Abstract
Sirtuin1 (SIRT1) and forkhead box transcription factor O subfamily 1 (FOXO1) play vital roles in the maintenance of hippocampal neuronal homeostasis during aging. Our previous study showed that melatonin, a hormone mainly secreted by the pineal gland, restored the impaired memory of aged mice. Age-related neuronal energy deficits contribute to the pathogenesis of several neurodegenerative disorders. An attempt has been made to determine whether the effect of melatonin is mediated through the SIRT1-FOXO1 pathways. The present results showed that aged mice (22 months old) exhibited significantly downregulated SIRT1, FOXO1, and melatonin receptors MT1 and MT2 protein expression but upregulated tumor suppressor protein 53 (p53), acetyl-p53 protein (Ac-p53), mouse double minute 2 homolog (MDM2), Dickkopf-1 (DKK1) protein expression in mouse hippocampus compared with the young group. Melatonin treatment (10 mg/kg, daily in drinking water for 6 months) in aged mice significantly attenuated the age-induced downregulation of SIRT1, FOXO1, MT1 and MT2 protein expression and attenuated the age-induced increase in p53, ac-p53, MDM2, and DKK1 protein and mRNA expression. Melatonin decreased p53 and MDM2 expression, which led to a decrease in FOXO1 degradation. These present results suggest that melatonin may help the hippocampal neuronal homeostasis by increasing SIRT1, FOXO1 and melatonin receptors expression while decreasing DKK1 expression in the aging hippocampus. DKK1 can be induced by the accumulation of amyloid β (Aβ) which is the major hallmark of Alzheimer's disease.
Keywords: melatonin, aging, hippocampus, sirtuin1, FOXO1, melatonin receptor
Introduction
Brain aging is linked to certain types of neurodegenerative diseases and has become critical for the identification of new therapeutic targets. It is currently known that the pathological processes in aging brain are associated with molecules and signaling pathways that sense and influence energy metabolism, e.g., sirtuins (SIRT1), forkhead box transcription factor O subfamily (FOXOs). SIRT1 and the FOXOs are the focus of many brain aging studies because of their interaction with multiple target genes. The protective mechanisms of SIRT1 are closely related to many substrates. FOXOs, one of SIRT1 targets, play a vital role in cellular homeostasis. FOXO1 is highly expressed in the hippocampus and plays an important role in modulating hippocampal neuronal homeostasis (Abbas et al., 2009[1]).
Tumor suppressor protein 53 (p53) is another SIRT1 target that plays a vital role in the apoptosis pathway during aging. SIRT1 suppresses apoptosis through deacetylated p53 and p53-related factors in many senescence models (Arunachalam et al., 2014[2]; Engel and Mahlknecht, 2008[19]; Rahman et al., 2012[47]; Rodella et al., 2013[50]). In addition, the p53 pathway controls FOXOs expression. The mouse double minute 2 homolog (Mdm2) and the E3 ubiquitin-protein ligase are important negative autoregulators of p53. MDM2 binds to FOXO1 and FOXO3A and promotes their ubiquitination and subsequent degradation (Chung et al., 2015[13]; Fu et al., 2009[21]; Huang and Tindall, 2011[25]). The Wnt/β-catenin pathway regulates FOXO1 activities. In the nucleus, β-catenin acts as a transcriptional co-regulator with FOXOs, binding to specific DNA-binding transcription factors. Therefore, β-catenin enhances FOXO1 transcriptional activity (Cadigan, 2008[5]). Cellular oxidative stress simultaneously increases binding between β-catenin and FOXOs, which triggers stress response cascades (Purro et al., 2014[46]).
Melatonin, a hormone mainly secreted by the pineal gland, is another anti-aging agent that could potentially be used to treat neurodegenerative diseases (Hardeland, 2013[24]; Karasek, 2004[28], 2007[27]). Our recent study showed that melatonin restored the impaired memory and attenuated the alteration of the amyloid precursor protein (APP) proteolytic processing enzyme (α- and β-secretases) expression in the hippocampus of aged mice (Mukda et al., 2016[40]). Melatonin preserved the relative protein levels of SIRT1 in the senescence-accelerated mice (SAMP8) (Cristofol et al., 2012[14]; Gutierrez-Cuesta et al., 2008[23]; Tajes Orduna et al., 2009[55]) and in the hippocampus of total sleep-deprived rats (Chang et al., 2009[12]). Age-related neuronal energy deficits contribute to the pathogenesis of several neurodegenerative disorders, such as Alzheimer's disease. Understanding of how melatonin together with molecular, cellular and systemic energy metabolisms may lead to greater understanding of anti-aging mechanisms to increase life span under healthy condition. The present study aimed to answer 3 questions. Firstly, whether melatonin promoted FOXO1 expression and activities via an increase in SIRT1 expression, secondly, whether melatonin promoted FOXO1-β-catenin activities through decreasing DKK1 and thirdly, we also asked whether melatonin increased FOXO1 expression via a p53-MDM2 interaction and decreased the degradation of FOXO1.
Material and Methods
Animals
Male mice (Mlac:ICR, outbreed, Mus musculus) used in the present experiment were obtained from the National Experimental Animals Center of Mahidol University, Salaya Campus, Thailand. All animal procedures were approved through the Laboratory Animal Care and Use Committee of Mahidol University. We certify that we carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996. All efforts were made to minimize the number of animals used and their suffering. The animals had free access to water and food and were maintained in a 12 h light/dark cycle (light on at 7.00 a.m.). The mice were divided into three treatment groups: (i) control young adult mice (2 months of age, body weight = 30-35 g), (ii) aged group (22 months old mice, body weight = 45-50 g), and (iii) aged mice + melatonin. Melatonin (Sigma Aldrich, St Louis, MO) solution was freshly prepared daily. The long term melatonin-treated mice received melatonin in drinking water from 16 to 22 months of age (for a period of 6 months) according to our previous study protocol (Mukda et al., 2016[40]). The weight of each mouse and the amount of water containing melatonin intake for each mouse were measured. The daily melatonin intake for each mouse was approximately 10 mg/kg (Cavallo and Hassan, 1995[11]; Daya et al., 2001[15]; Moussaoui and Bendriss, 2014[39]; Mukda et al., 2016[40]). Control mice were continued on normal drinking water. At 22 months of age, the mice were sacrificed, the brains were isolated and the hippocampus was dissected and stored at -80 °C until further use.
Semi-quantitative reverse transcription PCR (RT-PCR)
Total RNA was isolated using the TRIzolTM reagent (Invitrogen, Life Technologies, Carlsbad, CA, USA) according to the manufacturer's protocol. RNA samples with sufficient purity A260/A280 ratios at 1.8-2.0 were used for cDNA synthesis. Two microgram of total RNA was reverse transcribed by using reverse transcription kit (Promega, Madison, WI, USA) with an MJ Mini™ Gradient thermal cycler (Bio-Rad, Hercules, CA, USA). PCR amplification was carried out using primer sets specific for SIRT1, FOXO1, p53, Mdm2, and Gapdh. The primer sequences are shown in Table 1(Tab. 1). PCR amplification was carried out at 95 °C for 40 s, 55 or 60 °C for 45 s, and 72 °C for 50 s, which was repeated for 30-35 cycles followed by incubation at 72 °C for 10 min. The amplified products were electrophoresed on 1 % agarose gels in TBE buffer (89 mM Tris-base pH 7.6, 89 mM boric acid, 2 mM EDTA). The gel was stained with RedSafeTM Nucleic Acid Stained (iNtRON, South Korea). The quantity and base pair size of the PCR products were estimated relative to 100 bp DNA ladder marker (Thermo Fisher Scientific Inc., Waltham, Massachusetts, USA). The gel images were photographed with an image analysis system (GelDoc 1000; Bio-Rad, Hercules, CA, USA). Using the Gene Tools analysis software (Syngene, Cambridge, UK), the intensities of specific PCR bands were quantified in relation to the Gapdh bands that were amplified from the same cDNA.
