Abstract
Red blood cell (RBC) transfusions are critical for treatment and prevention of complications of sickle cell disease (SCD), and most SCD patients will receive 1 or more transfusions by age 20. However, SCD alloimmunization remains a serious complication of transfusions that can lead to life-threatening acute and delayed transfusion reactions. Alloimmunization rates are higher in SCD patients most likely due to RBC antigenic differences between largely white donors vs mainly African-American recipients and frequency of transfusions. However, it remains unclear why some but not all SCD patients develop alloantibodies. Cellular immune responses that differ between alloimmunized and nonalloimmunized SCD patients are beginning to be characterized. Altered CD4+ T helper cell responses, known to control immunoglobulin G production, have been identified in alloimmunized SCD patients, including abnormalities in regulatory T cells, as well as helper type 1 (TH1), TH17, and follicular helper T cells. Furthermore, heightened innate immune cell responses to cell free heme with cell polarization toward proinflammatory T cell profiles were recently reported in SCD antibody responders, suggesting that the ongoing hemolytic state in SCD may impair the ability of innate immune cells in these already alloimmunized patients to counter alloimmunization. Identification of molecular pathways in key cellular components that differ between alloimmunized and nonalloimmunized SCD patients is likely to lead to identification of biomarkers of alloimmunization and future design of targeted therapies to prevent or even dampen alloantibody responses in these highly susceptible patients.
Learning Objectives
To provide an overview of our current understanding of cellular immune responses in alloimmunized and nonalloimmunized patients with sickle cell disease
To review the effects of hemolytic state and extracellular free heme on the innate immune compartment in transfused patients with sickle cell disease
Introduction
Sickle cell disease (SCD) arising from a single β-globin mutation (Glu6Val) is the most common hemoglobinopathy and genetic blood disorder, affecting >90 000 people in the United States, mostly African Americans, and millions worldwide.1 Patients with SCD suffer from a range of clinical complications, including chronic hemolytic anemia, acute episodes of pain, stroke, acute chest syndrome, and chronic organ damage. Red blood cell (RBC) transfusion is the main treatment of acute complications of SCD including acute chest syndrome and splenic and hepatic sequestration and is administered for primary and secondary stroke prevention and treatment. Based on the study from the Cooperative study of sickle cell disease published in 1990, the majority of patients with SCD will have received transfusions by age 20.2 However, transfusions carry the risk of alloimmunization that can lead to acute and delayed hemolytic transfusion reactions and delays in obtaining compatible units. In the SCD patient population, delayed hemolytic transfusion reactions can trigger hyperhemolysis, a life-threatening poorly understood phenomena in which the transfused and the patient’s own RBCs are destroyed.3 The incidence of alloimmunization to RBC antigens in patients with SCD has been reported to range from 30% to 40%, which is markedly higher than what is seen in the general population (roughly 2-5%).3 The higher rates of alloimmunization in patients with SCD is in part due to RBC antigenic disparity between mostly white blood donors and transfusion recipients who are of African descent and partly due to the frequency of transfusions. Because most of the alloantibodies are against C, E, and K1 antigens, many centers in the United States have adopted the use of C, E, and K phenotypically matched units from primarily African-American donors for SCD patients. However, patients continue to develop alloantibodies because of genetic diversity of the RH locus in donors of African ancestry.4
T-cell regulation of SCD alloimmunization
