Abstract
If S-adenosylmethionine (SAM) is the direct precursor of ethylene as previously proposed, it is expected that 5′-S-methyl-5′-thioadenosine (MTA) would be the fragment nucleoside. When [Me-14C] or [35S]methionine was fed to climacteric apple (Malus sylvestris Mill) tissue, radioactive 5-S-methyl-5-thioribose (MTR) was identified as the predominant product and MTA as a minor one. When the conversion of methionine into ethylene was inhibited by l-2-amino-4-(2′-aminoethoxy)-trans-3-butenoic acid, the conversion of [35S] or [Me14C]methionine into MTR was similarly inhibited. Furthermore, the formation of MTA and MTR from [35S]methionine was observed only in climacteric tissue which produced ethylene and actively converted methionine to ethylene but not in preclimacteric tissue which did not produce ethylene or convert methionine to ethylene. These observations suggest that the conversion of methionine into MTA and MTR is closely related to ethylene biosynthesis and provide indirect evidence that SAM may be an intermediate in the conversion of methionine to ethylene.
When [35S]MTA was fed to climacteric or preclimacteric apple tissue, radioactivity was efficiently incorporated into MTR and methionine. However, when [35S]MTR was administered, radioactivity was efficiently incorporated into methionine but not MTA. This suggests that the sulfur of MTA is incorporated into methionine via MTR. A dual label experiment with [35S, Me-3H]MTA indicates that the CH3S group of MTA was transferred as a unit to form methionine.