Table 1. Primer sequences for semi-quantitative RT-PCR.
Western blot analysis
Protein extracts were prepared from tissues samples mixed with lysis buffer (150 mM NaCl, 50 mM Tris-base, 1 mM phenyl methanesulfonyl fluoride, 1 mM EDTA, 1 % Triton X-100, 0.5 % sodium deoxycholate, 0.1 % SDS, 1 % protease inhibitor and 1 % phosphatase inhibitor) and sonicated twice for 10 s each. The lysate was then centrifuged at 12,000 x g for 15 min at 4 °C, and the supernatant was collected and used for Western blot analysis. Protein concentration was determined using the Bradford protein assay. Samples were denatured after adding 2X SDS sample buffer. Samples were electrophoresed on 10-12 % SDS-PAGE gels and transferred onto PVDF membranes. The membranes were blocked with blocking buffer for 1 h at room temperature and then incubated overnight at 4 °C with different antibodies as shown in Table 2(Tab. 2); mouse monoclonal antibody against SIRT1 (1:2,000), rabbit polyclonal antibody against FOXO1 (1:2,000), rabbit polyclonal antibody against DKK1 (1:2,000), mouse monoclonal antibody against MDM2 (1:2,000), rabbit polyclonal antibody against p53 (1:2,000), and rabbit polyclonal antibody against acetylated p53 (Lys 379) (1:2,000). All antibodies were diluted using Can Get Signal™. After washing three times with TBST, the membranes were incubated with anti-mouse IgG antibody (1:5,000), or anti-ra:bit IgG antibody (1:5,000). The membranes were washed three times with Tris-Buffered Saline and Tween 20 (TBST) and processed for chemiluminescence detection using ECL Plus™. Western blotting detection reagents were added, and the membrane was exposed using the Azure c300 Chemiluminescent Western Blot Imaging System™ (Azure Biosystems, Inc., Dublin, CA, USA). The immunoblot band densities were quantified using a densitometer with the Scion image program (National Institutes of Health, Bethesda, MD).
Table 2. The antibodies used for Western blotting.
Statistical analysis
The data are expressed as means ± S.E.M. Significance was assessed using one-way analysis of variance (ANOVA), followed by Tukey-Kramer post-hoc tests, using GraphPad Prism version 5. P-values less than 0.05 were considered significant.
Results
SIRT1 and FOXO1 protein and mRNA expressions in the aged hippocampus
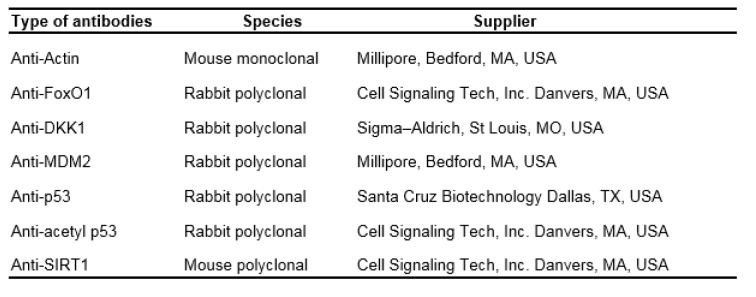
SIRT1 protein and mRNA expression were significantly decreased in the aged hippocampus when compared to the expression in young mice. The melatonin-treated group significantly increased SIRT1 protein and mRNA expression in aged mice compared with the aged untreated group (Figures 1A, B(Fig. 1)). FOXO1 protein and mRNA expression were significantly decreased in aged mice. The melatonin-treated group significantly upregulated FOXO1 protein and mRNA expression in the hippocampus of aged mice when compared with the aged control group (Figures 2A, B(Fig. 2)).
Figure 1. The effect of melatonin on sirtuin1 (SIRT1) protein (A) and mRNA (B) expression in the aging mouse hippocampus. SIRT1 protein expression in the hippocampus was compared between young and aged mice. Aged mice were treated with drinking water or with melatonin (Mel) at 10 mg/kg/day for 6 months. Representative bands from the different groups are shown. The bands from the semi-quantitative PCR were normalized to Gapdh, whereas the band densities from the Western blots were normalized to actin. The ratios were calculated as a percentage of the respective value of the control group. The values represent means ± SEM (n=4 for each group). (* and ** denote significant differences with p < 0.05 and p < 0.01, respectively, compared with young control group, and # denotes a significant difference with p < 0.05 compared with the aged control group).
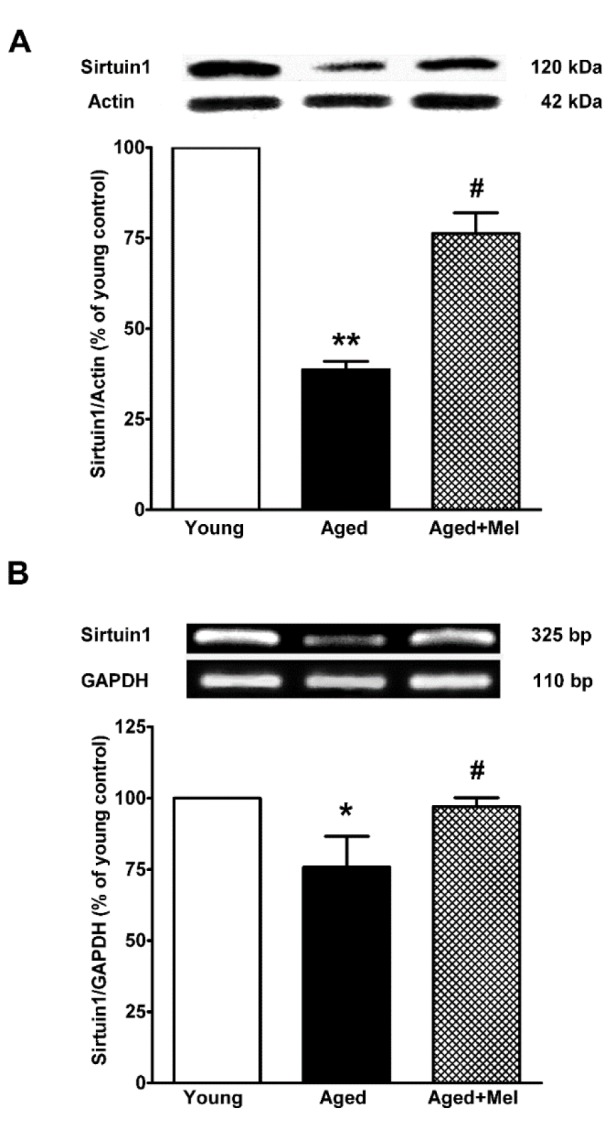
Figure 2. The effect of melatonin on FOXO1 protein (A) and mRNA (B) expression in the aging mouse hippocampus. The protein expression of FOXO1 was compared between young and aged mice. Aged mice were treated with drinking water or with melatonin (Mel) at 10 mg/kg/day for 6 months. Representative bands from the different groups are shown. The bands from the semi-quantitative PCR were normalized to Gapdh, whereas the band densities from the Western blots were normalized to actin. The ratios were calculated as a percentage of the respective value of the control group. The values represent means ± SEM (n=4 for each group). (* denotes a significant difference with p < 0.05 compared with the young control group, and # denotes a significant difference with p < 0.05 compared with the aged control group).
