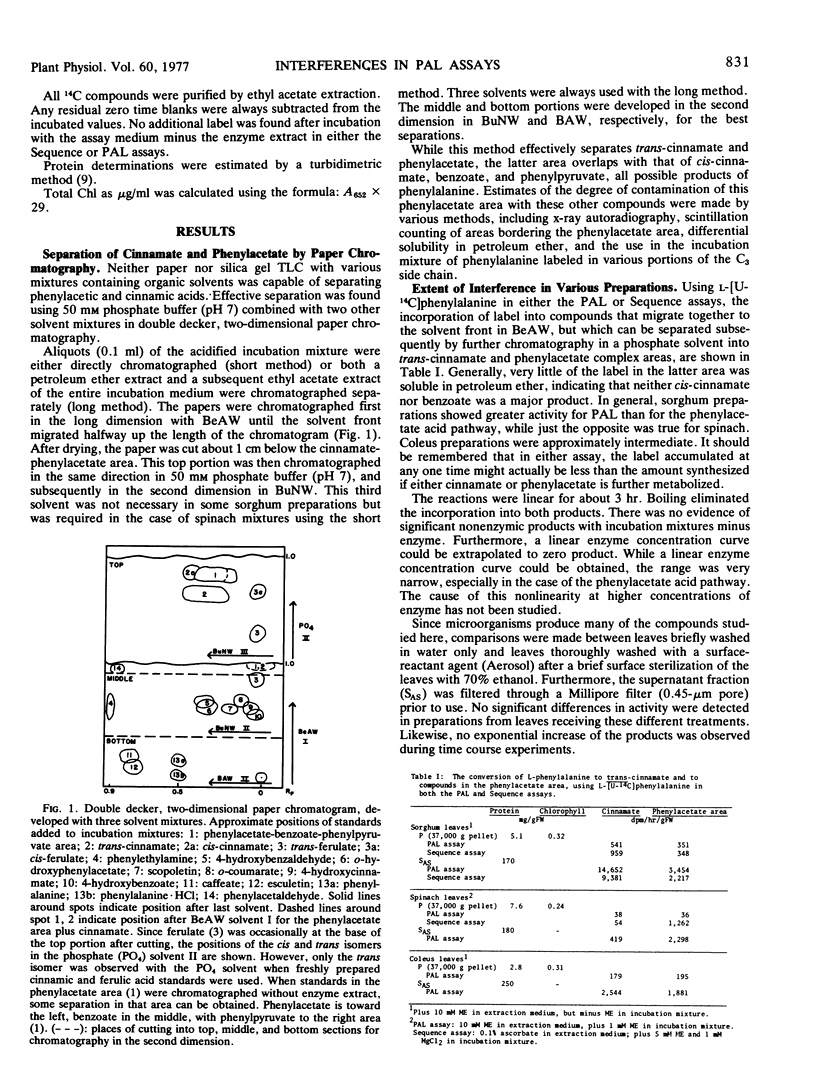
Abstract
Particulate and soluble fractions from leaves of Sorghum, Spinacia (spinach), and Coleus, capable of metabolizing l-phenylalanine to cinnamate or to caffeate, are also able to convert l-and d-phenylalanine to phenylacetate. Since cinnamate and phenylacetate are not effectively separated in commonly used chromatographic solvents, some of the isotropic assays used for phenylalanine ammonia-lyase are rendered ambiguous by the interference of this second pathway. Therefore, a “double decker,” two-dimensional paper chromatographic method was designed to separate cinnamate and phenylacetate. This was combined with the use of phenylalanine labeled randomly or just in either the carbon 1 or 2 position of the side chain.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Havir E. A., Hanson K. R. L-phenylalanine ammonia-lyase. II. Mechanism and kinetic properties of the enzyme from potato tubers. Biochemistry. 1968 May;7(5):1904–1914. doi: 10.1021/bi00845a039. [DOI] [PubMed] [Google Scholar]
- MAZELIS M. The pyridoxal phosphate-dependent oxidative decarboxylation of methionine by peroxidase. I. Characteristics and properties of the reaction. J Biol Chem. 1962 Jan;237:104–108. [PubMed] [Google Scholar]
- Stafford H. A., Baldy R. A monophenol oxidase activity in extracts of sorghum. Plant Physiol. 1970 Feb;45(2):215–222. doi: 10.1104/pp.45.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford H. A., Bliss M. The effect of greening of sorghum leaves on the molecular weight of a complex containing 4-hydroxycinnamic Acid hydroxylase activity. Plant Physiol. 1973 Nov;52(5):453–458. doi: 10.1104/pp.52.5.453. [DOI] [PMC free article] [PubMed] [Google Scholar]