Genetic and acquired patient-related factors influence alloimmunization. SCD is a chronic inflammatory condition, associated with marked inflammation and immune activation.3 Heightened immune effector cell responses and/or alterations in immune regulation of effector responses are likely to drive alloantibody production, but the immunoregulatory networks and cellular components remain largely uncharacterized in human SCD. Regulatory T cells (Tregs) can suppress B cells either directly5,6 or indirectly through suppression of effector T helper (TH) cells,7 which in turn control immunoglobulin G (IgG) antibody responses. It is therefore likely that any decrease in Treg numbers/activity will increase the likelihood of antibody production. In a small study of chronically transfused SCD patients, we observed reduced peripheral Treg suppressive function and altered TH responses with higher circulating interferon-γ (TH1) but lower interleukin 10 (IL-10; anti-inflammatory) cytokine levels in antibody responders compared with nonresponders.8 The Treg studies were performed using purified population of Tregs and effector T cells and in the absence of accessory cells, enabling the assessment of Treg functions in isolation. In contrast, when accessory cells were present in the assay conditions, differences in Treg activity between alloimmunized and nonalloimmunized SCD patients were no longer detectable,9 underscoring the importance of crosstalk between Tregs and accessory cells.10 In addition to impaired Treg function is alloimmunized SCD patients, we also found altered activity of B regulatory cells (Bregs) in these patients, specifically in the ability of their Bregs to inhibit proinflammatory cytokine expression by monocytes.11 Altogether, these data support our working hypothesis that SCD alloimmunized patients have an altered immunoregulatory compartment that puts them at a higher risk of alloimmunization. It remains to be determined whether this altered immunoregulatory state is genetically inherited as has been predicted by mathematical modeling12 or if the altered state is established only after the patient becomes alloimmunized. It is interesting to note that both in non-SCD and SCD settings, once a patient becomes alloimmunized, it is more likely that they make additional alloantibodies.2,13,14
Heightened effector T-cell responses and SCD alloimmunization
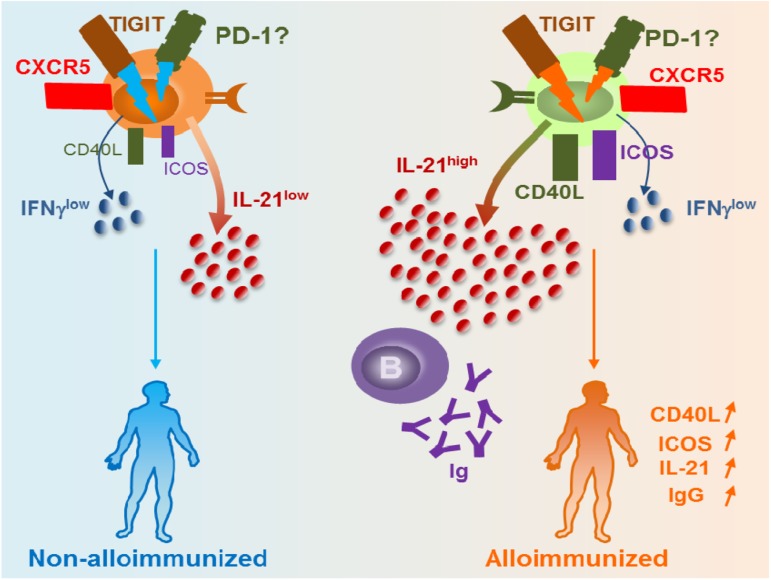
The result of a weakened immunoregulatory state is increased effector functions including heightened T-cell responses. By comparing SCD patients who had developed antibodies to Kidd system antigen, Jkb, to nonalloimmunized SCD patients, Vingert et al found that 5 of 9 of the responders had antigen-specific TH17 responses, and only 1 or 2 of them produced Jkb-specific TH1 or TH2 cytokine profiles; none of the nonalloimmunized or healthy donors elicited any response.15 These data, albeit in a small cohort, suggest heightened T-cell responses at least against Jkb antigen in SCD antibody (anti-Jkb) responders. Interestingly, antigen-specific follicular helper T (TFH) cell responses were detected in 3 of 9 patients. Of all the TH subsets, TFH cells have emerged as the key effector CD4+ T cells specialized in providing help to B cells to generate the initial wave of antibody response, as well as in supporting B-cell differentiation into high affinity antibody-producing B cells and long-lasting IgG antibodies.16 Recent studies have identified human blood TFH cells expressing similar surface markers as lymphoid TFH cells including the chemokine receptor CXCR5, high levels of inhibitory costimulatory molecule PD-1 (program death 1), and IL-21, a highly potent B-cell differentiation cytokine.17 Our group recently described a novel subset of TFH cells expressing TIGIT (T-cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory domains) that provides the most efficient B-cell help for IgG production.18 Furthermore, we showed that expression of TIGIT on TFH cells was necessary for mediating B-cell help responses because TIGIT blockade using anti-TIGIT antibodies in the T-B cocultures resulted in inhibition of IgG production.18 In the SCD setting, frequencies of TIGIT+ TFH cell and