p53 protein and mRNA expressions in the aged hippocampus
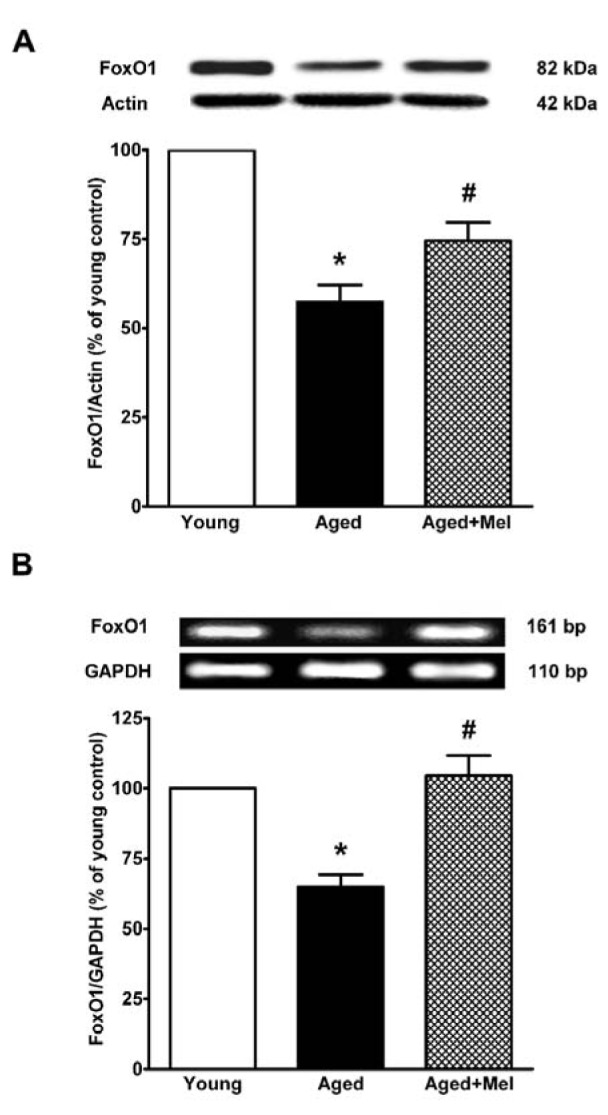
Because the p53-MDM2 regulatory pathway can modulate FOXO1 expression, we determined the effect of melatonin on p53 expression in the hippocampus of aged mice. Protein and mRNA expression of p53 in aged hippocampus significantly increased when compared to the young control group. Melatonin significantly decreased p53 protein and mRNA expression levels compared with those seen in the aged control group (Figures 3 A, B(Fig. 3)). Because p53 is a SIRT1 target, we explored SIRT1 activity by detecting the acetylated form of p53. Ac-p53 protein expression in aged mice was higher than that seen in the young control group. Melatonin significantly attenuated the increase in Ac-p53 expression in aged mice compared to the aged control group (Figure 3 C(Fig. 3)).
Figure 3. The effect of melatonin on p53 protein expression (A), p53 mRNA expression (B) and the acetylated form of p53 protein expression (C) in the aging mouse hippocampus. Aged mice were treated with drinking water or melatonin (Mel) at 10 mg/kg/day for 6 months. Representative bands from the different groups are shown. The bands from the semi-quantitative PCR were normalized to Gapdh, whereas the band densities from the Western blots were normalized to actin. The ratios were calculated as a percentage of the respective value of the control group. The values represent means ± SEM (n=4 for each group (* denotes a significant difference with p < 0.05 compared with the young control group, and # denotes a significant difference with p < 0.05 compared with the aged control group).
MDM2 protein and mRNA expressions in the aged hippocampus
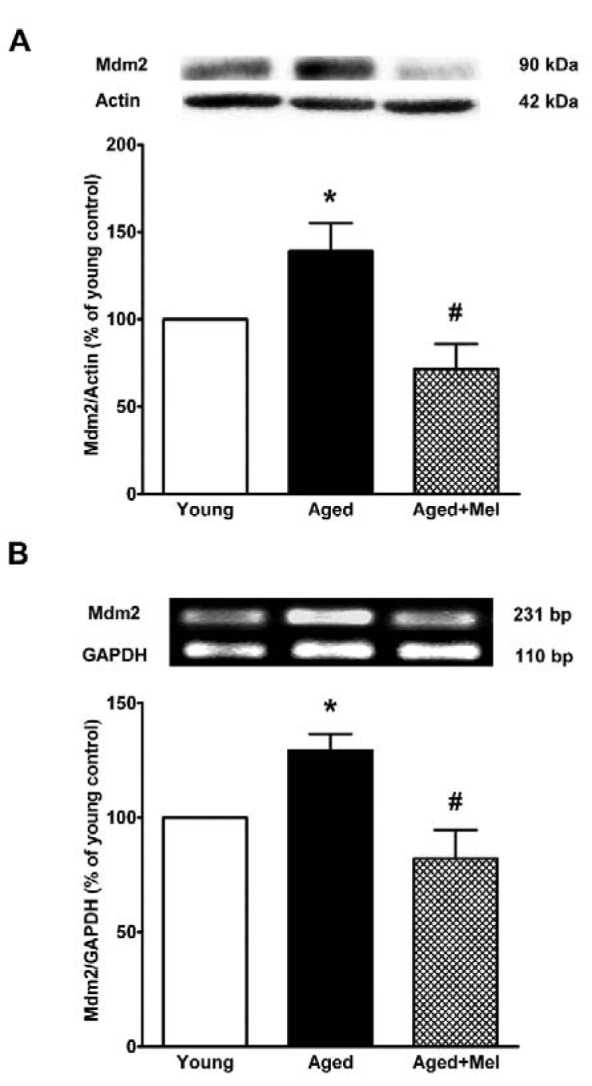
We determined whether MDM2, an ubiquitin E3 ligase for p53, can degrade FOXO1. Protein and mRNA expression levels of Mdm2 in the aged hippocampus were significantly increased when compared to the expression seen in the hippocampus of young mice. Melatonin treatment of the aged group significantly decreased MDM2 protein and mRNA expression compared with the expression levels in the aged control group (Figures 4A, B(Fig. 4)). The result suggested that the ubiquitination of FOXO1 in the aged hippocampus might be due to the activity of MDM2.
