryogenesis and blood vessel formation can proceed in its absence (44, 45). Likewise, whereas phagocytosis can proceed in the absence of EDB-containing fibronectin, the process is more efficient in the presence of EDB (11). A similar scheme is maintained in osteoblasts. Although differentiation takes place without EDB-containing fibronectin, its presence stimulates osteoblast mineralization in vitro (Fig. 1D). In contrast to the EDA domain, however, which can by itself bind to three integrin pairs, no direct binding of the EDB domain to an integrin has been reported (8, 10). Instead, stimulation of differentiation by EDB-containing fibronectin is dependent on its ability to bind through the RGD sequence to β3 integrin (Figs. 9, A and E). In this regard, the presence of the EDB domain might exert an indirect effect on integrin αvβ3 similar to what has been described for the presence of the EDA domain, leading to enhancement of binding of fibronectin through the RGD sequence to another integrin, α5β1 (9).
The role of αvβ3 integrin in osteoblasts remains poorly understood. Our data contrast with the conclusion by another group that β3 inhibits mineralization in an experimental setting in which overexpression of human αvβ3 integrin in a murine osteoblastic cell line leads to a decrease in mineralization (46). This is most likely due to the experimental design chosen by the other group as opposed to the use of primary knock-out osteoblasts. Our finding that wild-type osteoblasts indeed express β3 integrin is supported by the decrease in mineralization in vitro using β3 KO osteoblasts (Fig. 8). It is also in line with the decrease in circulating osteocalcin, a marker for osteoblast function in β3 KO mice, as found by others (Fig. 2 from Ref. 47).
Our data offer explanations for two more findings by others. αvβ3 integrin is required in osteoclasts for the formation of actin rings in vitro and ruffled membranes in vivo (47–49). Therefore, its deletion in transgenic mice led to osteopetrosis due to defective bone resorption (50). Of note, however, is that this osteopetrosis only became apparent after 4 months of age, although defects in osteoclast formation/resorption can already present briefly after birth, such as is the case for CSF1 (M-CSF) knock-out mice (51). This can be explained by the need for several integrin classes and/or defective osteoblast differentiation in the absence of β3 integrin, as we show here (52). Another issue that we help clarify is the effect of echistatin on bone. Echistatin on one hand prevents osteoclasts from attaching to the bone, diminishing their resorptive activity in vitro (53), but on the other hand, its use in vivo resulted in improved bone mineral density despite the fact that the number of osteoclasts in one study was not affected and that electron microscopic evaluation failed to show any osteoclast abnormalities (54). Thus, at least part of the effect of echistatin on bone might be attributed to its stimulation of osteoblast differentiation (Fig. 9, C and D).
Our report sheds new light on the complex differentiation of osteoblasts. We show that integrin-mediated activation of differentiation is involved in promoting mineralization by two isoforms of fibronectin produced by the osteoblasts themselves and characterize the isoforms and the mediating receptors. We conclude that engaging α4β1 is beneficial for osteoblast differentiation and that pharmacologic modification of αvβ3 should take into account its stimulatory role in osteoblasts.
