Abstract
For effective adaptive immunity to foreign antigens (Ag), secondary lymphoid organs (SLO) provide the confined environment in which Ag-restricted lymphocytes, with very low precursor frequencies, interact with Ag on Ag-presenting cells (APC). The spleen is the primordial SLO, arising in conjunction with adaptive immunity in early jawed vertebrates. The spleen, especially the spleen’s lymphoid compartment, the white pulp (WP), has undergone numerous modifications over evolutionary time. We describe the progressive advancement of splenic WP complexity, which evolved in parallel with the increasing functionality of adaptive immunity. The Ag-presenting function of follicular dendritic cells (FDC) also likely emerged at the inception of adaptive immunity, and we propose that a single type of hematopoietically derived APC displayed Ag to both T and B cells. A dedicated FDC, derived from a vascular precursor, is a recent evolutionary innovation that likely permitted the robust affinity maturation found in mammals.
Keywords: evolution, spleen, white pulp, adaptive immunity, antigen presentation
INTRODUCTION
The adaptive immune system emerged 500 million years ago (MYA) in a common ancestor of the jawed vertebrates (gnathostomes) and jawless vertebrates. B cells, which arise in the bone marrow of mammals and in various tissues in other vertebrates (Figure 1), express a B cell receptor [BCR, also termed antibody or immunoglobulin (Ig)] generated by a somatic gene rearrangement process that produces extremely high levels of diversity (Tonegawa 1983). T cells differentiate in the thymus or its equivalent in all vertebrates, and their antigen (Ag) receptor is the T cell receptor (TCR), also generated by gene rearrangement with the same enzymology as for the BCR. BCR generally recognize conformational epitopes on foreign Ag, whereas TCR recognize peptides that are derived from Ag bound to self major histocompatibility complex (MHC) class I or class II proteins.
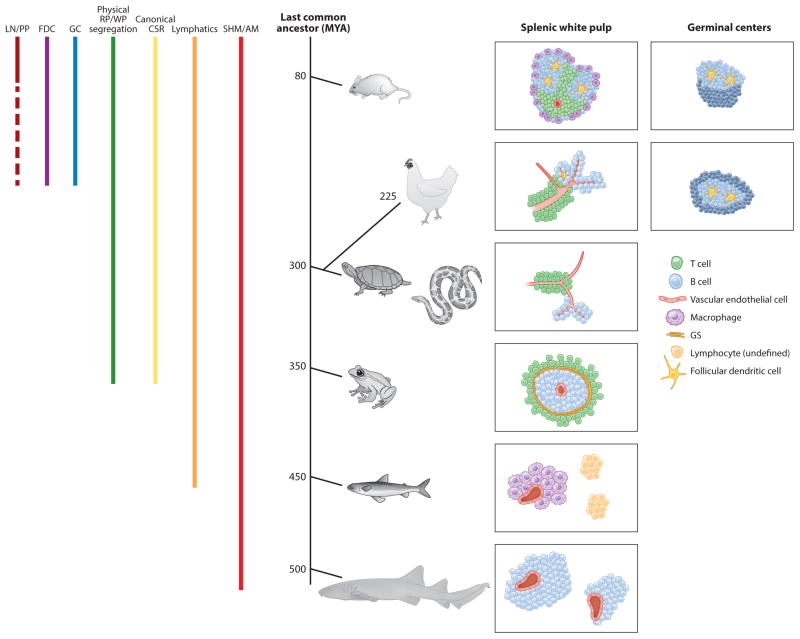
Figure 1.
Progressive accumulation of splenic white pulp complexity and functionality over the course of vertebrate evolution. The columns at left denote the presence of indicated cell types, substructures, or processes in the species shown in the middle of the figure. The timeline (middle) shows the estimated date of the last common ancestor shared between humans and the indicated species. At right, graphical representations of white pulp microarchitecture and cellular constituency (as well as germinal center composition, when present) are indicated in the species shown in the timeline. Abbreviations shown at left: CSR, class switch recombination; FDC, follicular dendritic cell; GC, germinal center; GS, Grenzschichtmembran of Sterba; LN, lymph node; MYA, million years ago; PP, Peyer’s patch; RP, red pulp; SHM/AM, somatic hypermutation/affinity maturation; WP, white pulp. Data from Mebius & Kraal (2005) for mammals; Yasuda et al. (1998, 2003) for birds; Leceta & Zapata (1985, 1991) for reptiles; Baldwin & Cohen (1981) for amphibians; Flajnik & Du Pasquier (2013) for bony fish; and Fange & Pulsford (1983), Rumfelt et al. (2002), and Castro et al. (2013) for cartilaginous fish.
Given the great diversity of somatically generated BCR and TCR in each individual, there is a requisite low precursor frequency of lymphocytes reactive with a given antigenic epitope ( Jenkins & Moon 2012). Thus, there is a requirement for a physical structure or location in which both Ag-restricted lymphocytes and foreign Ag can meet; without such a dedicated tissue site, the likelihood of Ag and one of the few potentially reactive lymphocytes encountering each other within the vertebrate body is extremely low. Secondary lymphoid organs (SLO) emerged in evolution to provide the structural framework necessary for the coconcentration of Ag and Ag-specific lymphocytes to initiate an efficient adaptive immune response (Hofmann et al. 2010). Not only must SLO provide this physical location and structural framework to promote lymphocyte:Ag encounters, they must also possess mechanisms for the recruitment of lymphocytes and for the acquisition and retention of Ag.
The spleen is the primordial SLO, and it evolved concurrently with adaptive immunity based on rearranging Ig and TCR Ag receptors (Boehm et al. 2012a). Indeed, it is the only SLO found in all jawed vertebrates. The spleen is unique among SLO in its functional and histological segregation into two discrete areas: the red pulp (RP) and the white pulp (WP) (Mebius & Kraal 2005). The RP is tasked with filtration of the blood, including removal of effete erythrocytes and free heme for iron recycling, as well as pathogen (especially bacterial) capture and clearance. The WP is the spleen’s lymphoid component, where T cells, B cells, and Ag-presenting cells (APC) reside under resting conditions. This architectural and functional dichotomy of RP and WP has been conserved since the emergence of the spleen in early jawed vertebrates approximately 500 MYA (Figure 1). Of note, the spleen is dispensable for the filtration of effete erythrocytes and heme from the blood; splenectomized or congenitally asplenic mammals are viable, as the liver is also capable of performing these duties. Thus, the spleen’s original primary function may have been as an SLO, with its blood filtration capacity arising later in evolution.