Figure 4. The effect of melatonin on MDM2 protein (A) and mRNA (B) expression in the aging mouse hippocampus. Aged mice were treated with drinking water or melatonin (Mel) at 10 mg/kg/day for 6 months. Representative bands from the different groups are shown. The bands from the semi-quantitative PCR were normalized to Gapdh, whereas the band densities from the Western blots were normalized to actin. The ratios were calculated as a percentage of the respective value of the control group. The values represent means ± SEM (n=4 for each group). (* denotes a significant difference with p < 0.05 compared with the young control group, and # denotes a significant difference with p < 0.05 compared with the aged control group).
DKK1 protein and mRNA expressions in the aged hippocampus
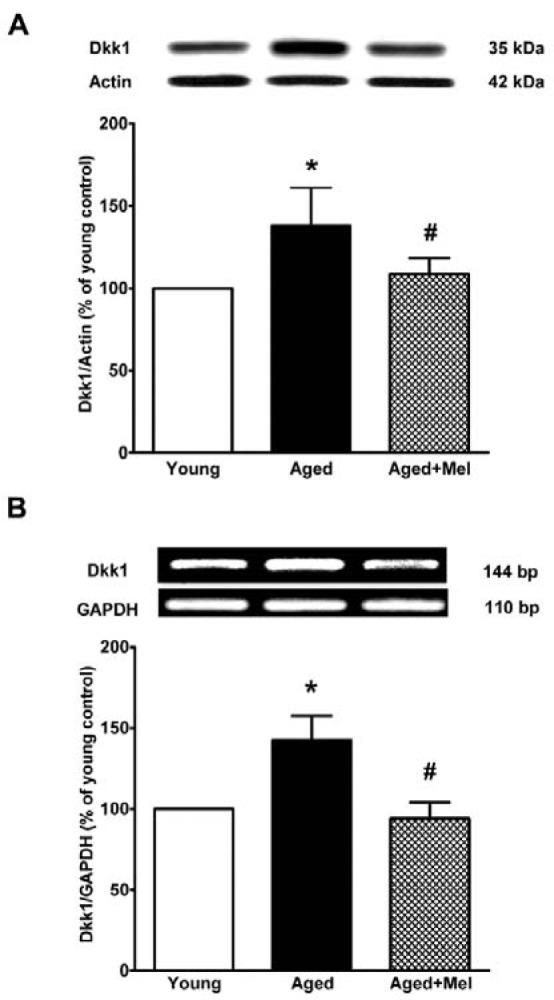
DKK1, a specific negative modulator of the Wnt pathway, was investigated in this study to determine whether the melatonin-induced increase in FOXO1 activity occurs via the Wnt/β-catenin pathway. The results showed that DKK1 protein and mRNA levels in the aged hippocampus were significantly increased when compared to those seen in the young group. The melatonin-treated group significantly decreased DKK1 protein and mRNA expression when compared to the levels seen in the aged control group (Figures 5A, B(Fig. 5)).
Figure 5. The effect of melatonin on DKK1 protein (A) and mRNA (B) expression in the aging mouse hippocampus. Aged mice were treated with drinking water or melatonin (Mel) at 10 mg/kg/day for 6 months. The representative bands from the different groups are shown. The bands from the semi-quantitative PCR were normalized to Gapdh, whereas the band densities from the Western blots were normalized to actin. The ratios were calculated as a percentage of the respective value of the control group. The values represent means ± SEM (n=4 for each group). (* denotes a significant difference with p < 0.05 compared with the young control group, and # denotes a significant difference with p < 0.05 compared with the aged control group).
MT1 and MT2 melatonin receptors proteins in the aged hippocampus
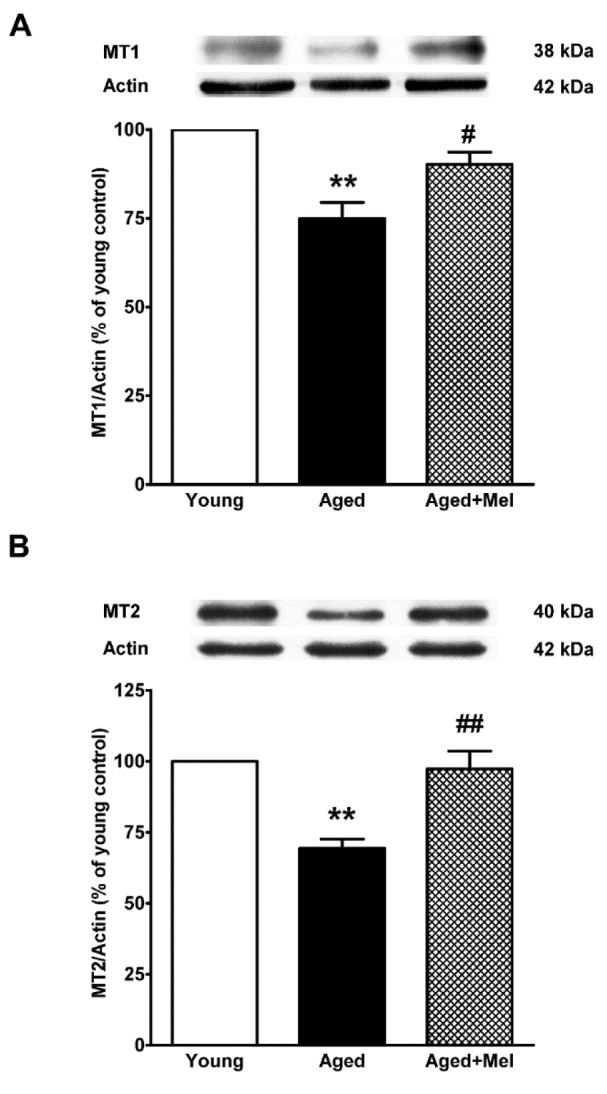
The levels of melatonin receptors MT1 and MT2 were examined (Figures 6A, B(Fig. 6)). In aging hippocampus, MT1 and MT2 levels were significantly downregulated when compared to those seen in the young group. Melatonin was able to significantly restore the reduction of melatonin receptors in the aged group.
Figure 6. The effect of melatonin on MT1 (A) and MT2 (B) expression in the aging mouse hippocampus. Aged mice were treated with drinking water or melatonin (Mel) at 10 mg/kg/day for 6 months. The representative bands from the different groups are shown. The band densities from the Western blots were normalized to actin. The ratios were calculated as a percentage of the respective value of the control group. The values represent means ± SEM (n=4 for each group). (** denotes significant difference with p < 0.01, compared with the young control group, # and ## denote significant difference with p < 0.05 and p < 0.01 compared with the aged control group).
