Abstract
An absolute requirement for divalent cations is reported for H14CO3− influx in Chara corallina. Effective substitution of eluted Ca2+ by Mg2+ and Sr2+ was observed, but Mn2+ was completely ineffective in restoring H14CO3− transport activity. Similarly, La3+ could not substitute for Ca2+ in this system. Low concentrations of ethylenediaminetetraacetate (0.01 to 0.06 mm) significantly enhanced the rate at which H14CO3− transport capacity was lost.
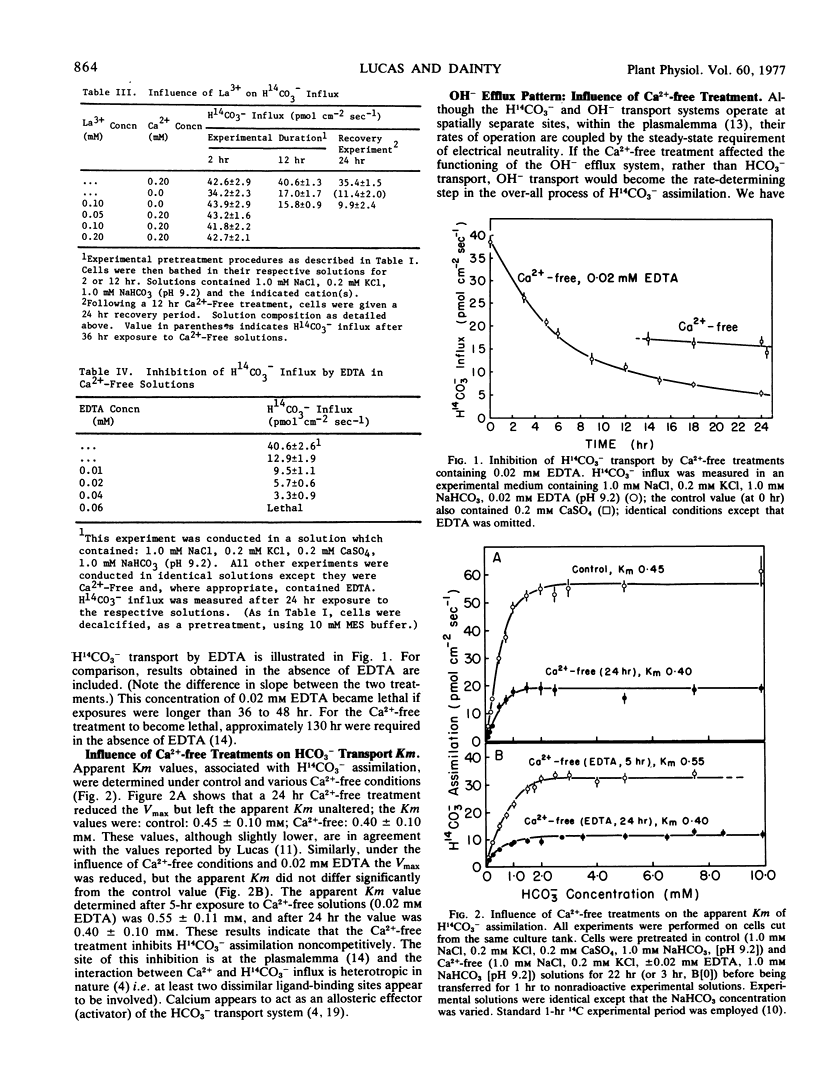
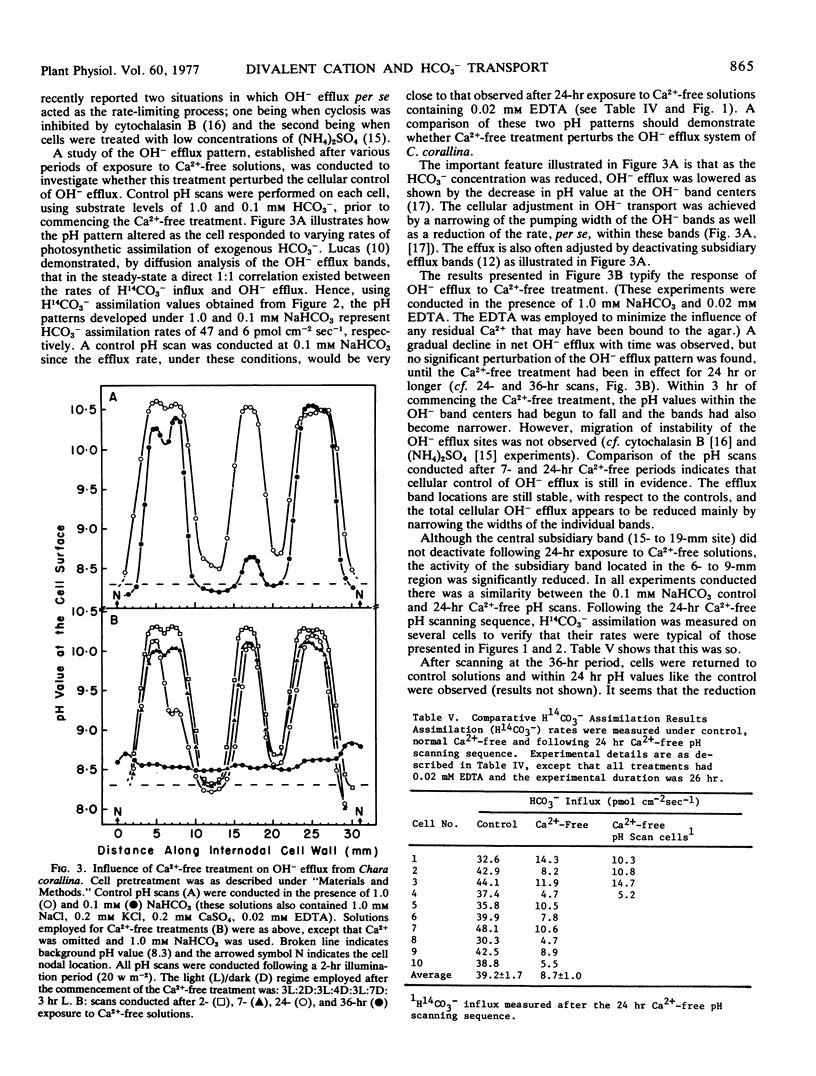
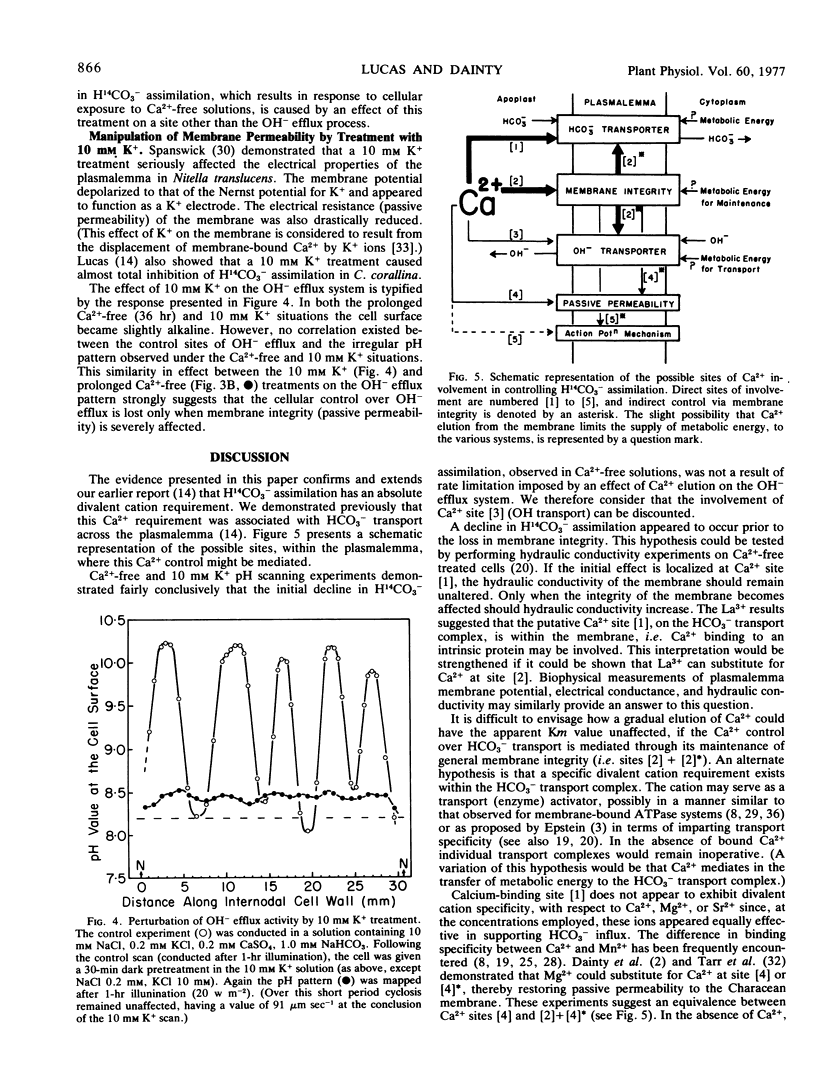
Examination of the response of OH− efflux, during Ca2+-free treatment, indicated that the cellular control over OH− efflux remained unaffected until membrane integrity became severely affected. This conclusion was supported by the response of OH− efflux to 10 mm K+. Therefore, assimilation of H14CO3− is not rate-limited by an effect of Ca2+ elution on the OH− transport system. Kinetic experiments indicated that Ca2+ removal from the membrane resulted in noncompetitive inhibition of H14CO3− assimilation; the apparent Michaelis constant remained unaltered over a wide range of conditions. An hypothesis is presented which suggests that membrane integrity is necessary for HCO3− transport to occur, but Ca2+ (Mg2+, Sr2+), per se, must be bound to the transport complex before activity is established.