Herein, we refer to bona fide SLO as structures that are dedicated to the coconcentration of Ag and Ag-restricted lymphocytes and that arise at a developmental time point and anatomical location specific to a given vertebrate species. Additionally, SLO have a defined, although species-specific, microarchitecture that is characterized by discrete areas for B cells and T cells. In mammals, lymph nodes (LN) and the Peyer’s patches (PP) of the gut also have these characteristics, whereas only the spleen does in lower vertebrates. Additional lymphoid structures exist in lower vertebrates (and mammals) and are referred to by different names, depending on their location and the context in which they appear. Mucosa-associated lymphoid tissue (MALT) is present in all vertebrates, although the structures observed prior to the evolution of PP and mesenteric LN in mammals lack the defining microarchitecture of a proper SLO (the nasal-associated lymphoid tissue may be an exception; see below). Furthermore, many of these poorly defined structures appear in the lower vertebrates only after antigenic stimulation.
Another central component of SLO is the presence of APC dedicated to the acquisition of Ag and its subsequent presentation to Ag-restricted lymphocytes in a context capable of eliciting a productive response. Dendritic cells (DC), both conventional (cDC) and follicular (FDC), have been extensively characterized in mammals and birds, and interest in their evolutionary origins is increasing. Naive αβ T cells require peptidic Ag presentation by another cell type, typically cDC, in the context of MHC (Steinman 2012). FDC, which have been positively identified only in birds and mammals, perform an Ag presentation function for B cells, although they present native, unprocessed Ag.
A progressive refinement of both adaptive immunity and SLO microarchitecture and function is evident during evolution of the vertebrates (Figure 1). The spleen of the cartilaginous fish accepts and filters only the blood, as cartilaginous fish lack a lymphatic system (Smith et al. 2015); lymphatics arose in teleost (bony) fish (Figure 1). Physical segregation of the splenic WP from the RP—defined by the presence of a discrete structure or cell population between the WP and RP—is first unambiguously observed in amphibians, along with the capacity for Ig genes to undergo canonical class switch recombination (CSR). Germinal centers (GC) are not evident in the spleen until the appearance of birds. Below, we review the spleen and its microarchitecture, cellular constituency, and functional capacity (with regard to the stimulation of adaptive immunity) throughout its evolution, alongside the evolution of adaptive immunity (Figure 1). We begin with the well-studied mammalian spleen, which has the most complex structure, to provide the frame of reference into which its evolutionary predecessors can be placed. This discussion is followed by a review of published data on the spleen, splenic WP, and splenic cellular constituency in all jawed vertebrate classes. We illustrate the progressive accumulation of splenic WP complexity and functional capacity that evolved in parallel with the adaptive immune system, complementing and enabling the progressive augmentation of complexity and potency of adaptive lymphocyte responses throughout vertebrate evolution.
THE MOUSE SPLEEN
The Mouse Splenic Red Pulp
The primary function of the splenic RP is filtration of the blood (reviewed in Cesta 2006, Mebius & Kraal 2005). The multiple splenic WP are associated with arterioles that eventually empty into the RP cords, which are composed of reticular fibers and fibroblasts forming an open blood system without an endothelial lining (Mebius & Kraal 2005). Blood is returned to the circulation via the venous sinuses of the RP, into which blood flows from the cords. Filtration of effete erythrocytes (i.e., aging erythrocytes that have lost membrane plasticity) is attributed to the ultrastructure of the venous sinuses of the RP, described as a “discontinuous endothelium” (den Haan et al. 2012). A lining of endothelial cells, positioned parallel to each other and connected by annular fibers and stress fibers, composes the sinuses (Mebius & Kraal 2005). The contractile stress fibers allow for adjustment of the positioning and proximity of the endothelial cells to each other, creating slits between the endothelial cells in which effete red blood cells (RBC) can be trapped/immobilized. RP macrophages then phagocytose and digest the RBC, ultimately resulting in the regeneration of free ferrous iron (Fe2+). RP macrophages also scavenge hemoglobin released after hemolysis of RBC outside the spleen.
The Mouse Splenic White Pulp
The term WP can refer to the entirety of the lymphoid compartment of the spleen or to an individual structure with the following characteristics: a central arteriole surrounded by a periarteriolar lymphoid sheath (PALS) of T cells, adjacent to which are one or more B cell follicles (FO), all of which is bounded by the marginal zone (MZ), which separates each individual WP from the RP (Figure 1). Hereafter, we use the term WP to refer to the individual structure, which is described in detail below.
The Mouse Splenic White Pulp: The T Cell Zone (Periarteriolar Lymphoid Sheath)
The PALS is populated by T cells, cDC, and stromal cells. T cell (and cDC) recruitment to the WP is governed by the chemokine receptor CCR7 on T cells in response to the chemokines CCL19 [originally named ELC (EBI1 ligand chemokine)] and CCL21 [originally named SLC (secondary lymphoid tissue chemokine)], which are produced most abundantly by a population of radioresistant, gp38+ stromal cells, specifically fibroblastic reticular cells (FRC) (Luther et al. 2000). FRC also produce the T cell survival factor cytokine IL-7 (Link et al. 2007). CCL19 is also produced, albeit at lower levels, by CD8+ DC within the PALS (Luther et al. 2000). The physical framework of the PALS is also established by the FRC; this framework provides structural integrity to the PALS and also allows for T cell entry into, and movement within and throughout, the PALS (Bajenoff et al. 2008). Entry from the RP, via the MZ, is facilitated by FRC-derived bridging channels. Within the PALS, a three-dimensional reticulum is formed by the interconnected FRC; lymphocytes fill the spaces within this network (den Haan et al. 2012). The T cells not only fill up the network but are in constant motion within it, sampling pMHC + Ag on cDC throughout the PALS. Ag acquisition by WP cDC, as well as migration of external cDC into the WP, is discussed below.
The Mouse Splenic White Pulp: The B Cell Zone (Follicle)
The B cell FO comprises B cells and FDC (see below). FDC produce the B cell chemoattractant cytokine (chemokine) CXCL13 (BLC), which, upon interacting with CXCR5, induces not only the migration of B cells into the FO, but also their expression of lymphotoxin (LT)α1β2, which in turn induces and maintains the maturation and differentiation of FDC (Ansel et al. 2000, Krautler et al. 2012). This positive feedback loop is necessary for the establishment and maintenance of FO integrity. Tumor necrosis factor (TNF)α and its receptor, TNFR1, are also necessary for the establishment and maintenance of FO and WP integrity, although the precise requirements for, and the effects of, TNFα and TNFR1 are not well understood. Genetic ablation of any member of these pathways results in an inability of the WP to properly form, and inhibition or blockade of these cytokines and receptors disrupts already established WP microarchitecture (Ansel et al. 2000, Koni et al. 1997, Pasparakis et al. 1996). FDC also express the cellular adhesion molecules VCAM-1 and ICAM-1 (Koopman et al. 1991), both of which are involved in FDC:B cell interaction and binding, as well as the B cell survival factor/cytokine BAFF (Gorelik et al. 2003). B cell migration within the quiescent splenic WP FO has not been studied as extensively as B cell migration within the quiescent LN FO; splenic FO B cells are nonetheless presumed to be in constant motion within the FO.