expression levels of TIGIT per cell basis were comparable between allo- vs nonalloimmunized patients. However, only TIGIT+ TFH cells from alloimmunized SCD patients were able to induce RBC-specific IgG production by B cells in T-B cocultures.18 Compared with TIGIT+ TFH cells from nonalloimmunized SCD patients, TIGIT+ TFH cells from alloimmunized SCD patients expressed significantly more IL-21 and higher levels of costimulation marker CD40L. These data suggest that, despite comparable levels of TIGIT TFH cells, the functional activity of TIGIT+ TFH cells differs between alloimmunized and nonalloimmunized SCD patients (Figure 1). Heightened TIGIT+ TFH responses in the alloimmunized group may be due to increased TIGIT signaling, or conversely, nonalloimmunized SCD patients may have impaired TIGIT-mediated responses and TIGIT signaling pathway(s). We are currently characterizing the differences in TIGIT-mediated responses between alloimmunized and nonalloimmunized SCD patients with the goal to identify potential TIGIT+ TFH-associated biomarkers of alloimmunization. These studies have the potential to aid development of targeted TIGIT+ TFH-based therapeutic strategies such as TIGIT blockade to prevent alloimmunization. Interestingly, many alloantibodies disappear (or at least fall in antibody titers to below detection levels, also termed evanescence) after several months in SCD patients,4 an incompletely understood phenomenon that in a recent study of general transfused population has an estimated 50% occurrence rate.19 Given their key role in providing help to B cells to support their activation, expansion, and differentiation, TFH cells are likely to be critical in the induction and persistence of the alloantibody response in responders. Studies to address the role of TIGIT+ TFH cells are ongoing, but it is also interesting to note that we found higher global IgG responses in allo- vs nonalloimmunized SCD patients, raising the provocative possibility that alloimmunized patients may display higher TIGIT+ TFH responses not just against RBC antigens but also nonspecifically, although larger studies are needed to confirm these findings.
Figure 1.
Differences in TIGIT+ TFH activity between alloimmunized and nonalloimmunized SCD patients. Our data suggest that alloimmunized patients with SCD have comparable levels of TIGIT and frequency of TIGIT+ TFH cells as nonalloimmunized SCD patients. However, functional activity of TIGIT+ TFH cells differs between the 2 groups: TIGIT+ TFH cells from nonalloimmunized SCD patients express less B-cell costimulatory markers such as Inducible T-cell COStimulator (ICOS) and CD40 ligand (CD40L) and produce less IL-21, which we hypothesize is due to differences in TIGIT signaling between the 2 groups. As a result, these cells from nonalloimmunized SCD patients are less effective in providing help to B cells to produce IgG compared with alloimmunized SCD patients who have more potent TIGIT+ TFH cells.
Role of hemolysis in SCD alloimmunization
SCD complications and clinical “subphenotypes” have traditionally been divided into those caused by hemolysis and those caused by vaso-occlusion. However, there is increasing evidence that free hemoglobin and heme play central roles in pathophysiology of its complications such as pain crisis and acute chest syndrome.20 The chronic hemolytic state of SCD results in substantial release of free hemoglobin and its oxidized form heme in the circulation.21,22 Free hemoglobin and heme cause pro-oxidant and cytotoxic damage through extravascular translocation; free radical generation and oxidative damage to lipids, proteins, and nucleic acids; and activation of inflammatory cascades.20 Heme triggers Toll-like receptor 4 signaling in endothelial cells, resulting in expression of adhesion molecules and vaso-occlusive crisis in mouse models of SCD.23 Interestingly, a heme homolog can trigger an anti-inflammatory response in innate immune cells in a non-SCD setting.24 It is therefore likely that the extent to which the innate immune cells of SCD patients can counter this potent inflammatory agonist will impact alloimmunization.
In a cross-sectional study, differences in monocyte control of Treg vs T-effector cells in alloimmunized vs nonalloimmunized SCD patients in response to extracellular heme were identified.25 In vitro Treg proliferation was increased, whereas TH1 polarization decreased in response to hemin, an oxidized form of heme, in nonalloimmunized patients in part through downregulation of IL-12 by monocytes.25 In contrast, monocytes from alloimmunized SCD patients did not induce an increase in Tregs and were not able to dampen IL-12 or TH1 responses following exposure to extracellular hemin. These data suggest that alloimmunized SCD patients may have a defective response to cell free heme with respect to their ability to switch off the heme-associated proinflammatory (IL-12) insult, resulting in a low Treg/high TH1 state.26 Due to the heightened proinflammatory state, we hypothesize that these patients will be more likely to mount an immune response against allogeneic RBCs. Consistent with the latter data, Fasano et al27 found that transfusions given to already alloimmunized patients during acute conditions associated with increased hemolysis such as acute chest syndrome and vaso-occlusive crisis resulted in a higher rate of new RBC alloantibody formation.
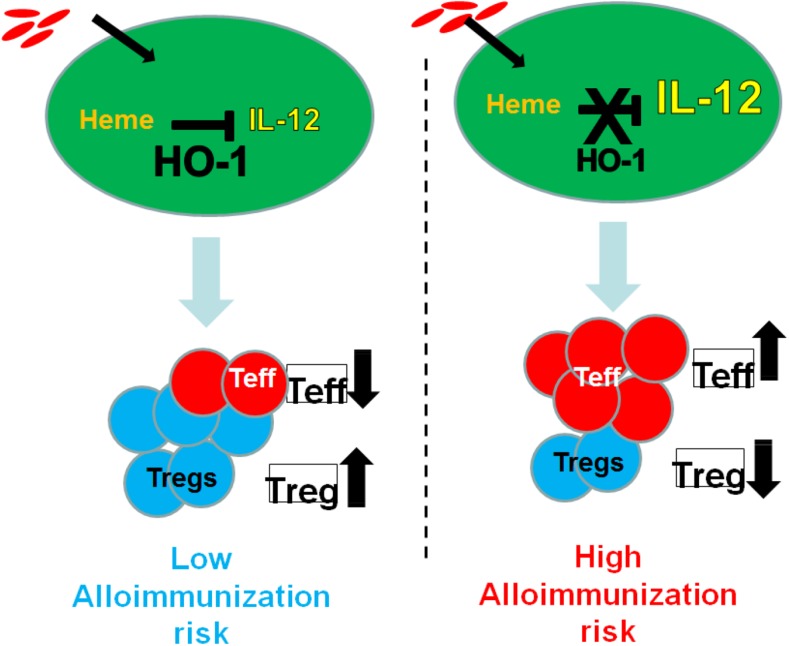
Patients with SCD have very low levels of haptoglobin and hemopexin, which normally remove free hemoglobin and free heme, respectively, from the circulation.27-29 Heme oxygenase 1 (HO-1), a cytoprotective enzyme induced by heme,30 is upregulated in SCD31,32 and is likely to play a major role when haptoglobin/hemopexin scavenging capacities are overwhelmed. HO-1 removes heme from the circulation by breaking it down into iron, which is rapidly bound by ferritin, and bilirubin and carbon monoxide, which confer cytoprotective and anti-inflammatory effects.30 Deficiency of HO-1 in mice and the 1 reported human case is associated with chronic inflammation.33 In non-SCD settings, HO-1 is immunosuppressive: it inhibits T-lymphocyte proliferation, blocks maturation of dendritic cells, and inhibits proinflammatory and allogeneic immune responses.24,34 In non-SCD myeloid-derived monocytes/macrophages/dendritic cells, HO-1 expression inhibits inflammatory cytokine (IL-6, IL-12, tumor necrosis factor α, IL-1β) and increases regulatory cytokine (IL-10) expression.35-37 Thus, HO-1 levels/activity in response to hemin may be a critical parameter to switch the activity of monocyte/macrophages from proinflammatory to immunoregulatory. In a small study of chronically transfused patients with SCD, we found lower levels of HO-1 in monocytes from alloimmunized than nonalloimmunized SCD patients.25 These data raise the possibility that low HO-1 levels/activity in innate immune cells of SCD patients who have chronic hemolysis leads to inability to effectively detoxify heme, leading to a proinflammatory state (high effector T cell/low Treg) and heightening their risk of alloimmunization (Figure 2). In contrast, effective removal of heme (in nonalloimmunized) patients ensures an anti-inflammatory immunoregulatory state (high Treg/low Teff) that is less conducive to alloimmunization (Figure 2). Prospective studies are ongoing to determine whether HO-1 is a precursor to alloimmunization or conversely if alloimmunization leads to suppressed HO-1 levels. Taking this one step further, one can speculate that impaired monocyte HO-1 levels in alloimmnized SCD patients will result in altered release of HO-1 catalytic byproducts. Carbon monoxide and biliverdin mediate antioxidative and anti-inflammatory effects, whereas iron can cause oxidative damage if not bound to ferritin.30 Interestingly, iron chelation and antioxidants can modify expression and activation of HO-1.38,39 Thus, more effective iron chelation and antioxidant treatment may overcome the altered Treg/Th polarized response to hemin in alloimmunized SCD patients, restoring their monocyte immunoregulatory activity and ultimately minimizing SCD alloimmunization. Some of these agents (CO, antioxidants, and iron chelators) have shown efficacy in patients with SCD or are being tested in clinical trials in SCD patients40 and may be used to inhibit and/or avert alloimmunization.
Figure 2.
HO-1 levels/activity in innate immune cells of SCD patients in response to ongoing hemolysis is a critical switch between proinflammatory vs immunoregulatory states, which in turn affect alloimmunization risk. We hypothesize that SCD patients with high HO-1 levels/activity will effectively inhibit the proinflammatory cytokine IL-12 in response to extracellular cell free heme. This will result in higher Treg/T effector ratios, which in turn will suppress B-cell responses in these individuals, lowering their risk of alloimmunization. In contrast, low HO-1 levels/activity will lead to inability to dampen IL-12, thereby lowering Treg/T effector ratios and increasing the risk of alloimmunization.
Acknowledgments
The authors thanks the members of the Laboratory of Complement Biology, Emmanuelle Godefroy, Hui Zhong, and Yunfeng Liu for helpful discussions and in preparing the figures.
This work was supported in part by the National Heart, Lung, and Blood Institute of the National Institutes of Health grants R01HL121415 and R01HL130139 and American Heart Association grant 14GRNT20480109.
