Abstract
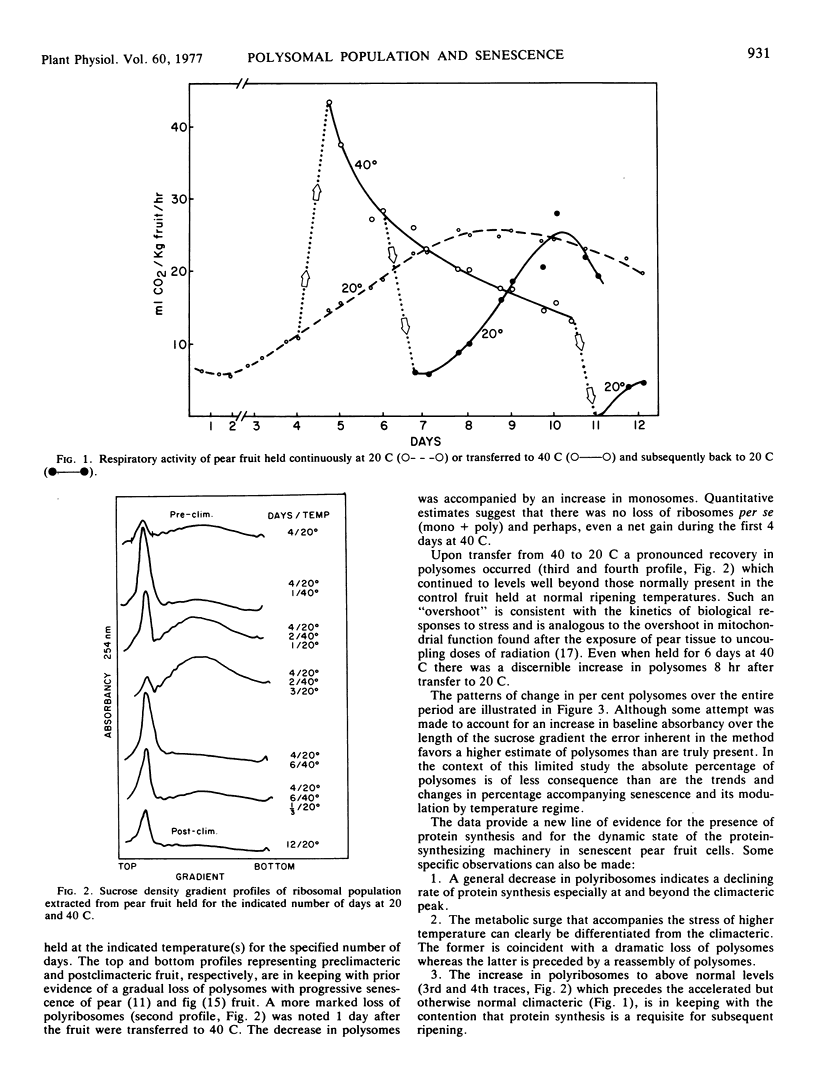
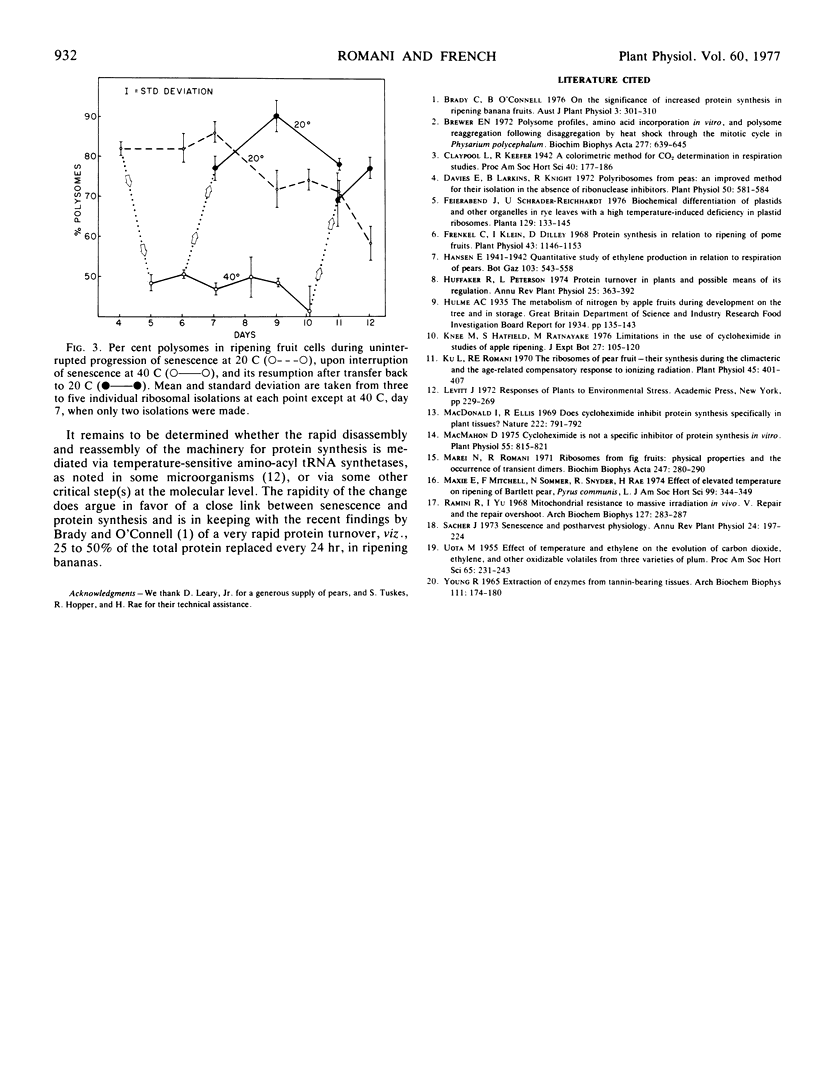
A loss and recovery of polysomes coincident with the temperature-dependent interruption and resumption of pear fruit (Pyrus communis, L.) senescence establishes a close correlation between the presence of protein-synthesizing machinery and the progression of senescence and ripening.
Full text
PDF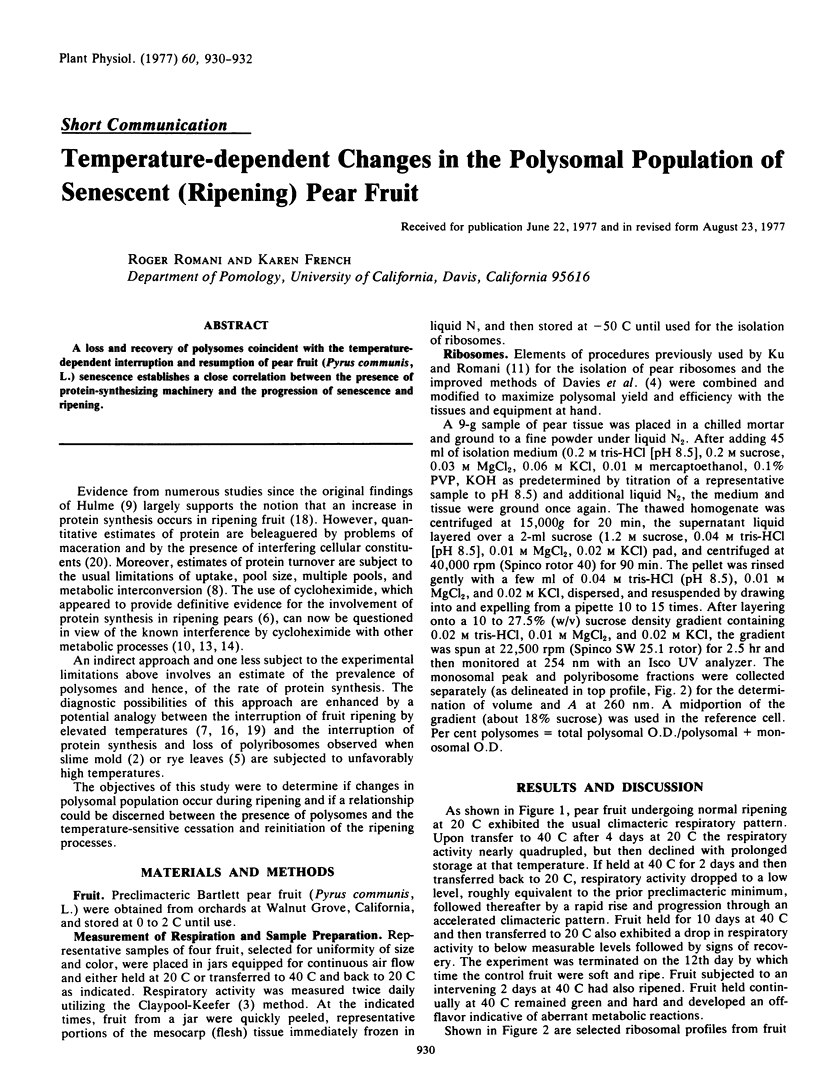
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brewer E. N. Polysome profiles, amino acid incorporation in vitro, and polysome reaggregation following disaggregation by heat shock through the mitotic cycle in Physarum polycephalum. Biochim Biophys Acta. 1972 Sep 14;277(3):639–645. doi: 10.1016/0005-2787(72)90108-6. [DOI] [PubMed] [Google Scholar]
- Bryla J., Kaniuga Z., Frackowiak B. On the nature of endogenous substrate in rat-liver mitochondria. Biochim Biophys Acta. 1967 Sep 6;143(2):285–291. doi: 10.1016/0005-2728(67)90082-5. [DOI] [PubMed] [Google Scholar]
- Crompton M., Palmieri F., Capano M., Quagliariello E. The transport of sulphate and sulphite in rat liver mitochondria. Biochem J. 1974 Jul;142(1):127–137. doi: 10.1042/bj1420127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies E., Larkins B. A., Knight R. H. Polyribosomes from peas: an improved method for their isolation in the absence of ribonuclease inhibitors. Plant Physiol. 1972 Nov;50(5):581–584. doi: 10.1104/pp.50.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day D. A., Hanson J. B. Effect of phosphate and uncouplers on substrate transport and oxidation by isolated corn mitochondria. Plant Physiol. 1977 Feb;59(2):139–144. doi: 10.1104/pp.59.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R., Mannella C. A., Bonner W. D., Jr The external NADH dehydrogenases of intact plant mitochondria. Biochim Biophys Acta. 1973 Jan 18;292(1):105–116. doi: 10.1016/0005-2728(73)90255-7. [DOI] [PubMed] [Google Scholar]
- Frenkel C., Klein I., Dilley D. R. Protein synthesis in relation to ripening of pome fruits. Plant Physiol. 1968 Jul;43(7):1146–1153. doi: 10.1104/pp.43.7.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J. B. Ion transport induced by polycations and its relationship to loose coupling of corn mitochondria. Plant Physiol. 1972 May;49(5):707–715. doi: 10.1104/pp.49.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg M., Palmieri F., Quagliariello E. Quantitative correlation between the distribution of anions and the pH difference across the mitochondrial membrane. Eur J Biochem. 1970 Dec;17(2):230–238. doi: 10.1111/j.1432-1033.1970.tb01158.x. [DOI] [PubMed] [Google Scholar]
- Ku L. L., Romani R. J. The ribosomes of pear fruit. Their synthesis during the climacteric and the age-related compensatory response to ionizing radiation. Plant Physiol. 1970 Apr;45(4):401–407. doi: 10.1104/pp.45.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer G. H., Miller R. J. The osmotic behavior of corn mitochondria. Plant Physiol. 1969 Jun;44(6):839–844. doi: 10.1104/pp.44.6.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marei N., Romani R. Ribosomes from fig fruits: physical properties and the occurrence of transient dimers. Biochim Biophys Acta. 1971 Oct 14;247(2):280–290. doi: 10.1016/0005-2787(71)90676-9. [DOI] [PubMed] [Google Scholar]
- Oestreicher G., Hogue P., Singer T. P. Regulation of Succinate Dehydrogenase in Higher Plants: II. Activation by Substrates, Reduced Coenzyme Q, Nucleotides, and Anions. Plant Physiol. 1973 Dec;52(6):622–626. doi: 10.1104/pp.52.6.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overman A. R., Lorimer G. H., Miller R. J. Diffusion and osmotic transfer in corn mitochondria. Plant Physiol. 1970 Feb;45(2):126–132. doi: 10.1104/pp.45.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani R. J., Yu I. K. Mitochondrial resistance to massive irradiation in vivo. V. Repair and the repair overshoot. Arch Biochem Biophys. 1968 Sep 20;127(1):283–287. doi: 10.1016/0003-9861(68)90227-0. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Takeda H., Kobayashi B. Accumulation of inorganic sulfate by isolated rat-liver mitochondria. J Biochem. 1969 Apr;65(4):641–643. doi: 10.1093/oxfordjournals.jbchem.a129058. [DOI] [PubMed] [Google Scholar]
- Wehrle J. P., Jurkowitz M., Scott K. M., Brierley G. P. Mg2+ and the permeability of heart mitochondria to monovalent cations. Arch Biochem Biophys. 1976 May;174(1):313–323. doi: 10.1016/0003-9861(76)90350-7. [DOI] [PubMed] [Google Scholar]
- Young R. E. Extraction of enzymes from tannin-bearing tissue. Arch Biochem Biophys. 1965 Jul;111(1):174–180. doi: 10.1016/0003-9861(65)90336-x. [DOI] [PubMed] [Google Scholar]