The Mouse Splenic White Pulp: Follicular Dendritic Cells
FDC were first identified by their branching morphology and capacity to trap immune complexes in SLO FO (Aguzzi et al. 2014, Klaus et al. 1980). FDC initiate humoral immune responses by retaining and presenting native Ag at their plasma membrane to B cells. FDC have long been known, on the basis of their radioresistance, to derive from a nonhematopoietic precursor and to be dependent upon LTα1β2 signaling via the LTβR (Endres et al. 1999, Ngo et al. 1999). The precursor cells of FDC (pre-FDC) were recently identified as ubiquitous, perivascular mural cells expressing platelet-derived growth factor receptor β (Krautler et al. 2012).
As mentioned above, the BCR recognizes epitopes on native (i.e., unprocessed) Ag. Engagement and cross-linking of multiple BCR on a single B cell are required for cellular activation. FDC provide a scaffolding platform upon which native Ag can be arrayed for efficient B cell activation, as FDC acquire and retain native Ag at their plasma membranes via complement receptors CR1 and CR2 (CD35 and CD21, respectively) and the Fc receptor CD32b (reviewed in Allen & Cyster 2008). The interdigitating network of the FDC’s dendritic processes permeates the FO, allowing Ag to be arrayed throughout the FO and maximizing the availability of FDC-associated Ag to B cells.
The Mouse Splenic White Pulp: The Marginal Zone and Antigen Trafficking
The MZ is composed of a unique subset of B cells (MZB cells); two unique subsets of macrophages, the marginal zone metallophilic macrophages (MMM) and marginal zone macrophages (MZM); and reticular fibroblasts. The MMM surround the WP, just inside the marginal sinus, which is lined by MADCAM1+ sinus-lining cells. Beyond the sinus are the MZB cells and the MZM, along with reticular fibroblasts, which give some level of structural integrity to the MZ as a whole. Also present in the MZ are a subset of cDC, characterized by their binding to a chimeric protein composed of the Fc region of human IgG1 fused to the cysteine-rich domain of the mannose receptor (termed CR-Fc) (Yu et al. 2002).
Because the MZ surrounds the WP, RP-derived Ag must cross the MZ to gain access to either the PALS or the FO. Small Ag (~65 kDa and smaller) can diffuse through the MZ, whereas larger Ag must be transported across or through by cells, including CR-Fc+ DC and cDC and MZM. Additionally, MZB cells transport Ag into the WP while migrating back and forth between the MZ and FO in a mechanism dependent upon reciprocal desensitization to S1P (abundant in the blood-rich RP) and CXCL13 (abundant in the FO) (Allen & Cyster 2008).
The Mouse Splenic White Pulp: The Secondary Follicle (Germinal Center)
Upon activation by cognate Ag (usually on the surface of an FDC), follicular B cells upregulate CCR7 and, in a CCL19/21-dependent manner, migrate to the edge of the FO that borders the T cell zone. T cells, stimulated by Ag on cDC in the PALS, upregulate CXCR5 and migrate in a CXCL13-dependent manner to the border of the PALS and FO. Ag-specific B cells also secrete chemokines (e.g., CCL3) that attract the recently stimulated T cells. In the event of a cognate T cell:B cell interaction at the T cell zone/B cell zone border (or in the interfollicular region), by mechanisms still not fully understood, a GC (also known as a secondary FO) forms (reviewed in De Silva & Klein 2015).
The GC is separated into two zones: the dark zone (DZ) and the light zone (LZ). Within the DZ, B cells known as centroblasts proliferate and mutate their BCR genes. This mutation, termed somatic hypermutation (SHM), underlies affinity maturation (AM) and is mediated by the activation-induced cytidine deaminase (AID)-dependent recognition and deamination of conserved motifs within the IgV region and by the consequent error-prone repair and mutation of nucleic acids within these motifs. CSR also occurs, and is mediated by AID, at regions downstream of the IgV gene and upstream and within the IgH gene complex. These regions, known as switch boxes, allow for the generation of AID-dependent double-strand breaks 5′ of the IgM C gene and the other switching C gene, resulting in germline recombination. After several rounds of proliferation and mutation, the B cells enter the LZ, where they are now known as centrocytes, for positive selection. The affinity of the mutated BCR is tested on FDC within the LZ, termed LZ FDC. LZ FDC, a further differentiated form of FDC, are characterized by increased expression of CR1 and -2, an enhanced ability to trap immune complexes, and augmented production of the milk fat globule–EGF factor 8 (Mfge8, also known as FDC-M1); Mfge8 is a phosphatidylserine-binding protein that promotes engulfment and phagocytosis of apoptotic GC B cells (Allen & Cyster 2008, Kranich et al. 2008). Selection upon cognate Ag on a LZ FDC, and subsequent internalization and processing of cognate Ag, is followed by interaction with CD4+ T follicular helper cells to ensure retention of specificity for the initially encountered Ag and presumably to prohibit autoimmune clones from being selected and perpetuated. Successful selection results in either reentry into the DZ (cyclic reentry) or egress from the GC as either a plasmablast or memory B cell.
To date, GC have been positively identified only in mammals and birds (ectothermic or warm-blooded vertebrates; Figure 1), despite the fact that SHM is found in all vertebrates with some level of AM. We discuss this conundrum below.
Ontogeny of the Mouse Splenic White Pulp
During murine ontogeny, the organogenesis of the spleen begins at E12 with the formation of the splanchnic mesodermal plate and progresses in homeobox transcription factor 11 (Hox11)-dependent fashion (Roberts et al. 1994). Colonization of the spleen by progenitor cells derived from erythroid and myeloid lineages begins as early as E13.5, along with colonization by lymphoid tissue inducer (LTi) cells; hematopoietic stem cell colonization follows at E14.5. Over the next several days of embryonic and fetal development, the spleen grows, and its cellular constituency increases in both number and cell type. Until birth, however, no RP/WP dichotomy is evident; rather, the spleen consists entirely of RP, and few scattered B cells are found through E17.5. At E18.5, splenic B cell numbers increase dramatically, but the cells do not localize around the splenic vasculature until immediately after birth (P0.5), despite the production of CXCL13 by perivascular pre-FDC as early as E17.5 (Neely & Flajnik 2015). Perivascular accumulation of B cells occurs concurrently with their acquisition of CXCL13 responsiveness, which occurs as the B cells progress from the early to late transitional stage of development. By P2, the B cells observed around the splenic vasculature have contracted into a nascent FO (Vondenhoff et al. 2008). At P4, the nascent FO has grown and is surrounded by MOMA-2+ cells (indicative of the appearance of the MZ), and a small number of T cells are detectable around the vasculature at its center. By P6, further displacement of the FO by the nascent PALS is evident, and the WP begins to display microarchitectural maturity. All the events in neonatal WP ontogeny are dependent upon LT:LTβR interactions, either directly or indirectly (Vondenhoff et al. 2008).