References
- 1.Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med. 2010;38(suppl 4):S512-S521. [DOI] [PubMed] [Google Scholar]
- 2.Rosse WF, Gallagher D, Kinney TR, et al. ; The Cooperative Study of Sickle Cell Disease. Transfusion and alloimmunization in sickle cell disease. Blood. 1990;76(7):1431-1437. [PubMed] [Google Scholar]
- 3.Yazdanbakhsh K, Ware RE, Noizat-Pirenne F. Red blood cell alloimmunization in sickle cell disease: pathophysiology, risk factors, and transfusion management. Blood. 2012;120(3):528-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou ST, Jackson T, Vege S, Smith-Whitley K, Friedman DF, Westhoff CM. High prevalence of red blood cell alloimmunization in sickle cell disease despite transfusion from Rh-matched minority donors. Blood. 2013;122(6):1062-1071. [DOI] [PubMed] [Google Scholar]
- 5.Lim HW, Hillsamer P, Banham AH, Kim CH. Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J Immunol. 2005;175(7):4180-4183. [DOI] [PubMed] [Google Scholar]
- 6.Iikuni N, Lourenço EV, Hahn BH, La Cava A. Cutting edge: Regulatory T cells directly suppress B cells in systemic lupus erythematosus. J Immunol. 2009;183(3):1518-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wing JB, Sakaguchi S. Multiple treg suppressive modules and their adaptability. Front Immunol. 2012;3:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bao W, Zhong H, Li X, et al. . Immune regulation in chronically transfused allo-antibody responder and nonresponder patients with sickle cell disease and β-thalassemia major. Am J Hematol. 2011;86(12):1001-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vingert B, Tamagne M, Desmarets M, et al. . Partial dysfunction of Treg activation in sickle cell disease. Am J Hematol. 2014;89(3):261-266. [DOI] [PubMed] [Google Scholar]
- 10.Kornete M, Piccirillo CA. Functional crosstalk between dendritic cells and Foxp3(+) regulatory T cells in the maintenance of immune tolerance. Front Immunol. 2012;3:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bao W, Zhong H, Manwani D, et al. . Regulatory B-cell compartment in transfused alloimmunized and non-alloimmunized patients with sickle cell disease. Am J Hematol. 2013;88(9):736-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JM, Sloan SR. Stochastic modeling of human RBC alloimmunization: evidence for a distinct population of immunologic responders. Blood. 2008;112(6):2546-2553. [DOI] [PubMed] [Google Scholar]
- 13.Schonewille H, van de Watering LM, Brand A. Additional red blood cell alloantibodies after blood transfusions in a nonhematologic alloimmunized patient cohort: is it time to take precautionary measures? Transfusion. 2006;46(4):630-635. [DOI] [PubMed] [Google Scholar]
- 14.Castro O, Sandler SG, Houston-Yu P, Rana S. Predicting the effect of transfusing only phenotype-matched RBCs to patients with sickle cell disease: theoretical and practical implications. Transfusion. 2002;42(6):684-690. [DOI] [PubMed] [Google Scholar]
- 15.Vingert B, Tamagne M, Habibi A, et al. . Phenotypic differences of CD4(+) T cells in response to red blood cell immunization in transfused sickle cell disease patients. Eur J Immunol. 2015;45(6):1868-1879. [DOI] [PubMed] [Google Scholar]
- 16.Crotty S. A brief history of T cell help to B cells. Nat Rev Immunol. 2015;15(3):185-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ueno H. Human circulating T follicular helper cell subsets in health and disease. J Clin Immunol. 2016;36(suppl 1):34-39. [DOI] [PubMed] [Google Scholar]
- 18.Godefroy E, Zhong H, Pham P, Friedman D, Yazdanbakhsh K. TIGIT-positive circulating follicular helper T cells display robust B-cell help functions: potential role in sickle cell alloimmunization. Haematologica. 2015;100(11):1415-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tormey CA, Stack G. The persistence and evanescence of blood group alloantibodies in men. Transfusion. 2009;49(3):505-512. [DOI] [PubMed] [Google Scholar]