A scheme is presented for the production of ethylene from methionine, the first step being the activation of methionine by ATP to give SAM. SAM is fragmented to give ethylene, MTA, and other products. MTA is then hydrolyzed to MTR which donates its methylthio group to a four-carbon acceptor to reform methionine.
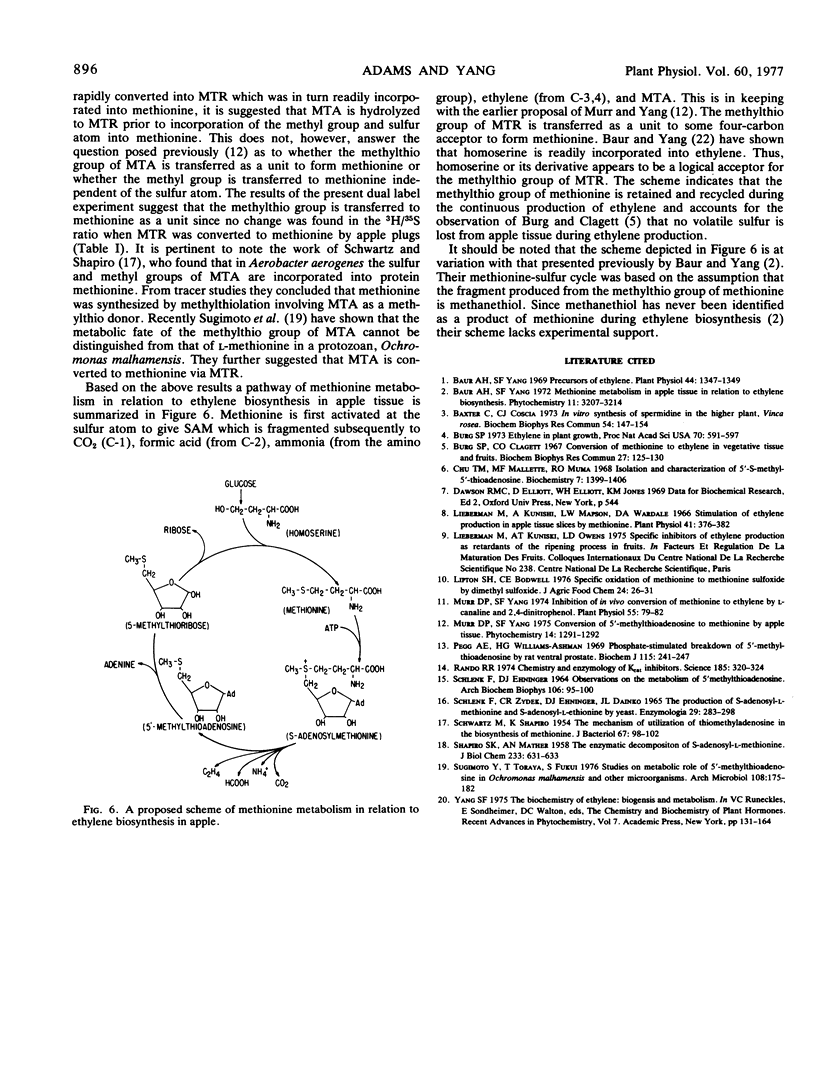
Full text
PDF
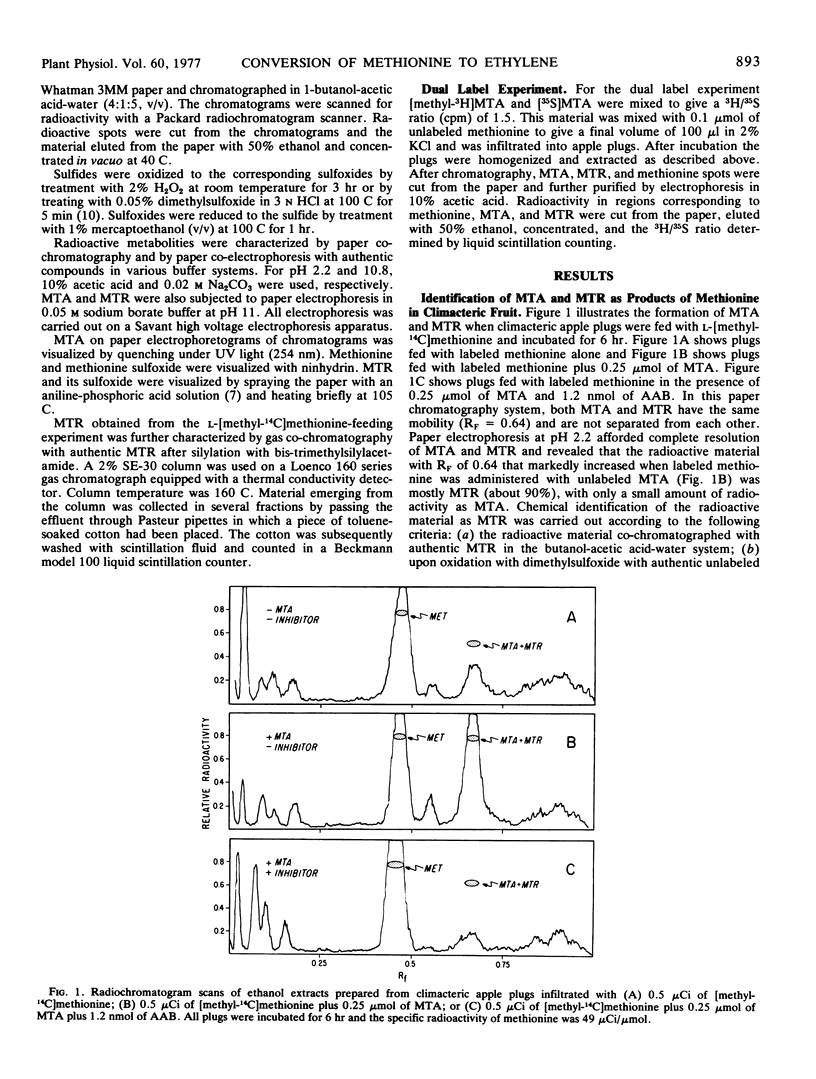
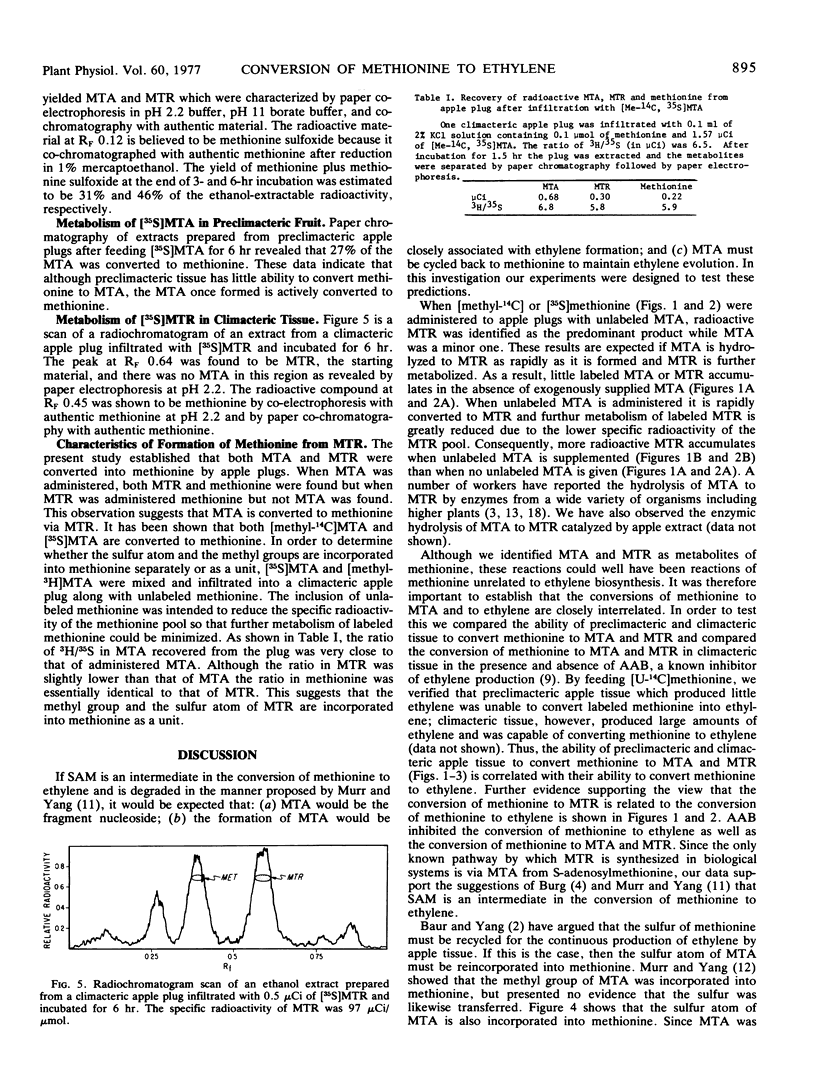
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baur A. H., Yang S. F. Precursors of ethylene. Plant Physiol. 1969 Sep;44(9):1347–1349. doi: 10.1104/pp.44.9.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Clagett C. O. Conversion of methionine to ethylene in vegetative tissue and fruits. Biochem Biophys Res Commun. 1967 Apr 20;27(2):125–130. doi: 10.1016/s0006-291x(67)80050-0. [DOI] [PubMed] [Google Scholar]
- Burg S. P. Ethylene in plant growth. Proc Natl Acad Sci U S A. 1973 Feb;70(2):591–597. doi: 10.1073/pnas.70.2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton S. H., Bodwell C. E. Specific oxidation of methionine to methionine sulfoxide by dimethyl sulfoxide. J Agric Food Chem. 1976 Jan-Feb;24(1):26–31. doi: 10.1021/jf60203a025. [DOI] [PubMed] [Google Scholar]
- Ming Chu T., Mallette M. F., Mumma R. O. Isolation and characterization of 5'-S-methyl-5'-thioadenosine from Escherichia coli. Biochemistry. 1968 Apr;7(4):1399–1406. doi: 10.1021/bi00844a023. [DOI] [PubMed] [Google Scholar]