The Mouse Spleen Versus the Human Spleen
The mouse spleen, specifically the spleen of the C57Bl/6J mouse, is not necessarily representative of all mammalian spleens. Indeed, the spleen from this strain is not even representative of all mouse spleens. Differences in the microarchitecture of the WP have been observed not only among different mouse species, but also within Mus musculus (A. Thiriot, personal communication). The architecture of human WP differs from that of mouse WP as well (Mebius & Kraal 2005, Steiniger 2015), although the characteristics that separate human WP from its evolutionary precursors remain poorly studied (see below). Moreover, the ontogeny of human WP differs from that of mouse WP: Ontogeny begins in utero, although its onset is still marked by the perivascular accumulation of B cells (Steiniger et al. 2007). The in utero ontogeny of human SLO likely contributes to the increased immunocompetence of human neonates relative to mouse neonates.
Additional Mammalian Secondary Lymphoid Organs: Lymph Nodes and Peyer’s Patches
The general architecture of the mammalian LN is highly reminiscent of the splenic WP. The central vasculature, in the form of high endothelial venules, is surrounded by the T cell zone in the paracortex. The B cell FO are situated in the cortex, which is adjacent and peripheral to the paracortex. Lymph-borne Ag flows into the node via the afferent lymphatics, through the planar conduit of the subcapsular sinus (SCS), and is transported into the cortex by a specialized subset of SCS macrophages (reviewed in Cyster 2010). Fluid and particulate matter not absorbed into the cortex flow through the SCS into the medullary sinuses and encounter another specialized subset of macrophages, the medullary macrophages, which are tasked with the internalization and destruction of Ag. The remaining fluid flows out of the LN via the efferent lymphatics, ultimately reentering circulation via the thoracic duct. The PP of the gut-associated lymphoid tissue share similar microarchitectural organization with both LN and the splenic WP; multiple B cell FO are situated around and separated by T cell areas (Suzuki et al. 2010). In both LN and PP, as in the splenic WP, B cell and T cell zone segregation and integrity are dependent upon CXCL13 and CCL19/21, respectively.
The ontogeny of the LN is similar to that of the splenic WP in that B cells first populate the node, and the mature architecture and cellularity are similarly dependent upon B cell–derived LTα1β2. However, the initiation of both LN ontogeny and PP ontogeny is distinct from that of the WP in that its dependence upon LTα1β2 is due to a unique subset of type 3 innate lymphoid cell (ILC3) (originally termed LTi cells), rather than B cells (Cherrier & Eberl 2012). Moreover, neither LN nor PP form in the absence of LTα1β2. These requirements highlight the evolutionary novelty of both LN and PP; as mentioned above, although the spleen itself develops in the absence of LTα1β2, there are defects in the characteristic microarchitecture of the splenic WP.
AGNATHANS
The first extant appearance of adaptive immunity is found in agnathans (jawless vertebrates, that is, lamprey and hagfish, with the last common ancestor with humans approximately 500 MYA). The Ag receptors expressed by agnathan lymphocytes, named the variable lymphocyte receptors (VLR), are composed of rearranging leucine-rich repeat (LRR) segments rather than of rearranging Ig segments (reviewed in Boehm et al. 2012b). Three distinct subsets of VLR- and VLR-bearing lymphocytes have been identified in lamprey: VLR-A, VLR-B, and VLR-C seem to correspond to αβ T cells, B cells, and γδ T cells of vertebrates, respectively (Flajnik 2014, Hirano et al. 2013, Li et al. 2013).
A primary lymphoid organ that seems to be the functional equivalent of the gnathostome thymus, the thymoid, has been identified in the sea lamprey (Petromyzon marinus) (Bajoghli et al. 2011) and is presumably present in all agnathans. However, there is no evidence of any SLO in the jawless fish. Likewise, “there has been no sign of MHC I or II genes in animals older than the cartilaginous fish” (Flajnik & Du Pasquier 2004), nor are there any orthologs of MHC-associated Ag-processing genes, which suggests an absence of Ag presentation and, by extension, of conventional APC. Whether any organ, tissue, or mechanism for the concentration of Ag, Ag-restricted lymphocytes, or both is present in the jawless vertebrates is an open question. Proliferating lymphocytes were found in the gills and ventral kidneys of immunized lamprey, but no architecture typical of SLO was detected in these tissues (Alder et al. 2008). Despite the absence of SLO, evidence exists for some level of AM over the course of an immune response, at least in the case of VLR-A (Deng et al. 2010). Whether this AM occurs in situ (i.e., at the site of VLR-A cell stimulation) or at a location dedicated to the support of VLR-A mutation is unknown.
ELASMOBRANCHS
Adaptive Immunity and Lymphopoiesis in Elasmobranchs
Adaptive immunity based on rearranging Ig/TCR:pMHC likely arose in the placoderms, an extinct vertebrate class. Chondrichthyes (cartilaginous fishes, i.e., chimeras, sharks, skates, and rays; the last common ancestor with humans lived ~500 MYA) is the oldest living group with Ig/TCR:pMHC-based immunity and is the oldest living group containing the primordial SLO, the spleen. Additionally, the nurse shark contains two distinct primary lymphoid organs: the epigonal organ (the bone marrow equivalent; some, but not all, elasmobranchs contain an additional bone marrow equivalent, the Leydig organ) and the thymus (Smith et al. 2015). As would be predicted, RAG1 expression is restricted to these organs (Rumfelt et al. 2002).
The genomic organization of elasmobranch IgH gene segments is unique among jawed vertebrates in that the segments are organized in clusters containing a single V segment, one or more D segments, a single J segment, and a dedicated constant region, in contrast to the conventional translocon organization found in all other jawed vertebrates (Flajnik 2002, Litman et al. 1985). RAG-dependent V(D)J recombination within a given cluster, however, occurs normally. Whereas SHM and AM occur in nurse shark B cells (Diaz et al. 1999), canonical CSR does not; no switch box regions or AID hot spots (RGYW) have been detected adjacent to IgH loci. However, evidence of a noncanonical form of CSR, perhaps orchestrated by a unique repetitive element, has been reported; such CSR has been studied only at the mRNA level (Zhu et al. 2012).
The Nurse Shark Spleen: Architecture and Ontogeny
As in all jawed vertebrates save mammals, in the nurse shark the only described bona fide SLO is the spleen. The spleen of the nurse shark is separated into distinct RP and WP, although no defining border or MZ exists between the two (Zapata et al. 1996). The RP of the nurse shark spleen contains macrophages and erythrocytes, as well as Ig-secreting plasma cells (Castro et al. 2013, Rumfelt et al. 2002), which are localized predominantly at the periphery of the WP and at the splenic capsule. The WP of the nurse shark contains densely packed lymphocytes that surround arterioles; the arterioles terminate into ellipsoids at the periphery of the WP (Rumfelt et al. 2002).
WP ontogeny begins in the neonatal nurse shark spleen and is marked by the accumulation of B cells around splenic vasculature, as in all other vertebrates tested (Rumfelt et al. 2002). DC are not evident in the nascent FO until a nurse shark is 2.5 months of age. A previous model in our laboratory showed that the nurse shark WP matures into a configuration characterized by a large, central T cell zone surrounded by a smaller B cell zone, with or without additional, smaller, adjacent B cell zones. However, recent data with in situ hybridization protocols demonstrated that the large splenic WP are clearly B cell zones (Castro et al. 2013). Whether T cell zones are adjacent to the B cell zones is currently under investigation.