Discussion
In the present study it has been shown that SIRT1 was downregulated in the aged hippocampus, while melatonin treatment for 6 months restored SIRT1 expression. Previous studies demonstrated that melatonin increased SIRT1 expression in a variety of models (Chang et al., 2009[12]; Tajes et al., 2009[54]). Melatonin protected neurons by preserving protein levels of SIRT1 in the dentate gyrus of aged rats (Kireev et al., 2013[29]), a total sleep-deprived model (Chang et al., 2009[12]), SAMP8 mice (Cristofol et al., 2012[14]; Gutierrez-Cuesta et al., 2008[23]; Tajes et al., 2009[54]) and neonatal rats subjected to hypoxia-ischemia (Carloni et al., 2014[10]). SIRT1 is NAD+-dependent deacetylase that senses changes in intracellular NAD+ levels (Canto and Auwerx, 2012[6]), the supportive effect of melatonin on SIRT1 may act via the nicotinamide adenine dinucleotide (NAD) system. Melatonin, an effective antioxidant, preserves NAD levels under oxidative stress. When melatonin was incubated with the PC12 cell line in an oxidative environment, the oxidation was reduced and melatonin donated an electron to the NAD radical, resulting in the generation of NAD (Tan et al., 2005[56]).
We found that FOXO1 was downregulated by aging, and the reduction could be prevented by melatonin administration. FOXO1 was predominantly expressed in the hippocampus and declined during aging (Zemva et al., 2012[61]). Melatonin may increase FOXO1 expression through the PI3K/Akt pathway. In an aged neuronal cell culture, melatonin increased Akt activation, leading to GSK3β inhibition and an increase in FOXO1 phosphorylation (Tajes et al., 2009[54]). Melatonin had protective effects against brain injury through activating Akt and its downstream targets in a middle cerebral artery occlusion model (Koh, 2008[31]). Melatonin increased the interaction of phosphorylated-FOXO1 (p-FOXO1) and 14-3-3 in a kainic acid-induced hippocampal excitotoxicity model (Lee et al., 2006[34]) and in focal cerebral ischemia in rats (Zheng et al., 2014[63]). The acetylation of FOXO1 decreased DNA binding and FOXOs activity (Bousiges et al., 2010[3]; Wang et al., 2012[59]). Melatonin upregulated the expression of SIRT1 and downregulated the expression of ac-FOXO1 in a cecal ligation brain injury model (Zhao et al., 2015[62]). Melatonin not only promotes FOXOs activities via SIRT1 deacetylation but also acts through CREB-binding protein (CBP) and p300. Melatonin suppressed p300 histone acetyltransferase (HAT) activity and p300 HAT-mediated NF-κB acetylation in the human vascular smooth muscle cell line CRL1999 (Shi et al., 2012[52]).
In the present study, melatonin treatment in aged animal decreased p53, acetyl-p53 compared with aged mice. This ameliorated the FOXO1 degradation process. Melatonin decreased p53 both in in vitro and in vivo studies (Gutierrez-Cuesta et al., 2008[23]). SIRT1 expression was lower in SAMP8 mice compared with SAMR1 mice, and these changes were prevented by melatonin (Gutierrez-Cuesta et al., 2008[23]). In a neuronal cell culture, melatonin treatment inhibited the Ataxia telangiectasia mutated (ATM) protein. ATM is a serine/threonine protein kinase that phosphorylates several key proteins that initiate the activation of the DNA damage checkpoint, including p53 and MDM2. Moreover, melatonin administration activated the prosurvival protein Akt, which resulted in GSK-3β inhibition and an increase in p-FOXO1 (Tajes et al., 2009[54]).
p53 activation is mainly regulated by MDM2 in the nucleus. MDM2 also acts as E3 ubiquitin ligase, promoting proteasome-dependent p53 degradation; therefore, p53 and MDM2 regulate each other in an autoregulatory loop. Melatonin drastically downregulated Mdm2 gene expression and inhibited MDM2 shuttling into the nucleus of MCF7 breast cancer cells. Because PI3K/Akt-mediated MDM2 phosphorylation on serine 166 is essential to enable MDM2 entry in the nucleus, melatonin slowed down the activity of the PI3K/AkT pathway, leading to a reduction in MDM2 levels. In addition, the inhibition of Akt-mediated MDM2 phosphorylation prevents the nuclear translocation of MDM2 and consequently impairs its ability to target and degrade p53 (Proietti et al., 2011[44], 2014[45]). Both MDM2 and p53 graphs showed the same trend of expression in the present results, since P53 and MDM2 regulate each other in an autoregulatory feedback loop. If the expression of MDM2 or p53 is out of the autoregulatory manner, this will trigger the DNA damage (Pant et al., 2013[41]).
The neuroprotective effects of melatonin may be due to a decrease in the acetylated form of p53. The acetylation of p53 on some residues such as lysine373, enhanced the stabilization/activity of p53 and increased the susceptibility of cells to stress. Acetylation of p53 was altered in the hippocampus following global cerebral ischemia (Raz et al., 2011[48]). In addition, the acetylated form of p53 attenuated mdm2-mediated ubiquitination, possibly through inducing a protein conformational change (Ito et al., 2002[26]; Li et al., 2002[36]).
To investigate whether melatonin affects Wnt/β-catenin signaling, we determined the expression of the specific inhibitor Dickkopf-1 (DKK1). We found that DKK1 protein and mRNA levels in the aged hippocampus were higher than those in the young group, and melatonin treatment lowered the level of DKK1 protein and mRNA. DKK1 is a specific inhibitor of the Wnt pathway, and antagonizes signaling by binding to low-density lipoprotein receptor-related protein 5/6 (LRP5/6). DKK1 is scarcely found in normal brain, but upregulation of DKK1 precedes neuronal death in models of neurodegenerative diseases (Caricasole et al., 2004[9]; Rosi et al., 2010[51]) and ischemic neuronal death (Cappuccio et al., 2005[7]). Melatonin may decrease DKK1 via downregulation of p53. DKK1 is induced by p53 because the Dkk1 transcription start site has a p53 responsive element (Caricasole et al., 2004[9]; Wang et al., 2000[58]), whereas DKK1 and Wnt inhibitory factor-1 (WIF1) significantly activated the transcription of p53 in ovarian carcinoma cells (Ko et al., 2014[30]). In addition, mature neurons exposed to DKK1 showed a decrease in the number of functional synaptic vesicle recycling sites, and DKK1 promoted the dispersion of pre- and postsynaptic proteins, indicating a loss of synapses (Purro et al., 2014[46]). The inhibition of the Wnt pathway in neurospheres by retroviral overexpression of Dkk1 robustly increased gliogenesis at the expense of neurogenesis (Kunke et al., 2009[32]). Aging rat hippocampus exhibited an upregulation of DKK1, while exercise could reverse this change in expression.
Several lines of evidence have suggested a pathophysiological relationship between reduction in melatonin and melatonin receptors with aging and neurodegenerative diseases. Our result here showed that MT1 and MT2 declined during aging while melatonin supplement was able to reverse this change. Melatonin transmits its action via the specific high affinity G-protein couple receptors (Reppert et al., 1994[49]) which are expressed in several areas of the brain, including hippocampus (Mazzucchelli et al., 1996[38]). Melatonin exerts its action on neurogenesis via the membrane-bound receptor both in the subventricular zone (Sotthibundhu et al., 2010[53]) and rat hippocampus (Tocharus et al., 2014[57]). In this study, we found an increase in MT1 and MT2 protein levels after melatonin treatment, which is consistent with our previous studies in cultured adult subventricular zone neurospheres (Sotthibundhu et al., 2010[53]) and in cultured adult hippocampal precursor cells pretreated with dexamethasone (Ekthuwapranee et al., 2015[18]). Activation of MT1 via Gαi-coupled melatonin receptors has been shown to produce the inhibitory responses in the cAMP signal transduction cascade, resulting in decreases in PKA activity and in CREB phosphorylation (Brydon et al., 1999[4]; Dubocovich et al., 2003[17]; Li et al., 1998[35]). Moreover, through the dissociation of βγ subunits of Gi, melatonin activates MAP kinases as well as the Ras-MEK pathway to stimulate ERK phosphorylation (Carbajo-Pescador et al., 2011[8]; Pearson et al., 2001[42]; Witt-Enderby et al., 2003[60]). Activation of the MT2 can also result in decreases in cGMP, decreases cAMP formation and PI hydrolysis via Gαi subunit activation (Dubocovich et al., 2010[16]; Markowska et al., 2004[37]). Melatonin acted through MT1 and MT2 receptors to activate hypothalamic Akt/PKB and protein kinase B to suppress hepatic gluconeogenesis in rats (Faria et al., 2013[20]).