Experimental procedures
Studies in osteoblasts and MC3T3
Newborn mouse osteoblasts were isolated from calvariae subjected to serial digestions and cultured in α-minimum Eagle's medium containing 10% fibronectin-depleted FCS, 50 IU/ml penicillin, and 50 μg/ml streptomycin). Differentiation of primary osteoblasts was induced by culture in mineralizing medium containing 50 μg/ml vitamin C, 5 mm β-glycerophosphate, and 10 nm dexamethasone added fresh with each medium change (3 times/week) as described (3). Mineralization of MC3T3 was induced with 50 μg/ml vitamin C and 10 mm β-glycerophosphate with each medium change (3 times/week). Nodules stained with von Kossa stain after 14–21 days were quantified using ImageJ (Wayne Rasband, National Institutes of Health). An alkaline phosphatase activity assay was performed by a colorimetric method as described except for the use of ZnCl2 in the substrate solution (55). For immunofluorescence staining, osteoblasts were fixed with 4% paraformaldehyde at the end of mineralization and stained for α4 (Millipore, catalog no. AB1924) using donkey anti-rabbit Alexa 488 (Molecular Probes, catalog no. A21206) or for β3 (Millipore, catalog no. AB1932) with anti-rabbit Alexa 647 (Abcam, catalog no. ab150079). Nuclei were stained with DAPI. FCS was depleted of fibronectin as described and tested by ELISA (3). To isolate the recombinant isoforms used in the stimulation experiments, FCS-free conditioned medium from MDA-MB-231-B/luc+ FN KO cells, which stably express cDNA pcDNA3.1-hygro constructs for FN, FN-EDA, or FN-EDB, were concentrated using Amicon 100,000 nominal molecular weight limit centrifugal devices. The concentration of the various fibronectin isoforms was analyzed by specific ELISA. Osteoblasts were stimulated for 15 min with 200 ng/ml rFN, rFN-EDA, or rFN-EDB after starving the cells for 8 h in medium without FCS and harvested for immune blotting. The CS1 peptide (DELPQLVTLPHPNLHGPEILDVPST) and the scrambled CT peptide (GDPELNITLSVPLPTHLQEPDPVLH) (added at 25 μg/ml) were synthesized on an ABI 433 peptide synthesizer (Life Technologies) using standard Fmoc (N-(9-fluorenyl)methoxycarbonyl) chemistry on Rink amide resin (Merck KGaA). Peptide purification was by RP-HPLC. Purity and identity of the peptides were verified by RP-HPLC and ESI-TOF mass spectrometry. The α4β1 inhibitor (BIO 5192, Tocris) was used at a concentration of 1.8 nm, and echistatin α1 isoform (Tocris) was used at a concentration of 0.27 nm. For analysis of FAK, AKT, and ERK phosphorylation, cells were transfected (TurboFect, Thermo Fisher) according to the manufacturer's instructions with the different constructs or siRNAs and harvested for protein analysis after culturing for 2 days in medium containing fibronectin-depleted FCS. In the case of FAK phosphorylation in response to echistatin treatment, echistatin was added at 0.27 nm to osteoblasts exposed overnight to FCS-free medium, and cell lysates were collected 15 min later. Additionally, transfected osteoblasts were differentiated for 2–3 weeks and stained with von Kossa stain, used for mRNA expression analysis or for an alkaline phosphatase activity assay in cell lysates or medium. Both results in medium and cell lysates are shown in Fig. 1. After that, all figures show only alkaline phosphatase activity in medium. The following transgenic mouse lines were used to generate osteoblasts: fibronectin fl/fl mated with osterix-Cre-expressing mice over two generations, β1 integrin fl/fl mated with osterix-Cre-expressing mice over two generations, and β3 complete knock-out (β3−/−) mice generated from matings of β3+/− mice (14–16, 28, 30). Controls always represent the littermates that do not carry the CRE recombinase gene and, in the case of β3, the wild-type littermates. Studies using primary β3−/− osteoblasts were approved by the Regierungspräsidium Karlsruhe of the state of Baden-Württemberg in Germany (G303/14).
Flow cytometry
For flow cytometry, cells were dissociated using dissociation buffer (Gibco) with 0.1% collagenase, resuspended in 2.5% FCS/PBS, and stained using phycoerythrin (PE) rat IgG2aκ anti-CD49d (integrin α4), clone 9C10; Alexa 647 rat IgG2bκ anti-mouse CD49e (integrin α5), clone 5H10–27; phycoerythrin rat IgG1κ anti-mouse CD51 (integrin αv), clone RMV-7; FITC Armenian hamster anti-mouse CD29 (integrin β1), clone HMβ1–1; goat anti-integrin α9 (R&D), and goat anti-mouse CD 61 (integrin β3, AbD Serotec). Cells were then analyzed by flow cytometry on an LSR II flow cytometer (BD Biosciences). Data are presented as MFI after exclusion of the background. To obtain integrin α4 and α9 (−) or (+) cell populations, osteoblasts were stained for CD49d or Integrin α9, respectively, and sorted for low and high expression using FACSAria-III (BD Biosciences) for each twice. For later differentiation experiments and repeat flow cytometry on α4-enriched and -depleted cells, antibody-coated magnetic beads were used based on the manufacturer's protocol (Thermo Fisher).