Dendritic and Antigen-Presenting Cells in the Nurse Shark
Along with describing lymphocytes in the WP of the quiescent nurse shark (Ginglymostoma cirratum) spleen, we described large cells expressing high levels of MHC class II and displaying dendritic processes (Rumfelt et al. 2002; L.L. Rumfelt & M.F. Flajnik, unpublished). Immunization of adult nurse sharks with biotinylated bovine serum albumin resulted in an accumulation of immunogen in the splenic RP within one week; four weeks after immunization, the immunogen had localized to the splenic WP and was observed predominantly at the plasma membranes of large cells with dendritic processes (L.L. Rumfelt & M.F. Flajnik, unpublished). This retention of immunogen at the DC’s plasma membrane suggests Ag presentation to B cells, although the high levels of MHC class II suggest that these cells are of a conventional, hematopoietic lineage (i.e., they are not bona fide FDC).
Effector Sites of Humoral Immunity in the Nurse Shark
Although the nurse shark spleen, as the only SLO, is the “major site for Ag stimulation leading to antibody … synthesis” (Rumfelt et al. 2002), it is not the only site of Ig expression or secretion. 19S IgM- and IgW-secreting cells populate the epigonal organ (Castro et al. 2013) in a manner reminiscent of mammalian bone marrow population by both B-1a lineage Ig-secreting cells (Choi et al. 2012) and long-lived plasma cells (Tangye 2011). Additionally, significant Ig transcription has been detected in the pancreas, gill, liver, kidney, and olfactory organ (Rumfelt et al. 2004). Ig secretion has been confirmed in both the pancreas and olfactory organ (H.R. Neely, I. Salinas & M.F. Flajnik, unpublished).
TELEOST FISH: THE TELEOST SPLEEN
The WP of the teleost (bony fish) spleen is poorly studied relative to the spleens of cartilaginous fish and amphibians. In the zebrafish (Danio rerio) spleen, there is a separation of RP from WP, but the WP contains few cells; macrophages surround the ellipsoids, with accumulations of lymphocytes nearby. No resolution between B and T cell zones has been reported in the zebrafish WP. However, this lack of resolution does not rule out the possibility that such segregation exists; B cell/T cell zone segregation has been suggested in the rainbow trout (O. Sunyer, personal communication) and medaka (B. Bajoghli, personal communication).
cDC were recently identified and characterized in teleost fish. Employing a cell-sorting method based on a combination of a light scatter profile characteristic of myelomonocytes and binding of peanut agglutinin, Traver and colleagues (Lugo-Villarino et al. 2010) isolated cells with canonical DC morphology, expression of DC-associated transcripts, and Ag-specific induction of T cell proliferation. These DC were found not only in the spleen, but in the peritoneal cavity, gut, thymus, and skin (Wittamer et al. 2011). Similar populations of cDC-like cells were identified in the Atlantic salmon (Salmo salar) (Haugland et al. 2012) and medaka (Aghaallaei et al. 2010).
Although the teleost spleen lacks well-defined lymphoid microarchitecture, it does support adaptive immune responses. Expression of AID has been exclusively observed in the spleen in the context of an adaptive immune response (Saunders et al. 2010), suggesting that the spleen is a locale for Ag and lymphocyte concentration and promotes adaptive humoral immunity. Interest in teleost immunity is increasing, largely due to a rise in large-scale aquaculture. Our knowledge and understanding of the teleost spleen will likely grow in the coming years.
Although the spleen is the only bona fide SLO in teleost fish, aggregations of lymphocytes and the induction and execution of adaptive immune responses have been reported in multiple MALT, including the gut, skin, gill, and nasopharynx (Salinas 2015). The recently discovered nasopharynx-associated lymphoid tissue (NALT) provides an interesting view of the evolutionary progression of non-SLO lymphoid aggregates. The lymphocyte component of teleost NALT is diffuse. In lungfish, which are members of the living phylogenetic fish group with the closest ties to amphibians, aggregations of lymphocytes in NALT demonstrate some level of structure and architecture and represent the first appearance of organized NALT (Sepahi & Salinas 2016), which culminates in the mammalian tonsil.
AMPHIBIANS
Primary Lymphoid Organs and Lymphopoiesis in Xenopus
In the adult African clawed frog (Xenopus laevis), primary lymphopoiesis occurs in the bone marrow and thymus. The genomic organization of Ig loci in Xenopus is comparable to that of mammals: Multiple V, D, and J segments are encoded in translocon organization, are upstream of IgM and IgD constant regions, and have additional isotype regions downstream (Ohta & Flajnik 2006). The additional isotypes are IgX (an IgA homolog), IgY (an IgG homolog; also the first to appear in the evolution of adaptive humoral immunity), and IgF (which is strongly related to IgY) (Sun et al. 2013).
Humoral Adaptive Immunity in Amphibians
Amphibians represent an inflection point in the evolution of humoral adaptive immunity; they are the first class of vertebrates in which canonical CSR, characterized by the AID-mediated recombination of the rearranged primary isotype to a downstream (non-IgM) constant region, occurs (reviewed in Du Pasquier et al. 2000). As in mammals, this rearrangement is guided by switch box regions downstream of the rearranged V region and upstream of the target C region (Mussmann et al. 1997). Additionally, CSR in Xenopus can be either T cell independent, in the case of the switch from IgM to IgX, or T cell dependent, in the case of the switch from IgM to IgY; serum IgY is undetectable in thymectomized (and therefore T cell–deficient) animals (Mussmann et al. 1996). Despite the presence of canonical CSR, GC do not form in the Xenopus spleen (Du Pasquier et al. 2000). The absence of GC is reflected in the limited extent of AM observed in class-switched B cell–derived Ig; increases in affinity after SHM in Xenopus range between 10-and 100-fold for a hapten-specific response compared with an affinity increase of 1,000 or more in mammals. Moreover, bona fide FDC have not been identified in the Xenopus WP, although a population of cells with an apparent capacity to present native Ag to B cells has been described (see below).
Splenic White Pulp Ontogeny and Microarchitecture in Xenopus
The splenic anlage in X. laevis is first evident at developmental stage 45 (Manning & Horton 1969). The spleen, as a discrete organ, appears by stage 47, with large, immature lymphoblasts at the center of the spleen and a small number of erythrocytes at the periphery of the spleen. The relative positioning of erythrocytes and lymphoblasts suggests that WP/RP segregation may begin as early as when the spleen is first populated by hematopoietic lineage cells. Late in developmental stage 50, small, mature lymphocytes aggregate at the center of the spleen, with a detectable “double layer of elongated cells,” the Grenzschichtmembran of Sterba (GS), surrounding them (Manning & Horton 1969). The GS becomes more apparent by stage 51. The spleen continues to grow, “with branching of the central WP until 3–5 WP areas are seen” (Manning & Horton 1969), until metamorphosis, at which point nearly all splenic cellularity is lost, to be regained during the growth of the frog. The mechanism regulating cell egress from the tadpole spleen, as well as the mechanism that reestablishes the adult animal’s splenic lymphocyte compartment after metamorphosis, is unknown.