- Murr D. P., Yang S. F. Inhibition of in Vivo Conversion of Methionine to Ethylene by l-Canaline and 2,4-Dinitrophenol. Plant Physiol. 1975 Jan;55(1):79–82. doi: 10.1104/pp.55.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. Phosphate-stimulated breakdown of 5'-methylthioadenosine by rat ventral prostate. Biochem J. 1969 Nov;115(2):241–247. doi: 10.1042/bj1150241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando R. R. Chemistry and enzymology of kcat inhibitors. Science. 1974 Jul 26;185(4148):320–324. doi: 10.1126/science.185.4148.320. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ M., SHAPIRO S. K. The mechanism of utilization of thiomethyladenosine in the biosynthesis of methionine. J Bacteriol. 1954 Jan;67(1):98–102. doi: 10.1128/jb.67.1.98-102.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAPIRO S. K., MATHER A. N. The enzymatic decomposition of S-adenosyl-L-methionine. J Biol Chem. 1958 Sep;233(3):631–633. [PubMed] [Google Scholar]
- Schlenk F., Zydek C. R., Ehninger D. J., Dainko J. L. The production of S-adenosyl-L-methionine and S-adenosyl-L-ethionine by yeast. Enzymologia. 1965 Nov 6;29(3):283–298. [PubMed] [Google Scholar]
- Sugimoto Y., Toraya T., Fukui S. Studies on metabolic role of 5'-Methylthioadenosine in Ochromonas malhamensis and other microorganisms. Arch Microbiol. 1976 Jun;108(2):175–182. doi: 10.1007/BF00428948. [DOI] [PubMed] [Google Scholar]