Aging decreases the Wnt pathway activities. Melatonin may help to enhance the Wnt pathway through different pathways. For example; melatonin decreased reactive oxygen species (ROS) and decreased GSK-3β. Increases in GSK-3β activity during aging inactivate β-catenin through phosphorylation. Melatonin increased phosphorylated GSK-3β in primary cultures of cerebellar granule neurons (Tajes et al., 2009[54]), in a SAMP8 mice aging model (Gutierrez-Cuesta et al., 2007[22]) and in an Alzheimer's animal model (Kwon et al., 2010[33]; Peng et al., 2013[43]). Melatonin may act through PI3K-Akt-GSK3β pathway via MT1 and MT2 receptors. In addition, melatonin decreases some factors that are related to neurodegenerative diseases, such as amyloid β (Aβ) since Aβ accumulation induces Dkk1 production.
Conclusion
In summary, the current study suggested possible mechanisms of the neuroprotective effects of melatonin on the FOXO1 pathway during aging. Melatonin significantly upregulated SIRT1, FOXO1, MT1 and MT2 expression but downregulated p53, ac-p53 and MDM2, the ligase enzyme that ubiquitinates FOXO1. Melatonin may increase FOXO1 expression via downregulation of the p53-MDM2 pathway. The expression of DKK1, a specific inhibitor of the canonical Wnt pathway, was downregulated by melatonin. Melatonin may promote FOXO1 activities by inducing β-catenin, the co-transcription factor of FOXO1.
Acknowledgements
This study was supported by the Thailand Research Fund (TRF) (DPG5780001 and IRG5780009) and a Mahidol University Research Grant to PG.
References
- 1.Abbas AK, Dozmorov M, Li R, Huang FS, Hellberg F, Danielson J, et al. Persistent LTP without triggered protein synthesis. Neurosci Res. 2009;63:59–65. doi: 10.1016/j.neures.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Arunachalam G, Samuel SM, Marei I, Ding H, Triggle CR. Metformin modulates hyperglycaemia-induced endothelial senescence and apoptosis through SIRT1. Br J Pharmacol. 2014;171:523–535. doi: 10.1111/bph.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bousiges O, Vasconcelos AP, Neidl R, Cosquer B, Herbeaux K, Panteleeva I, et al. Spatial memory consolidation is associated with induction of several lysine-acetyltransferase (histone acetyltransferase) expression levels and H2B/H4 acetylation-dependent transcriptional events in the rat hippocampus. Neuropsychopharmacology. 2010;35:2521–2537. doi: 10.1038/npp.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brydon L, Roka F, Petit L, de Coppet P, Tissot M, Barrett P, et al. Dual signaling of human Mel1a melatonin receptors via G(i2), G(i3), and G(q/11) proteins. Mol Endocrinol. 1999;13:2025–2038. doi: 10.1210/mend.13.12.0390. [DOI] [PubMed] [Google Scholar]
- 5.Cadigan KM. Wnt-beta-catenin signaling. Curr Biol. 2008;18:R943–R947. doi: 10.1016/j.cub.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 6.Canto C, Auwerx J. Targeting sirtuin 1 to improve metabolism: all you need is NAD(+)? Pharmacol Rev. 2012;64:166–187. doi: 10.1124/pr.110.003905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cappuccio I, Calderone A, Busceti CL, Biagioni F, Pontarelli F, Bruno V, et al. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is required for the development of ischemic neuronal death. J Neurosci. 2005;25:2647–2657. doi: 10.1523/JNEUROSCI.5230-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carbajo-Pescador S, Garcia-Palomo A, Martin-Renedo J, Piva M, Gonzalez-Gallego J, Mauriz JL. Melatonin modulation of intracellular signaling pathways in hepatocarcinoma HepG2 cell line: role of the MT1 receptor. J Pineal Res. 2011;51:463–471. doi: 10.1111/j.1600-079X.2011.00910.x. [DOI] [PubMed] [Google Scholar]
- 9.Caricasole A, Copani A, Caraci F, Aronica E, Rozemuller AJ, Caruso A, et al. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is associated with neuronal degeneration in Alzheimer's brain. J Neurosci. 2004;24:6021–6027. doi: 10.1523/JNEUROSCI.1381-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carloni S, Albertini MC, Galluzzi L, Buonocore G, Proietti F, Balduini W. Melatonin reduces endoplasmic reticulum stress and preserves sirtuin 1 expression in neuronal cells of newborn rats after hypoxia-ischemia. J Pineal Res. 2014;57:192–199. doi: 10.1111/jpi.12156. [DOI] [PubMed] [Google Scholar]
- 11.Cavallo A, Hassan M. Stability of melatonin in aqueous solution. J Pineal Res. 1995;18:90–92. doi: 10.1111/j.1600-079x.1995.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 12.Chang HM, Wu UI, Lan CT. Melatonin preserves longevity protein (sirtuin 1) expression in the hippocampus of total sleep-deprived rats. J Pineal Res. 2009;47:211–220. doi: 10.1111/j.1600-079X.2009.00704.x. [DOI] [PubMed] [Google Scholar]
- 13.Chung KW, Choi YJ, Park MH, Jang EJ, Kim DH, Park BH, et al. Molecular insights into SIRT1 protection against UVB-induced skin fibroblast senescence by suppression of oxidative stress and p53 acetylation. J Gerontol A Biol Sci Med Sci. 2015;70:959–968. doi: 10.1093/gerona/glu137. [DOI] [PubMed] [Google Scholar]
- 14.Cristofol R, Porquet D, Corpas R, Coto-Montes A, Serret J, Camins A, et al. Neurons from senescence-accelerated SAMP8 mice are protected against frailty by the sirtuin 1 promoting agents melatonin and resveratrol. J Pineal Res. 2012;52:271–281. doi: 10.1111/j.1600-079X.2011.00939.x. [DOI] [PubMed] [Google Scholar]
- 15.Daya S, Walker RB, Glass BD, Anoopkumar-Dukie S. The effect of variations in pH and temperature on stability of melatonin in aqueous solution. J Pineal Res. 2001;31:155–158. doi: 10.1034/j.1600-079x.2001.310209.x. [DOI] [PubMed] [Google Scholar]
- 16.Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev. 2010;62:343–380. doi: 10.1124/pr.110.002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubocovich ML, Rivera-Bermudez MA, Gerdin MJ, Masana MI. Molecular pharmacology, regulation and function of mammalian melatonin receptors. Front Biosci. 2003;8:d1093–d1108. doi: 10.2741/1089. [DOI] [PubMed] [Google Scholar]
- 18.Ekthuwapranee K, Sotthibundhu A, Tocharus C, Govitrapong P. Melatonin ameliorates dexamethasone-induced inhibitory effects on the proliferation of cultured progenitor cells obtained from adult rat hippocampus. J Steroid Biochem Mol Biol. 2015;145:38–48. doi: 10.1016/j.jsbmb.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Engel N, Mahlknecht U. Aging and anti-aging: unexpected side effects of everyday medication through sirtuin1 modulation. Int J Mol Med. 2008;21:223–232. [PubMed] [Google Scholar]
- 20.Faria JA, Kinote A, Ignacio-Souza LM, de Araujo TM, Razolli DS, Doneda DL, et al. Melatonin acts through MT1/MT2 receptors to activate hypothalamic Akt and suppress hepatic gluconeogenesis in rats. Am J Physiol Endocrinol Metab. 2013;305:E230–E242. doi: 10.1152/ajpendo.00094.2013. [DOI] [PubMed] [Google Scholar]
- 21.Fu W, Ma Q, Chen L, Li P, Zhang M, Ramamoorthy S, et al. MDM2 acts downstream of p53 as an E3 ligase to promote FOXO ubiquitination and degradation. J Biol Chem. 2009;284:13987–14000. doi: 10.1074/jbc.M901758200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutierrez-Cuesta J, Sureda FX, Romeu M, Canudas AM, Caballero B, Coto-Montes A, et al. Chronic administration of melatonin reduces cerebral injury biomarkers in SAMP8. J Pineal Res. 2007;42:394–402. doi: 10.1111/j.1600-079X.2007.00433.x. [DOI] [PubMed] [Google Scholar]
- 23.Gutierrez-Cuesta J, Tajes M, Jimenez A, Coto-Montes A, Camins A, Pallas M. Evaluation of potential pro-survival pathways regulated by melatonin in a murine senescence model. J Pineal Res. 2008;45:497–505. doi: 10.1111/j.1600-079X.2008.00626.x. [DOI] [PubMed] [Google Scholar]
- 24.Hardeland R. Melatonin and the theories of aging: a critical appraisal of melatonin's role in antiaging mechanisms. J Pineal Res. 2013;55:325–356. doi: 10.1111/jpi.12090. [DOI] [PubMed] [Google Scholar]
- 25.Huang H, Tindall DJ. Regulation of FOXO protein stability via ubiquitination and proteasome degradation. Biochim Biophys Acta. 2011;1813:1961–1964. doi: 10.1016/j.bbamcr.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito A, Kawaguchi Y, Lai CH, Kovacs JJ, Higashimoto Y, Appella E, et al. MDM2-HDAC1-mediated deacetylation of p53 is required for its degradation. Embo J. 2002;21:6236–6245. doi: 10.1093/emboj/cdf616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karasek M. Does melatonin play a role in aging processes? J Physiol Pharmacol. 2007;58(Suppl 6):105–113. [PubMed] [Google Scholar]
- 28.Karasek M. Melatonin, human aging, and age-related diseases. Exp Gerontol. 2004;39:1723–1729. doi: 10.1016/j.exger.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 29.Kireev RA, Vara E, Tresguerres JA. Growth hormone and melatonin prevent age-related alteration in apoptosis processes in the dentate gyrus of male rats. Biogerontology. 2013;14:431–442. doi: 10.1007/s10522-013-9443-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ko YB, Kim BR, Yoon K, Choi EK, Seo SH, Lee Y, et al. WIF1 can effectively co-regulate pro-apoptotic activity through the combination with DKK1. Cell Signal. 2014;26:2562–2572. doi: 10.1016/j.cellsig.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 31.Koh PO. Melatonin prevents the injury-induced decline of Akt/forkhead transcription factors phosphorylation. J Pineal Res. 2008;45:199–203. doi: 10.1111/j.1600-079X.2008.00577.x. [DOI] [PubMed] [Google Scholar]
- 32.Kunke D, Bryja V, Mygland L, Arenas E, Krauss S. Inhibition of canonical Wnt signaling promotes gliogenesis in P0-NSCs. Biochem Biophys Res Commun. 2009;386:628–633. doi: 10.1016/j.bbrc.2009.06.084. [DOI] [PubMed] [Google Scholar]
- 33.Kwon KJ, Kim HJ, Shin CY, Han SH. Melatonin potentiates the neuroprotective properties of resveratrol against beta-amyloid-induced neurodegeneration by modulating amp-activated protein kinase pathways. J Clin Neurol. 2010;6:127–137. doi: 10.3988/jcn.2010.6.3.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee SH, Chun W, Kong PJ, Han JA, Cho BP, Kwon OY, et al. Sustained activation of Akt by melatonin contributes to the protection against kainic acid-induced neuronal death in hippocampus. J Pineal Res. 2006;40:79–85. doi: 10.1111/j.1600-079X.2005.00283.x. [DOI] [PubMed] [Google Scholar]
- 35.Li L, Xu JN, Wong YH, Wong JT, Pang SF, Shiu SY. Molecular and cellular analyses of melatonin receptor-mediated cAMP signaling in rat corpus epididymis. J Pineal Res. 1998;25:219–228. doi: 10.1111/j.1600-079x.1998.tb00391.x. [DOI] [PubMed] [Google Scholar]
- 36.Li M, Luo J, Brooks CL, Gu W. Acetylation of p53 inhibits its ubiquitination by Mdm2. J Biol Chem. 2002;277:50607–50611. doi: 10.1074/jbc.C200578200. [DOI] [PubMed] [Google Scholar]
- 37.Markowska M, Mrozkowiak A, Pawlak J, Skwarlo-Sonta K. Intracellular second messengers involved in melatonin signal transduction in chicken splenocytes in vitro. J Pineal Res. 2004;37:207–212. doi: 10.1111/j.1600-079X.2004.00154.x. [DOI] [PubMed] [Google Scholar]
- 38.Mazzucchelli C, Pannacci M, Nonno R, Lucini V, Fraschini F, Stankov BM. The melatonin receptor in the human brain: cloning experiments and distribution studies. Brain Res Mol Brain Res. 1996;39:117–126. doi: 10.1016/0169-328x(96)00017-4. [DOI] [PubMed] [Google Scholar]
- 39.Moussaoui NE, Bendriss A. The influence of storage conditions on melatonin stability. Int J Eng Res Tech. 2014;3:2243–2246. [Google Scholar]