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hammes G. G., Wu C. W. Kinetics of allosteric enzymes. Annu Rev Biophys Bioeng. 1974;3(0):1–33. doi: 10.1146/annurev.bb.03.060174.000245. [DOI] [PubMed] [Google Scholar]
- Leonard R. T., Hotchkiss C. W. Cation-stimulated Adenosine Triphosphatase Activity and Cation Transport in Corn Roots. Plant Physiol. 1976 Sep;58(3):331–335. doi: 10.1104/pp.58.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Nagahashi G., Thomson W. W. Effect of lanthanum on ion absorption in corn roots. Plant Physiol. 1975 Mar;55(3):542–546. doi: 10.1104/pp.55.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas W. J., Dainty J. Spatial distribution of functional OH- carriers along a characean internodal cell: determined by the effect of cytochalasin B on H14CO3- assimilation. J Membr Biol. 1977 Apr 7;32(1-2):75–92. doi: 10.1007/BF01905210. [DOI] [PubMed] [Google Scholar]
- Lucas W. J., Ferrier J. M., Dainty J. Plasmalemma transport of OH- in Chara corallina: dynamics of activation and deactivation. J Membr Biol. 1977 Apr 7;32(1-2):49–73. doi: 10.1007/BF01905209. [DOI] [PubMed] [Google Scholar]
- Maas E. V., Moore D. P., Mason B. J. Influence of calcium and magnesium on manganese absorption. Plant Physiol. 1969 Jun;44(6):796–800. doi: 10.1104/pp.44.6.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manery J. F. Effects of Ca ions on membranes. Fed Proc. 1966 Nov-Dec;25(6):1804–1810. [PubMed] [Google Scholar]
- Mela L. Interactions of La3+ and local anesthetic drugs with mitochondrial Ca++ and Mn++ uptake. Arch Biochem Biophys. 1968 Feb;123(2):286–293. doi: 10.1016/0003-9861(68)90136-7. [DOI] [PubMed] [Google Scholar]
- Murakami S., Packer L. The role of cations in the organization of chloroplast membranes. Arch Biochem Biophys. 1971 Sep;146(1):337–347. doi: 10.1016/s0003-9861(71)80072-3. [DOI] [PubMed] [Google Scholar]
- Poovaiah B. W., Leopold A. C. Effects of inorganic salts on tissue permeability. Plant Physiol. 1976 Aug;58(2):182–185. doi: 10.1104/pp.58.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff O. Ca2+ activation of membrane-bound (Ca2++Mg2+)-dependent ATPase from human erythrocytes prepared in the presence or absence of Ca2+. Biochim Biophys Acta. 1976 Aug 16;443(2):206–218. doi: 10.1016/0005-2736(76)90504-6. [DOI] [PubMed] [Google Scholar]
- Spanswick R. M. Evidence for an electrogenic ion pump in Nitella translucens. I. The effects of pH, K + , Na + , light and temperature on the membrane potential and resistance. Biochim Biophys Acta. 1972 Oct 23;288(1):73–89. doi: 10.1016/0005-2736(72)90224-6. [DOI] [PubMed] [Google Scholar]