The WP of the mature, quiescent, adult Xenopus spleen is composed of a central arteriole that is surrounded by B cells and bounded by the GS (Manning & Horton 1969). Thus, the WP retains the embryonic feature described above in the Ontogeny of the Mouse Splenic White Pulp section. Few T cells are present within the GS-bounded WP; rather, T cells surround individual WP in a corona. In the quiescent spleen, a single, morphologically homogeneous population of DC and APC has been described, and this population is termed XL cells (Baldwin & Cohen 1981).
Dendritic and Antigen-Presenting Cells and Antigen Transport in Xenopus
Xenopus XL cells have the morphological characteristics of DC: abundant cytoplasm; dendritic processes; and large, multilobed nuclei. These XL cells are also mitotically active and may be capable of migrating into the WP during acute immune responses (Baldwin & Cohen 1981). The trafficking of Ag into the WP of the Xenopus spleen is a thymus-dependent event (Horton & Manning 1974). In wild-type, unmanipulated animals, immunization with human IgG resulted in the localization of the Ag at the internal perimeter of the WP, with the position of Ag similar to that of the XL cells (Horton & Manning 1974). This finding led to the speculation that XL cells are involved in the trafficking of Ag into the WP (Baldwin 1981), and this possibility is supported by the observation that XL cells are capable of trapping Ag at their plasma membranes (Baldwin 1983). Surprisingly, thymectomized animals show no localization of immunogen to the WP and little, if any, immunogen localization to the spleen (Horton & Manning 1974; H.R. Neely & M.F. Flajnik, unpublished).
In the periphery, DC, identified by canonical morphology and expression of MHC class II, have been described in Xenopus thymus and skin (Du Pasquier & Flajnik 1990, Turpen & Smith 1986). The langerin- and vimentin-expressing DC of the skin resemble Langerhans cells, suggesting that DC specialization occurred no later than in amphibians (Du Pasquier & Flajnik 1990, Mescher et al. 2007).
REPTILES
The Reptilian White Pulp
The reptilian spleen is segregated into RP and WP. The WP is further segregated, in some but not all reptiles, into two discrete areas: (a) a PALS and (b) a peri-ellipsoid lymphoid sheath (PELS) at the periphery of the PALS, where splenic arterioles terminate into the splenic cords. However, both a PALS and a PELS are not observed in all reptiles; variations in splenic microarchitecture among reptilian species have been described (Leceta & Zapata 1991).
The microarchitecture of the reptilian PELS appears to be somewhat similar to that of the amphibian WP in that the more central cells are surface Ig+ B cells, with Ig− lymphocytes, presumably T cells, at the periphery (Leceta & Zapata 1991). In contrast, the lymphocyte compartment of the reptilian PALS is presumed to be dominated by T cells; the PALS cells have the characteristic morphology of lymphocytes but are negative for surface Ig (Leceta & Zapata 1991). Furthermore, the cellularity of the PALS is substantially reduced upon adult thymectomy or treatment with antithymocyte antiserum (Pitchappan & Muthukkaruppan 1977), and a less pronounced loss in cellularity is observed after adult thymic involution (Borysenko & Cooper 1972). As such, the reptilian PALS seems more similar to the mammalian WP.
As in fish and amphibian spleens, GC have not been observed in the reptilian spleen (Leceta & Zapata 1991). However, the reptilian spleen has not been studied as extensively; further analysis, particularly in the context of acute immune responses, is certainly warranted. Additionally, “lymphoid aggregates” in the axillary and inguinal regions of the snapping turtle (Chelydra serpentina) have been reported and have been suggested to be the precursors to LN (Borysenko & Cooper 1972). Further study of this species and other reptiles will undoubtedly shed light on the earliest origins of LN and their phylogenetic ancestors.
Dendritic and Antigen-Presenting Cells in Reptiles
The WP of the snake Python reticulatus contains “a framework of irregularly shaped, ramifying reticulum cells, forming a network wherein the lymphoid elements [are] localized” (Kroese et al. 1985). Moreover, these cells display long processes that are in close contact with collagen fibers and that are reminiscent of the mammalian FRC and their associated framework. Two additional nonlymphoid cell types were observed in the WP of the snake: highly phagocytic macrophages and Ag-trapping DC (which are also capable of, to a lesser extent than macrophages, internalization of Ag). These DC showed a localization pattern similar to that of Xenopus XL cells. This similarity to XL cells, rather than canonical FDC, argues for a lack of canonical GC.
BIRDS: THE AVIAN SPLEEN
The microarchitecture of the avian WP is similar to that of the reptilian WP; segregation of the WP into discrete PALS and PELS is observed (Yasuda et al. 1998, 2003). Furthermore, as in reptiles, the PALS is composed primarily of T cells, whereas the PELS consists primarily of B cells. Some additional WP—containing both B and T cells—is observed between and adjacent to the PALS and PELS, the regions where canonical GC, the first observed in vertebrates, form (Yasuda et al. 1998, 2003).
In the PALS of the chicken spleen, CD83+ CD45+ interdigitating DC are observed in intimate contact with T cells; this arrangement is highly reminiscent of the mammalian T cell zone (Olah & Nagy 2013). Within the PELS, cells with the canonical morphology, surface phenotype, and function of mammalian FDC have been identified. These chicken FDC are stellate, express VCAM-1 and ICAM-1 (along with other cellular adhesion molecules), stain as positive for surface Ig (IgM and IgG/Y), and have a demonstrated capacity to stimulate both B cell proliferation and CSR to IgG/Y without the ability to stimulate T cell proliferation (Del Cacho et al. 2009). The lineage of these cells is controversial; conflicting reports regarding their expression of both CD45 and MHC class II (with positivity of either indicating a hematopoietic lineage) have been published.
The microarchitectural organization of the chicken WP, and particularly the presence of GC, is surprising, given that the chicken genome has lost TNFα, LTα, and LTβ [along with many other immune genes (Magor et al. 2013)], all of which are necessary for the establishment and maintenance of mammalian WP and GC. Also surprising is the report of LN in the absence of TNF superfamily members in the white Pekin duck (Anas platyrhynchos) (Sugimura et al. 1977). LN have not been reported in other avian species.
CONCLUDING REMARKS
The data above demonstrate a clear and progressive accumulation of splenic WP complexity and functionality over the course of vertebrate evolution, but our knowledge of SLO evolution is far from complete. Specifically determining the positioning of the T cell zone in the elasmobranch spleen (relative to the perivascular B cell FO), both in the steady state and in the context of acute immune responses, will fully demonstrate the ancestral microarchitecture of the spleen and splenic WP and may also offer insight into the ancestral nature of T cell:B cell interaction.