- 20.Schaer DJ, Buehler PW, Alayash AI, Belcher JD, Vercellotti GM. Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood. 2013;121(8):1276-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lezcano NE, Odo N, Kutlar A, Brambilla D, Adams RJ. Regular transfusion lowers plasma free hemoglobin in children with sickle-cell disease at risk for stroke. Stroke. 2006;37(6):1424-1426. [DOI] [PubMed] [Google Scholar]
- 22.Reiter CD, Wang X, Tanus-Santos JE, et al. . Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8(12):1383-1389. [DOI] [PubMed] [Google Scholar]
- 23.Belcher JD, Chen C, Nguyen J, et al. . Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood. 2014;123(3):377-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chauveau C, Rémy S, Royer PJ, et al. . Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL-10 expression. Blood. 2005;106(5):1694-1702. [DOI] [PubMed] [Google Scholar]
- 25.Zhong H, Bao W, Friedman D, Yazdanbakhsh K. Hemin controls T cell polarization in sickle cell alloimmunization. J Immunol. 2014;193(1):102-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong H, Yazdanbakhsh K. Differential control of Helios(+/-) Treg development by monocyte subsets through disparate inflammatory cytokines. Blood. 2013;121(13):2494-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bourantas KL, Dalekos GN, Makis A, Chaidos A, Tsiara S, Mavridis A. Acute phase proteins and interleukins in steady state sickle cell disease. Eur J Haematol. 1998;61(1):49-54. [DOI] [PubMed] [Google Scholar]
- 28.Muller-Eberhard U, Javid J, Liem HH, Hanstein A, Hanna M. Plasma concentrations of hemopexin, haptoglobin and heme in patients with various hemolytic diseases. Blood. 1968;32(5):811-815. [PubMed] [Google Scholar]
- 29.Naumann HN, Diggs LW, Barreras L, Williams BJ. Plasma hemoglobin and hemoglobin fractions in sickle cell crisis. Am J Clin Pathol. 1971;56(2):137-147. [DOI] [PubMed] [Google Scholar]
- 30.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86(2):583-650. [DOI] [PubMed] [Google Scholar]
- 31.Nath KA, Grande JP, Haggard JJ, et al. . Oxidative stress and induction of heme oxygenase-1 in the kidney in sickle cell disease. Am J Pathol. 2001;158(3):893-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jison ML, Munson PJ, Barb JJ, et al. . Blood mononuclear cell gene expression profiles characterize the oxidant, hemolytic, and inflammatory stress of sickle cell disease. Blood. 2004;104(1):270-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci USA. 1997;94(20):10919-10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rémy S, Blancou P, Tesson L, et al. . Carbon monoxide inhibits TLR-induced dendritic cell immunogenicity. J Immunol. 2009;182(4):1877-1884. [DOI] [PubMed] [Google Scholar]
- 35.Ma JL, Yang PY, Rui YC, Lu L, Kang H, Zhang J. Hemin modulates cytokine expressions in macrophage-derived foam cells via heme oxygenase-1 induction. J Pharmacol Sci. 2007;103(3):261-266. [DOI] [PubMed] [Google Scholar]
- 36.Kapturczak MH, Wasserfall C, Brusko T, et al. . Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse. Am J Pathol. 2004;165(3):1045-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Listopad J, Asadullah K, Sievers C, et al. . Heme oxygenase-1 inhibits T cell-dependent skin inflammation and differentiation and function of antigen-presenting cells. Exp Dermatol. 2007;16(8):661-670. [DOI] [PubMed] [Google Scholar]
- 38.Kruger AL, Peterson SJ, Schwartzman ML, et al. . Up-regulation of heme oxygenase provides vascular protection in an animal model of diabetes through its antioxidant and antiapoptotic effects. J Pharmacol Exp Ther. 2006;319(3):1144-1152. [DOI] [PubMed] [Google Scholar]
- 39.Suttner DM, Dennery PA. Reversal of HO-1 related cytoprotection with increased expression is due to reactive iron. FASEB J. 1999;13(13):1800-1809. [DOI] [PubMed] [Google Scholar]
- 40.Vichinsky E. Emerging ‘A' therapies in hemoglobinopathies: agonists, antagonists, antioxidants, and arginine. Hematol Am Soc Hematol Educ Prog. 2012;2012:271-275. [DOI] [PubMed]