- 40.Mukda S, Panmanee J, Boontem P, Govitrapong P. Melatonin administration reverses the alteration of amyloid precursor protein-cleaving secretases expression in aged mouse hippocampus. Neurosci Lett. 2016;621:39–46. doi: 10.1016/j.neulet.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 41.Pant V, Xiong S, Jackson JG, Post SM, Abbas HA, Quintas-Cardama A, et al. The p53-Mdm2 feedback loop protects against DNA damage by inhibiting p53 activity but is dispensable for p53 stability, development, and longevity. Genes Dev. 2013;27:1857–1867. doi: 10.1101/gad.227249.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 43.Peng CX, Hu J, Liu D, Hong XP, Wu YY, Zhu LQ, et al. Disease-modified glycogen synthase kinase-3beta intervention by melatonin arrests the pathology and memory deficits in an Alzheimer's animal model. Neurobiol Aging. 2013;34:1555–1563. doi: 10.1016/j.neurobiolaging.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 44.Proietti S, Cucina A, D'Anselmi F, Dinicola S, Pasqualato A, Lisi E, et al. Melatonin and vitamin D3 synergistically down-regulate Akt and MDM2 leading to TGFbeta-1-dependent growth inhibition of breast cancer cells. J Pineal Res. 2011;50:150–158. doi: 10.1111/j.1600-079X.2010.00824.x. [DOI] [PubMed] [Google Scholar]
- 45.Proietti S, Cucina A, Dobrowolny G, D'Anselmi F, Dinicola S, Masiello MG, et al. Melatonin down-regulates MDM2 gene expression and enhances p53 acetylation in MCF-7 cells. J Pineal Res. 2014;57:120–129. doi: 10.1111/jpi.12150. [DOI] [PubMed] [Google Scholar]
- 46.Purro SA, Galli S, Salinas PC. Dysfunction of Wnt signaling and synaptic disassembly in neurodegenerative diseases. J Mol Cell Biol. 2014;6:75–80. doi: 10.1093/jmcb/mjt049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rahman I, Kinnula VL, Gorbunova V, Yao H. SIRT1 as a therapeutic target in inflammaging of the pulmonary disease. Prev Med. 2012;54(Suppl):S20–S28. doi: 10.1016/j.ypmed.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raz L, Zhang QG, Han D, Dong Y, De Sevilla L, Brann DW. Acetylation of the pro-apoptotic factor, p53 in the hippocampus following cerebral ischemia and modulation by estrogen. PLoS One. 2011;6:e27039. doi: 10.1371/journal.pone.0027039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reppert SM, Weaver DR, Ebisawa T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron. 1994;13:1177–1185. doi: 10.1016/0896-6273(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 50.Rodella LF, Favero G, Rossini C, Foglio E, Bonomini F, Reiter RJ, et al. Aging and vascular dysfunction: beneficial melatonin effects. Age (Dordr) 2013;35:103–115. doi: 10.1007/s11357-011-9336-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosi MC, Luccarini I, Grossi C, Fiorentini A, Spillantini MG, Prisco A, et al. Increased Dickkopf-1 expression in transgenic mouse models of neurodegenerative disease. J Neurochem. 2010;112:1539–1551. doi: 10.1111/j.1471-4159.2009.06566.x. [DOI] [PubMed] [Google Scholar]
- 52.Shi D, Xiao X, Wang J, Liu L, Chen W, Fu L, et al. Melatonin suppresses proinflammatory mediators in lipopolysaccharide-stimulated CRL1999 cells via targeting MAPK, NF-kappaB, c/EBPbeta, and p300 signaling. J Pineal Res. 2012;53:154–165. doi: 10.1111/j.1600-079X.2012.00982.x. [DOI] [PubMed] [Google Scholar]
- 53.Sotthibundhu A, Phansuwan-Pujito P, Govitrapong P. Melatonin increases proliferation of cultured neural stem cells obtained from adult mouse subventricular zone. J Pineal Res. 2010;49:291–300. doi: 10.1111/j.1600-079X.2010.00794.x. [DOI] [PubMed] [Google Scholar]
- 54.Tajes M, Gutierrez-Cuesta J, Ortuno-Sahagun D, Camins A, Pallas M. Anti-aging properties of melatonin in an in vitro murine senescence model: involvement of the sirtuin 1 pathway. J Pineal Res. 2009;47:228–237. doi: 10.1111/j.1600-079X.2009.00706.x. [DOI] [PubMed] [Google Scholar]
- 55.Tajes Orduna M, Pelegri Gabalda C, Vilaplana Hortensi J, Pallas Lliberia M, Camins Espuny A. An evaluation of the neuroprotective effects of melatonin in an in vitro experimental model of age-induced neuronal apoptosis. J Pineal Res. 2009;46:262–267. doi: 10.1111/j.1600-079X.2008.00656.x. [DOI] [PubMed] [Google Scholar]
- 56.Tan DX, Manchester LC, Sainz RM, Mayo JC, Leon J, Hardeland R, et al. Interactions between melatonin and nicotinamide nucleotide: NADH preservation in cells and in cell-free systems by melatonin. J Pineal Res. 2005;39:185–194. doi: 10.1111/j.1600-079X.2005.00234.x. [DOI] [PubMed] [Google Scholar]
- 57.Tocharus C, Puriboriboon Y, Junmanee T, Tocharus J, Ekthuwapranee K, Govitrapong P. Melatonin enhances adult rat hippocampal progenitor cell proliferation via ERK signaling pathway through melatonin receptor. Neuroscience. 2014;275:314–321. doi: 10.1016/j.neuroscience.2014.06.026. [DOI] [PubMed] [Google Scholar]
- 58.Wang J, Shou J, Chen X. Dickkopf-1, an inhibitor of the Wnt signaling pathway, is induced by p53. Oncogene. 2000;19:1843–1848. doi: 10.1038/sj.onc.1203503. [DOI] [PubMed] [Google Scholar]
- 59.Wang J, Xiao X, Zhang Y, Shi D, Chen W, Fu L, et al. Simultaneous modulation of COX-2, p300, Akt, and Apaf-1 signaling by melatonin to inhibit proliferation and induce apoptosis in breast cancer cells. J Pineal Res. 2012;53:77–90. doi: 10.1111/j.1600-079X.2012.00973.x. [DOI] [PubMed] [Google Scholar]
- 60.Witt-Enderby PA, Bennett J, Jarzynka MJ, Firestine S, Melan MA. Melatonin receptors and their regulation: biochemical and structural mechanisms. Life Sci. 2003;72:2183–2198. doi: 10.1016/s0024-3205(03)00098-5. [DOI] [PubMed] [Google Scholar]
- 61.Zemva J, Schilbach K, Stohr O, Moll L, Franko A, Krone W, et al. Central FOXO3a and FOXO6 expression is down-regulated in obesity induced diabetes but not in aging. Exp Clin Endocrinol Diabetes. 2012;120:340–350. doi: 10.1055/s-0031-1297970. [DOI] [PubMed] [Google Scholar]
- 62.Zhao L, An R, Yang Y, Yang X, Liu H, Yue L, et al. Melatonin alleviates brain injury in mice subjected to cecal ligation and puncture via attenuating inflammation, apoptosis, and oxidative stress: the role of SIRT1 signaling. J Pineal Res. 2015;59:230–239. doi: 10.1111/jpi.12254. [DOI] [PubMed] [Google Scholar]
- 63.Zheng Y, Hou J, Liu J, Yao M, Li L, Zhang B, et al. Inhibition of autophagy contributes to melatonin-mediated neuroprotection against transient focal cerebral ischemia in rats. J Pharmacol Sci. 2014;124:354–364. doi: 10.1254/jphs.13220fp. [DOI] [PubMed] [Google Scholar]