Constructs and siRNAs used
Human plasma fibronectin cDNA clone (DKFZp686M04163) and a cDNA fibronectin clone containing the EDA and the EDB domain (DKFZp696O1166) were used to generate our expression constructs. The cDNA constructs were cloned into the pGEMT easy cloning vector (Promega). The EDA or EDB domains were cloned into the plasma fibronectin cDNA construct via the BamHI and NotI or the NheI and BamHI restriction sites, respectively. For expression in eukaryotic cells, the fibronectin cDNAs were cloned into the pmax cloning vector, and transfection efficiency of osteoblasts was determined using the pmax vector encoding GFP (Lonza, catalog no. VDC-1040). The pcDNA3.1-hygro vector was used for generation of recombinant isoforms (Thermo Fisher, V87520) as described (21). The RGD motif in the FN-EDB construct was selectively mutated to the non-functional RGE sequence using site-directed mutagenesis (Stratagene). For shRNA-mediated knockdown in MC3T3-E1 clone 4 cells, shRNA pLKO.1 constructs (Sigma) were used for integrin β3 (TRCN0000009620) and, as a control, empty pLKO.1 vector. Knockdown efficiency was analyzed by flow cytometry. The siRNAs used were as published (18). The constructs used were as follows: EDA siRNA, 5′ siRNA (5′-CAUUGAUCGCCCUAAAGGAdTdT-3′) and 3′ siRNA (5′-AGGAAAUCCCGCUAGUUACdTdT-3′); EDA siRNA 2, 5′ siRNA (5′-GGGUUCUGAGUACACAGUCAGUGUGdTdT-3′) and 3′ siRNA (5′-GUGUGACUGACACAUGAGUCUUGGdTdT-3′); EDA siRNA 3, 5′ siRNA (5′-UCAGUGUGGUUGCCUUGCACGAUGAdTdT-3′) and 3′ siRNA (5′-AGUAGCACGUUCCGUUGGUGUGACUdTdT-3′); EDB siRNA, 5′ siRNA (5′-GCAUCGGCCUGAGGUGGACdTdT-3′) and 3′ siRNA (5′- CAGGUGGAGUCCGGCUACGdTdT-3′); EDB siRNA 2, 5′ siRNA (5′-GCGGCAGGAGAAGGUAUCCCUAUUUdTdT-3′) and 3′ siRNA (5′-UUUAUCCCUAUGGAAGAGGACGGCGdTdT-3′); EDB siRNA 3, 5′ siRNA (5′-GGCAUUGACUAUGAUAUCAGCGUUAdTdT-3′) and 3′ siRNA (5′-AUUGCGACUAUAGUAUCAGUUACGGdTdT-3′); EDA scrambled siRNA, 5′ siRNA (5′-GCGUUGGCGUCGUCGUUACAUUAGAdTdT-3′) and 3′ siRNA (5′-AGAUUACAUUGCUGCUGCGGUUGCGdTdT-3′); EDB scrambled siRNA, 5′ siRNA (5′-GUAGGACAUGCUUAUCGUGAAUCUA-dTdT-3′) and 3′ siRNA (5′- AUCUAAGUGCUAUUCGUACAGGAUGdTdT-3′). For transduction, cells were plated in microtiter plates and the next day transduced with 2 μl of lentiviral particles (5 × 106 transducing units/ml) in the presence of 8 μg/ml Polybrene. 12 h after the transduction, the medium was replaced, and the cells were stained or starved for 8 h before they were exposed to CS1 or the scrambled peptides (25 μg/ml). The shRNAs used were as follows: β3 shRNA, TRCN0000009620; β3 shRNA 2, TRCN0000009619; α4 shRNA, TRCN0000066043; α4 shRNA 2, TRCN0000066044; α4 shRNA 3, TRCN0000066045; α4 shRNA 4, TRCN0000066046; α4 shRNA 5, TRCN0000066047; CT shRNA, Sigma SHC001V.