The teleost spleen presents challenges and opportunities. As multiple teleost models of immunity are studied (and interest in teleost immunity is booming, in part due to increases in large-scale aquaculture and the use of zebrafish and medaka as vertebrate model systems), a comparative analysis of spleens within the Teleostei will allow for the characterization of the general bony fish spleen; these basic characteristics may then be compared with those of the spleens of other vertebrates to provide a clearer picture of the progression of splenic and SLO evolution through the earliest gnathostomes.
The presence of canonical CSR in the absence of GC makes the amphibian spleen a uniquely fascinating organ, as does the emergence of the first described physical structure that segregates B cell zones from the rest of the spleen. In addition, amphibians are the oldest group with an IgG equivalent (IgY) and with clear humoral memory responses with this isotype. Multiple questions are raised by these advances in WP complexity and adaptive immunity. What is the nature of the T cell:B cell collaboration required for CSR to IgY, and where does it occur? Are APC (XL cells) in the spleen capable of double duty, presenting Ag to both T and B cells (i.e., performing both cDC and FDC functions), and could they be models for Ag presentation in all cold-blooded vertebrates?
Preliminary evidence, from studies performed long ago, suggests that the mammalian-type splenic organization first appears in reptiles. Several genome and gene expression projects in model species will allow for a reexamination of the basic findings described above.
As is found for other avian immune features, including the generation of B cell diversity, a dedicated tissue for B cell development, and an unusual MHC with loss of the immuno- and thymoproteasome (among other aspects), SLO pose major questions (Magor et al. 2013). Although a lack of LN is consistent with the loss of the MHC-linked TNF family members, the presence of FDC and GC must be regulated by other factors yet to be defined. Genome projects in chickens and other birds will permit analysis of expression of the chemokine and cytokine network in this vertebrate class (Cheng et al. 2013).
Lastly, the emergence of FDC and GC in the endothermic vertebrates was a huge step forward in AM and memory formation (Figure 1). What selective forces drove this great enhancement of immunity? Clearly, the SHM mechanism was in place since the origins of vertebrates (even in agnathans), and emerging evidence suggests that cDC may present Ag to B cells, at least in amphibians. One possibility is that endothermy allows pathogens to divide at a faster rate and the GC required to “keep up,” whereas in cold-blooded animals “time is not of the essence” in immunity specific for slowly replicating pathogens (Hsu 1998).
We look forward to the next wave of study of SLO structure and function in nonmammalian vertebrates. Advances in knockout technologies and genome projects in model species of all vertebrate classes should allow for steady progress on all the questions raised in this review.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Aghaallaei N, Bajoghli B, Schwarz H, Schorpp M, Boehm T. Characterization of mononuclear phagocytic cells in medaka fish transgenic for a cxcr3a:gfp reporter. PNAS. 2010;107:18079–84. doi: 10.1073/pnas.1000467107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguzzi A, Kranich J, Krautler NJ. Follicular dendritic cells: origin, phenotype, and function in health and disease. Trends Immunol. 2014;35:105–13. doi: 10.1016/j.it.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Alder MN, Herrin BR, Sadlonova A, Stockard CR, Grizzle WE, et al. Antibody responses of variable lymphocyte receptors in the lamprey. Nat Immunol. 2008;9:319–27. doi: 10.1038/ni1562. [DOI] [PubMed] [Google Scholar]
- Allen CD, Cyster JG. Follicular dendritic cell networks of primary follicles and germinal centers: phenotype and function. Semin Immunol. 2008;20:14–25. doi: 10.1016/j.smim.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansel KM, Ngo VN, Hyman PL, Luther SA, Forster R, et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–14. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- Bajenoff M, Glaichenhaus N, Germain RN. Fibroblastic reticular cells guide T lymphocyte entry into and migration within the splenic T cell zone. J Immunol. 2008;181:3947–54. doi: 10.4049/jimmunol.181.6.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajoghli B, Guo P, Aghaallaei N, Hirano M, Strohmeier C, et al. A thymus candidate in lampreys. Nature. 2011;470:90–94. doi: 10.1038/nature09655. [DOI] [PubMed] [Google Scholar]
- Baldwin WM., 3rd Antigen trapping cells in Xenopus laevis: tissue distribution. Dev Comp Immunol. 1983;7:709–10. [Google Scholar]
- Baldwin WM, 3rd, Cohen N. A giant cell with dendritic cell properties in spleens of the anuran amphibian Xenopus laevis. Dev Comp Immunol. 1981;5:461–73. doi: 10.1016/s0145-305x(81)80058-4. [DOI] [PubMed] [Google Scholar]
- Boehm T, Hess I, Swann JB. Evolution of lymphoid tissues. Trends Immunol. 2012a;33:315–21. doi: 10.1016/j.it.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Boehm T, McCurley N, Sutoh Y, Schorpp M, Kasahara M, Cooper MD. VLR-based adaptive immunity. Annu Rev Immunol. 2012b;30:203–20. doi: 10.1146/annurev-immunol-020711-075038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borysenko M, Cooper EL. Lymphoid tissue in the snapping turtle, Chelydra serpentina. J Morphol. 1972;138:487–97. doi: 10.1002/jmor.1051380408. [DOI] [PubMed] [Google Scholar]
- Castro CD, Ohta Y, Dooley H, Flajnik MF. Noncoordinate expression of J-chain and Blimp-1 define nurse shark plasma cell populations during ontogeny. Eur J Immunol. 2013;43:3061–75. doi: 10.1002/eji.201343416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesta MF. Normal structure, function, and histology of the spleen. Toxicol Pathol. 2006;34:455–65. doi: 10.1080/01926230600867743. [DOI] [PubMed] [Google Scholar]
- Cheng HH, Kaiser P, Lamont SJ. Integrated genomic approaches to enhance genetic resistance in chickens. Annu Rev Anim Biosci. 2013;1:239–60. doi: 10.1146/annurev-animal-031412-103701. [DOI] [PubMed] [Google Scholar]
- Cherrier M, Eberl G. The development of LTi cells. Curr Opin Immunol. 2012;24:178–83. doi: 10.1016/j.coi.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Choi YS, Dieter JA, Rothaeusler K, Luo Z, Baumgarth N. B-1 cells in the bone marrow are a significant source of natural IgM. Eur J Immunol. 2012;42:120–29. doi: 10.1002/eji.201141890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyster JG. B cell follicles and antigen encounters of the third kind. Nat Immunol. 2010;11:989–96. doi: 10.1038/ni.1946. [DOI] [PubMed] [Google Scholar]
- De Silva NS, Klein U. Dynamics of B cells in germinal centres. Nat Rev Immunol. 2015;15:137–48. doi: 10.1038/nri3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Cacho E, Gallego M, Lillehoj HS, Lopez-Bernard F, Sanchez-Acedo C. Avian follicular and interdigitating dendritic cells: isolation and morphologic, phenotypic, and functional analyses. Vet Immunol Immunopathol. 2009;129:66–75. doi: 10.1016/j.vetimm.2008.12.015. [DOI] [PubMed] [Google Scholar]
- den Haan JM, Mebius RE, Kraal G. Stromal cells of the mouse spleen. Front Immunol. 2012;3:201. doi: 10.3389/fimmu.2012.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Velikovsky CA, Xu G, Iyer LM, Tasumi S, et al. A structural basis for antigen recognition by the T cell–like lymphocytes of sea lamprey. PNAS. 2010;107:13408–13. doi: 10.1073/pnas.1005475107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz M, Velez J, Singh M, Cerny J, Flajnik MF. Mutational pattern of the nurse shark antigen receptor gene (NAR) is similar to that of mammalian Ig genes and to spontaneous mutations in evolution: the translesion synthesis model of somatic hypermutation. Int Immunol. 1999;11:825–33. doi: 10.1093/intimm/11.5.825. [DOI] [PubMed] [Google Scholar]
- Du Pasquier L, Flajnik MF. Expression of MHC class II antigens during Xenopus development. Dev Immunol. 1990;1:85–95. doi: 10.1155/1990/67913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Pasquier L, Robert J, Courtet M, Mussmann R. B-cell development in the amphibian Xenopus. Immunol Rev. 2000;175:201–13. doi: 10.1111/j.1600-065x.2000.imr017501.x. [DOI] [PubMed] [Google Scholar]
- Endres R, Alimzhanov MB, Plitz T, Futterer A, Kosco-Vilbois MH, et al. Mature follicular dendritic cell networks depend on expression of lymphotoxin beta receptor by radioresistant stromal cells and of lymphotoxin beta and tumor necrosis factor by B cells. J Exp Med. 1999;189:159–68. doi: 10.1084/jem.189.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fange R, Pulsford A. Structural studies on lymphomyeloid tissues of the dogfish, Scyliorhinus canicula L. Cell Tissue Res. 1983;230:337–51. doi: 10.1007/BF00213808. [DOI] [PubMed] [Google Scholar]
- Flajnik MF. Comparative analyses of immunoglobulin genes: surprises and portents. Nat Rev Immunol. 2002;2:688–98. doi: 10.1038/nri889. [DOI] [PubMed] [Google Scholar]
- Flajnik MF. Re-evaluation of the immunological Big Bang. Curr Biol. 2014;24:R1060–65. doi: 10.1016/j.cub.2014.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flajnik MF, Du Pasquier L. Evolution of innate and adaptive immunity: Can we draw a line? Trends Immunol. 2004;25:640–44. doi: 10.1016/j.it.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Flajnik MF, Du Pasquier L. Evolution of the immune system. In: Paul WE, editor. Fundamental Immunology. 7 Philadelphia: Lippincroft-Raven; 2013. pp. 67–128. [Google Scholar]
- Gorelik L, Gilbride K, Dobles M, Kalled SL, Zandman D, Scott ML. Normal B cell homeostasis requires B cell activation factor production by radiation-resistant cells. J Exp Med. 2003;198:937–45. doi: 10.1084/jem.20030789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugland GT, Jordal AE, Wergeland HI. Characterization of small, mononuclear blood cells from salmon having high phagocytic capacity and ability to differentiate into dendritic like cells. PLOS ONE. 2012;7:e49260. doi: 10.1371/journal.pone.0049260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano M, Guo P, McCurley N, Schorpp M, Das S, et al. Evolutionary implications of a third lymphocyte lineage in lampreys. Nature. 2013;501:435–38. doi: 10.1038/nature12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann J, Greter M, Du Pasquier L, Becher B. B-cells need a proper house, whereas T-cells are happy in a cave: the dependence of lymphocytes on secondary lymphoid tissues during evolution. Trends Immunol. 2010;31:144–53. doi: 10.1016/j.it.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Horton JD, Manning MJ. Effect of early thymectomy on the cellular changes occurring in the spleen of the clawed toad following administration of soluble antigen. Immunology. 1974;26:797–807. [PMC free article] [PubMed] [Google Scholar]
- Hsu E. Mutation, selection, and memory in B lymphocytes of exothermic vertebrates. Immunol Rev. 1998;162:25–36. doi: 10.1111/j.1600-065x.1998.tb01426.x. [DOI] [PubMed] [Google Scholar]
- Jenkins MK, Moon JJ. The role of naive T cell precursor frequency and recruitment in dictating immune response magnitude. J Immunol. 2012;188:4135–40. doi: 10.4049/jimmunol.1102661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus GG, Humphrey JH, Kunkl A, Dongworth DW. The follicular dendritic cell: its role in antigen presentation in the generation of immunological memory. Immunol Rev. 1980;53:3–28. doi: 10.1111/j.1600-065x.1980.tb01038.x. [DOI] [PubMed] [Google Scholar]
- Koni PA, Sacca R, Lawton P, Browning JL, Ruddle NH, Flavell RA. Distinct roles in lymphoid organo-genesis for lymphotoxins alpha and beta revealed in lymphotoxin beta-deficient mice. Immunity. 1997;6:491–500. doi: 10.1016/s1074-7613(00)80292-7. [DOI] [PubMed] [Google Scholar]
- Koopman G, Parmentier HK, Schuurman HJ, Newman W, Meijer CJ, Pals ST. Adhesion of human B cells to follicular dendritic cells involves both the lymphocyte function-associated antigen 1/intercellular adhesion molecule 1 and very late antigen 4/vascular cell adhesion molecule 1 pathways. J Exp Med. 1991;173:1297–304. doi: 10.1084/jem.173.6.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranich J, Krautler NJ, Heinen E, Polymenidou M, Bridel C, et al. Follicular dendritic cells control engulfment of apoptotic bodies by secreting Mfge8. J Exp Med. 2008;205:1293–302. doi: 10.1084/jem.20071019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krautler NJ, Kana V, Kranich J, Tian Y, Perera D, et al. Follicular dendritic cells emerge from ubiquitous perivascular precursors. Cell. 2012;150:194–206. doi: 10.1016/j.cell.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroese FG, Leceta J, Dopp EA, Herraez MP, Nieuwenhuis P, Zapata A. Dendritic immune complex trapping cells in the spleen of the snake, Python reticulatus. Dev Comp Immunol. 1985;9:641–52. doi: 10.1016/0145-305x(85)90029-1. [DOI] [PubMed] [Google Scholar]
- Leceta J, Zapata A. Seasonal changes in the thymus and spleen of the turtle, Mauremys caspica. A morphometrical, light microscopical study. Dev Comp Immunol. 1985;9:653–68. doi: 10.1016/0145-305x(85)90030-8. [DOI] [PubMed] [Google Scholar]
- Leceta J, Zapata AG. White pulp compartments in the spleen of the turtle Mauremys caspica. Cell Tissue Res. 1991;266:605–13. [Google Scholar]
- Li J, Das S, Herrin BR, Hirano M, Cooper MD. