Abstract
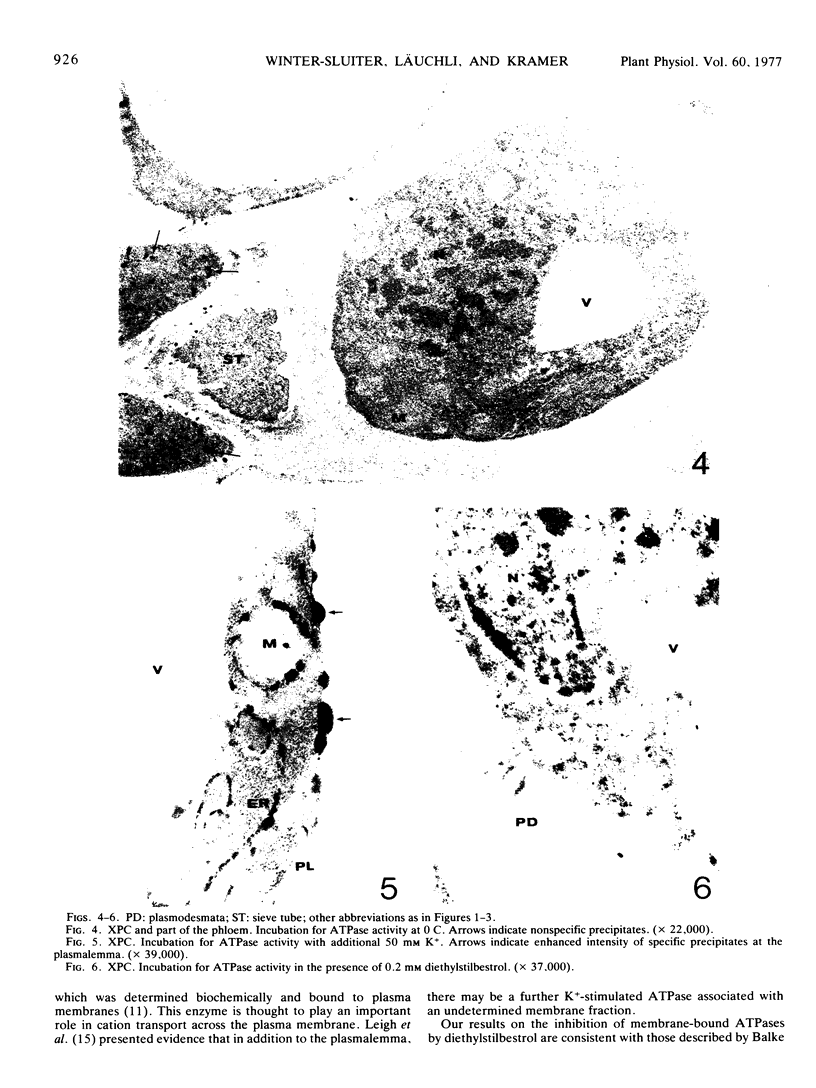
ATPase activity in xylem parenchyma cells of barley (Hordeum vulgare L.) roots was demonstrated cytochemically with a lead precipitation reaction. The methodical parameters of this cytochemical test were optimized for distinction between ATPase-specific and nonspecific precipitates. Optimum conditions were prefixation in 1% glutaraldehyde for 1 hour and incubation for 2 hours in a medium containing 2 mm each of ATP, Ca2+, and Pb2+ at pH 7 and 25 C. Problems of cytochemical localizations are discussed.
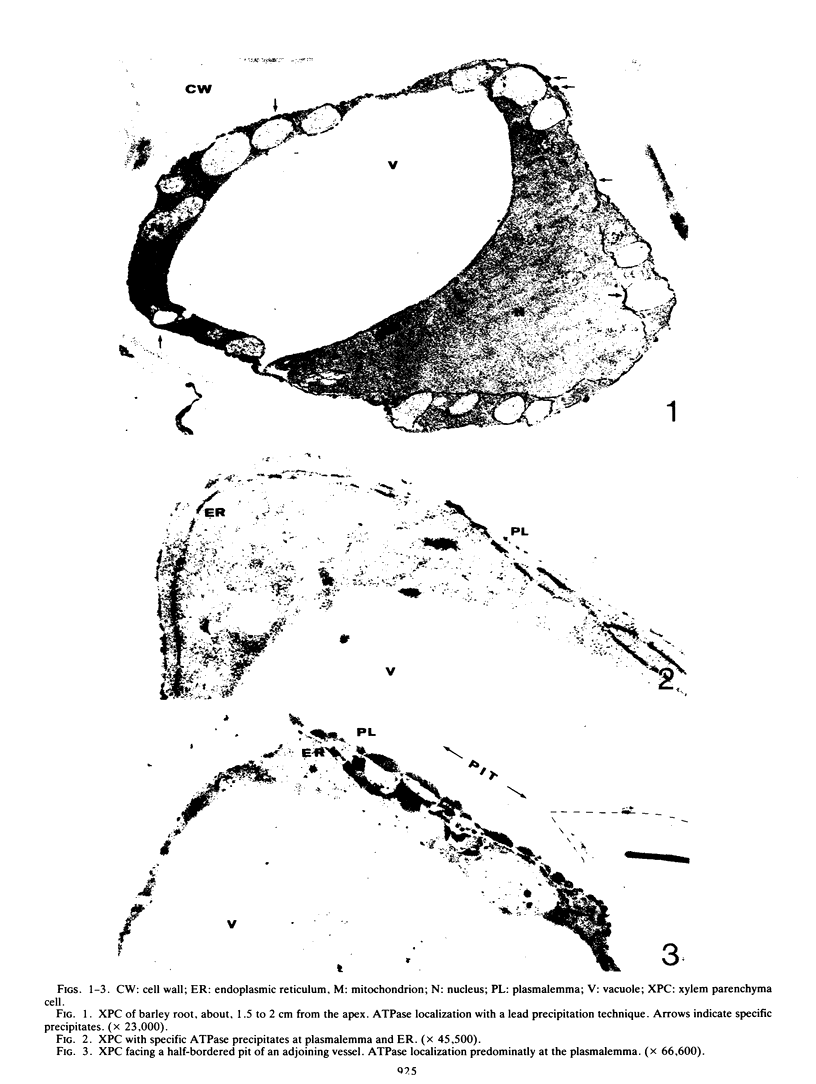
ATPase activity occurred mainly at the plasmalemma, the endoplasmic reticulum nuclear envelope, and outer mitochondrial membranes of xylem parenchyma cells. The tonoplast of these cells showed only little ATPase activity. High K+ concentrations stimulated ATPase activity, particularly at the plasmalemma. Diethylstilbestrol prevented the formation of ATPase-specific precipitates. The cytochemical demonstration of a K+-stimulated ATPase at the plasmalemma of xylem parenchyma cells is discussed in relation to the possible role of this membrane in ion transport to the vessels.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. J. Purification and quantitation of glutaraldehyde and its effect on several enzyme activities in skeletal muscle. J Histochem Cytochem. 1967 Aug;15(11):652–661. doi: 10.1177/15.11.652. [DOI] [PubMed] [Google Scholar]
- Balke N. E., Hodges T. K. Plasma membrane adenosine triphosphatase of oat roots: activation and inhibition by mg and ATP. Plant Physiol. 1975 Jan;55(1):83–86. doi: 10.1104/pp.55.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byington K. H., Smoly J. M., Morey A. V., Green D. E. On the fragmentation of mitochondria by diethylstilbesterol. I. Conditions for maximizing fragmentation. Arch Biochem Biophys. 1968 Dec;128(3):762–773. doi: 10.1016/0003-9861(68)90085-4. [DOI] [PubMed] [Google Scholar]
- Davis R. F., Higinbotham N. Electrochemical gradients and k and cl fluxes in excised corn roots. Plant Physiol. 1976 Feb;57(2):129–136. doi: 10.1104/pp.57.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh R. A., Williamson F. A., Jones R. G. Presence of Two Different Membrane-bound, KCl-stimulated Adenosine Triphosphatase Activities in Maize Roots. Plant Physiol. 1975 Apr;55(4):678–685. doi: 10.1104/pp.55.4.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Hotchkiss C. W. Cation-stimulated Adenosine Triphosphatase Activity and Cation Transport in Corn Roots. Plant Physiol. 1976 Sep;58(3):331–335. doi: 10.1104/pp.58.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- WACHSTEIN M., MEISEL E. Histochemistry of hepatic phosphatases of a physiologic pH; with special reference to the demonstration of bile canaliculi. Am J Clin Pathol. 1957 Jan;27(1):13–23. doi: 10.1093/ajcp/27.1.13. [DOI] [PubMed] [Google Scholar]