RNA analysis
RNA was isolated using RNAzol RT (Sigma) and reverse-transcribed. Quantitative PCR (qPCR) was performed using SensiFast NoRox (Bioline) in a LightCycler I system (Roche Applied Science). Results were normalized to HPRT. The primers and probes used were those suggested by the Roche library for HPRT (number 95), ITGA4 (number 97), ITGA5 (number 76), ITGA9 (number 66), ITGAV (number 21), ITGB1 (number 109), ITGB3 (number 31), and osteocalcin (number 32). Mouse EDA and EDB fibronectin primer were as follows: EDA, 5′-TTGCACGATGATATGGAGAG-3′ and 5′-AGGCATAAAGCCACTGTTCC-3′ (number 77); EDB, 5′-CCCCTATCTCTGATACCGTTGT-3′ and 5′-GAATCACAGTAGTTGCGGCA-3′ (number 31).
Western blotting
The following antibodies were used: GAPDH (Sigma), pERK, ERK, pAKT, AKT, pFAK Tyr-397 (Cell Signaling), FAK (Millipore), and integrin β1 (clone MB 1.2, Millipore). The secondary antibodies were as follows: goat anti-rabbit IgG-HRP and goat anti-rat IgG-HRP (Dianova). Densitometry was analyzed using ImageJ.
ELISA
Total fibronectin and EDA- and EDB-containing fibronectin were quantified by ELISA as reported (56). The antibodies used were anti-human FN (Sigma), FN-3E2 (EDA fibronectin), SIP L-19 (EDB fibronectin), and rabbit polyclonal antibody conjugated with horseradish peroxidase (Dako) as described (11, 56).
Statistical analyses
Analyses were performed using GraphPad Prism (version 14). Analysis of variance tests were used as appropriate. If global probability values were smaller than 5%, subsequent comparisons between selected group pairs were then performed using Student's t, Mann-Whitney, or Wilcoxon paired tests as appropriate. Results are expressed as mean ± S.D.
Author contributions
C. S. designed and performed experiments, analyzed data, and prepared figures; K. H. designed and generated reagents, performed experiments, and analyzed data; S. P. and S. U. designed reagents; G. W. helped with flow cytometry evaluation; M. M. suggested experiments and provided experimental advice; I. A. N. designed experiments, analyzed data, and wrote the manuscript; C. S., K. H., and I. A. N. take responsibility for the integrity of the data analysis.
Acknowledgment
We thank Reinhard Fässler for invaluable input.
This work was supported by the Max-Planck Society and the German Science Foundation (DFG). The authors declare that they have no conflicts of interest with the contents of this article.
I. A. Nakchbandi, unpublished data.
- EDA and EDB
- extradomain A and B, respectively
- FN
- fibronectin
- CT
- control(s)
- rFN-EDA
- rFN-EDB, and rFN, recombinant FN-EDA, FN-EDB, and FN, respectively
- MFI
- mean fluorescence intensity
- KD
- knockdown
- qPCR
- quantitative PCR
- HPRT
- hypoxanthine-guanine phosphoribosyltransferase
- pERK
- pAKT, and pFAK, phosphorylated ERK, AKT, and FAK, respectively.
References
- 1. Neve A., Corrado A., and Cantatore F. P. (2011) Osteoblast physiology in normal and pathological conditions. Cell Tissue Res. 343, 289–302 [DOI] [PubMed] [Google Scholar]
- 2. Bentmann A., Kawelke N., Moss D., Zentgraf H., Bala Y., Berger I., Gasser J. A., and Nakchbandi I. A. (2010) Circulating fibronectin affects bone matrix, whereas osteoblast fibronectin modulates osteoblast function. J. Bone Miner. Res. 25, 706–715 [DOI] [PubMed] [Google Scholar]
- 3. Kawelke N., Bentmann A., Hackl N., Hager H. D., Feick P., Geursen A., Singer M. V., and Nakchbandi I. A. (2008) Isoform of fibronectin mediates bone loss in patients with primary biliary cirrhosis by suppressing bone formation. J. Bone Miner. Res. 23, 1278–1286 [DOI] [PubMed] [Google Scholar]
- 4. Moursi A. M., Globus R. K., and Damsky C. H. (1997) Interactions between integrin receptors and fibronectin are required for calvarial osteoblast differentiation in vitro. J. Cell Sci. 110, 2187–2196 [DOI] [PubMed] [Google Scholar]
- 5. Legate K. R., Wickström S. A., and Fässler R. (2009) Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 23, 397–418 [DOI] [PubMed] [Google Scholar]
- 6. Sottile J., and Hocking D. C. (2002) Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol. Biol. Cell 13, 3546–3559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leiss M., Beckmann K., Girós A., Costell M., and Fässler R. (2008) The role of integrin binding sites in fibronectin matrix assembly in vivo. Curr. Opin. Cell Biol. 20, 502–507 [DOI] [PubMed] [Google Scholar]
- 8. Liao Y. F., Gotwals P. J., Koteliansky V. E., Sheppard D., and Van De Water L. (2002) The EIIIA segment of fibronectin is a ligand for integrins α9β1 and α4β1 providing a novel mechanism for regulating cell adhesion by alternative splicing. J. Biol. Chem. 277, 14467–14474 [DOI] [PubMed] [Google Scholar]
- 9. Manabe R., Ohe N., Maeda T., Fukuda T., and Sekiguchi K. (1997) Modulation of cell-adhesive activity of fibronectin by the alternatively spliced EDA segment. J. Cell Biol. 139, 295–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shinde A. V., Bystroff C., Wang C., Vogelezang M. G., Vincent P. A., Hynes R. O., and Van De Water L. (2008) Identification of the peptide sequences within the EIIIA (EDA) segment of fibronectin that mediate integrin α9β1-dependent cellular activities. J. Biol. Chem. 283, 2858–2870 [DOI] [PubMed] [Google Scholar]
- 11. Kraft S., Klemis V., Sens C., Lenhard T., Jacobi C., Samstag Y., Wabnitz G., Kirschfink M., Wallich R., Hänsch G. M., and Nakchbandi I. A. (2016) Identification and characterization of a unique role for EDB fibronectin in phagocytosis. J. Mol. Med. 94, 567–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hynes R. (1990) Structure of fibronectins. in Fibronectins (Rich A., ed) pp. 113–175, Springer, New York [Google Scholar]
- 13. Schwarzbauer J. E., Spencer C. S., and Wilson C. L. (1989) Selective secretion of alternatively spliced fibronectin variants. J. Cell Biol. 109, 3445–3453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kawelke N., Vasel M., Sens C., von Au A., Dooley S., and Nakchbandi I. A. (2011) Fibronectin protects from excessive liver fibrosis by modulating the availability of and responsiveness of stellate cells to active TGF-β. PLoS One 6, e28181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rodda S. J., and McMahon A. P. (2006) Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development 133, 3231–3244 [DOI] [PubMed] [Google Scholar]
- 16. Sakai T., Johnson K. J., Murozono M., Sakai K., Magnuson M. A., Wieloch T., Cronberg T., Isshiki A., Erickson H. P., and Fässler R. (2001) Plasma fibronectin supports neuronal survival and reduces brain injury following transient focal cerebral ischemia but is not essential for skin-wound healing and hemostasis. Nat. Med. 7, 324–330 [DOI] [PubMed] [Google Scholar]
- 17. Su X., Yu M., Qiu G., Zheng Y., Chen Y., Wen R., Fu G., Zhu W., Chen J., Wu N., Ma P., Chen W., Wu Z., and Wang D. (2016) Evaluation of nestin or osterix promoter-driven cre/loxp system in studying the biological functions of murine osteoblastic cells. Am. J. Transl. Res. 8, 1447–1459 [PMC free article] [PubMed] [Google Scholar]
- 18. Cseh B., Fernandez-Sauze S., Grall D., Schaub S., Doma E., and Van Obberghen-Schilling E. (2010) Autocrine fibronectin directs matrix assembly and crosstalk between cell-matrix and cell-cell adhesion in vascular endothelial cells. J. Cell Sci. 123, 3989–3999 [DOI] [PubMed] [Google Scholar]
- 19. Johansson S., Svineng G., Wennerberg K., Armulik A., and Lohikangas L. (1997) Fibronectin-integrin interactions. Front. Biosci. 2, d126–d146 [DOI] [PubMed] [Google Scholar]
- 20. Kohan M., Muro A. F., White E. S., and Berkman N. (2010) EDA-containing cellular fibronectin induces fibroblast differentiation through binding to α4β7 integrin receptor and MAPK/Erk 1/2-dependent signaling. FASEB J. 24, 4503–4512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rossnagl S., Altrock E., Sens C., Kraft S., Rau K., Milsom M. D., Giese T., Samstag Y., and Nakchbandi I. A. (2016) EDA-fibronectin originating from osteoblasts inhibits the immune response against cancer. PLoS Biol. 14, e1002562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Postigo A. A., Sánchez-Mateos P., Lazarovits A. I., Sánchez-Madrid F., and de Landázuri M. O. (1993) α4β7 integrin mediates B cell binding to fibronectin and vascular cell adhesion molecule-1: expression and function of α4 integrins on human B lymphocytes. J. Immunol. 151, 2471–2483 [PubMed] [Google Scholar]
- 23. Adair B. D., Xiong J. P., Maddock C., Goodman S. L., Arnaout M. A., and Yeager M. (2005) Three-dimensional EM structure of the ectodomain of integrin αVβ3 in a complex with fibronectin. J. Cell Biol. 168, 1109–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sens C., Altrock E., Rau K., Klemis V., von Au A., Pettera S., Uebel S., Damm T., Tiwari S., Moser M., and Nakchbandi I. A. (2017) An O-glycosylation of fibronectin mediates hepatic osteodystrophy through α4β1 integrin. J. Bone Miner. Res. 32, 70–81 [DOI] [PubMed] [Google Scholar]
- 25. Makarem R., and Humphries M. J. (1991) LDV: a novel cell adhesion motif recognized by the integrin α4β1. Biochem. Soc. Trans. 19, 380S. [DOI] [PubMed] [Google Scholar]
- 26. Bouvard D., Aszodi A., Kostka G., Block M. R., Albigès-Rizo C., and Fässler R. (2007) Defective osteoblast function in ICAP-1-deficient mice. Development 134, 2615–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hultenby K., Reinholt F. P., and Heinegård D. (1993) Distribution of integrin subunits on rat metaphyseal osteoclasts and osteoblasts. Eur. J. Cell Biol. 62, 86–93 [PubMed] [Google Scholar]
- 28. Bungartz G., Stiller S., Bauer M., Müller W., Schippers A., Wagner N., Fässler R., and Brakebusch C. (2006) Adult murine hematopoiesis can proceed without β1 and β7 integrins. Blood 108, 1857–1864 [DOI] [PubMed] [Google Scholar]
- 29. Leone D. R., Giza K., Gill A., Dolinski B. M., Yang W., Perper S., Scott D. M., Lee W. C., Cornebise M., Wortham K., Nickerson-Nutter C., Chen L. L., LePage D., Spell J. C., Whalley E. T., et al. (2003) An assessment of the mechanistic differences between two integrin alpha 4 beta 1 inhibitors, the monoclonal antibody TA-2 and the small molecule BIO5192, in rat experimental autoimmune encephalomyelitis. J. Pharmacol. Exp. Ther. 305, 1150–1162 [DOI] [PubMed] [Google Scholar]
- 30. Hodivala-Dilke K. M., McHugh K. P., Tsakiris D. A., Rayburn H., Crowley D., Ullman-Culleré M., Ross F. P., Coller B. S., Teitelbaum S., and Hynes R. O. (1999) β3-Integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J. Clin. Invest. 103, 229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kumar C. C., Nie H., Rogers C. P., Malkowski M., Maxwell E., Catino J. J., and Armstrong L. (1997) Biochemical characterization of the binding of echistatin to integrin αvβ3 receptor. J. Pharmacol. Exp. Ther. 283, 843–853 [PubMed] [Google Scholar]
- 32. Manabe R., Oh-e N., and Sekiguchi K. (1999) Alternatively spliced EDA segment regulates fibronectin-dependent cell cycle progression and mitogenic signal transduction. J. Biol. Chem. 274, 5919–5924 [DOI] [PubMed] [Google Scholar]
- 33. Muro A. F., Moretti F. A., Moore B. B., Yan M., Atrasz R. G., Wilke C. A., Flaherty K. R., Martinez F. J., Tsui J. L., Sheppard D., Baralle F. E., Toews G. B., and White E. S. (2008) An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 177, 638–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Clover J., Dodds R. A., and Gowen M. (1992) Integrin subunit expression by human osteoblasts and osteoclasts in situ and in culture. J. Cell Sci. 103, 267–271 [DOI] [PubMed] [Google Scholar]
- 35. Gronthos S., Stewart K., Graves S. E., Hay S., and Simmons P. J. (1997) Integrin expression and function on human osteoblast-like cells. J Bone Miner. Res. 12, 1189–1197 [DOI] [PubMed] [Google Scholar]
- 36. Grzesik W. J., and Robey P. G. (1994) Bone matrix RGD glycoproteins: immunolocalization and interaction with human primary osteoblastic bone cells in vitro. J. Bone Miner. Res. 9, 487–496 [DOI] [PubMed] [Google Scholar]
- 37. Hughes D. E., Salter D. M., Dedhar S., and Simpson R. (1993) Integrin expression in human bone. J. Bone Miner. Res. 8, 527–533 [DOI] [PubMed] [Google Scholar]
- 38. Pistone M., Sanguineti C., Federici A., Sanguineti F., Defilippi P., Santolini F., Querzé G., Marchisio P. C., and Manduca P. (1996) Integrin synthesis and utilization in cultured human osteoblasts. Cell Biol. Int 20, 471–479 [DOI] [PubMed] [Google Scholar]
- 39. Marie P. J. (2013) Targeting integrins to promote bone formation and repair. Nat. Rev. Endocrinol. 9, 288–295 [DOI] [PubMed] [Google Scholar]
- 40. Hoepner R., Faissner S., Salmen A., Gold R., and Chan A. (2014) Efficacy and side effects of natalizumab therapy in patients with multiple sclerosis. J. Cent. Nerv. Syst. Dis. 6, 41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saruta M., and Papadakis K. A. (2014) Lymphocyte homing antagonists in the treatment of inflammatory bowel diseases. Gastroenterol. Clin. North Am. 43, 581–601 [DOI] [PubMed] [Google Scholar]
- 42. Trachsel E., Kaspar M., Bootz F., Detmar M., and Neri D. (2007) A human mAb specific to oncofetal fibronectin selectively targets chronic skin inflammation in vivo. J. Invest. Dermatol. 127, 881–886 [DOI] [PubMed] [Google Scholar]
- 43. Palumbo A., Hauler F., Dziunycz P., Schwager K., Soltermann A., Pretto F., Alonso C., Hofbauer G. F., Boyle R. W., and Neri D. (2011) A chemically modified antibody mediates complete eradication of tumours by selective disruption of tumour blood vessels. Br. J. Cancer 104, 1106–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Astrof S., Crowley D., George E. L., Fukuda T., Sekiguchi K., Hanahan D., and Hynes R. O. (2004) Direct test of potential roles of EIIIA and EIIIB alternatively spliced segments of fibronectin in physiological and tumor angiogenesis. Mol. Cell. Biol. 24, 8662–8670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fukuda T., Yoshida N., Kataoka Y., Manabe R., Mizuno-Horikawa Y., Sato M., Kuriyama K., Yasui N., and Sekiguchi K. (2002) Mice lacking the EDB segment of fibronectin develop normally but exhibit reduced cell growth and fibronectin matrix assembly in vitro. Cancer Res. 62, 5603–5610 [PubMed] [Google Scholar]
- 46. Cheng S. L., Lai C. F., Blystone S. D., and Avioli L. V. (2001) Bone mineralization and osteoblast differentiation are negatively modulated by integrin αvβ3. J. Bone Miner Res. 16, 277–288 [DOI] [PubMed] [Google Scholar]
- 47. Zou W., and Teitelbaum S. L. (2015) Absence of Dap12 and the alphavbeta3 integrin causes severe osteopetrosis. J. Cell Biol. 208, 125–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jung Y. K., Han S. W., Kim G. W., Jeong J. H., Kim H. J., and Choi J. Y. (2012) DICAM inhibits osteoclast differentiation through attenuation of the integrin αVβ3 pathway. J. Bone Miner. Res. 27, 2024–2034 [DOI] [PubMed] [Google Scholar]
- 49. Nakamura I., Pilkington M. F., Lakkakorpi P. T., Lipfert L., Sims S. M., Dixon S. J., Rodan G. A., and Duong L. T. (1999) Role of α(v)β(3) integrin in osteoclast migration and formation of the sealing zone. J. Cell Sci. 112, 3985–3993 [DOI] [PubMed] [Google Scholar]
- 50. McHugh K. P., Hodivala-Dilke K., Zheng M. H., Namba N., Lam J., Novack D., Feng X., Ross F. P., Hynes R. O., and Teitelbaum S. L. (2000) Mice lacking β3 integrins are osteosclerotic because of dysfunctional osteoclasts. J. Clin. Invest. 105, 433–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yoshida H., Hayashi S., Kunisada T., Ogawa M., Nishikawa S., Okamura H., Sudo T., Shultz L. D., and Nishikawa S. (1990) The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 345, 442–444 [DOI] [PubMed] [Google Scholar]
- 52. Schmidt S., Nakchbandi I., Ruppert R., Kawelke N., Hess M. W., Pfaller K., Jurdic P., Fässler R., and Moser M. (2011) Kindlin-3-mediated signaling from multiple integrin classes is required for osteoclast-mediated bone resorption. J. Cell Biol. 192, 883–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sato M., Sardana M. K., Grasser W. A., Garsky V. M., Murray J. M., and Gould R. J. (1990) Echistatin is a potent inhibitor of bone resorption in culture. J. Cell Biol. 111, 1713–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yamamoto M., Fisher J. E., Gentile M., Seedor J. G., Leu C. T., Rodan S. B., and Rodan G. A. (1998) The integrin ligand echistatin prevents bone loss in ovariectomized mice and rats. Endocrinology 139, 1411–1419 [DOI] [PubMed] [Google Scholar]
- 55. Bessey O. A., Lowry O. H., and Brock M. J. (1946) A method for the rapid determination of alkaline phosphates with five cubic millimeters of serum. J. Biol. Chem. 164, 321–329 [PubMed] [Google Scholar]
- 56. Hackl N. J., Bersch C., Feick P., Antoni C., Franke A., Singer M. V., and Nakchbandi I. A. (2010) Circulating fibronectin isoforms predict the degree of fibrosis in chronic hepatitis C. Scand. J. Gastroenterol. 45, 349–356 [DOI] [PubMed] [Google Scholar]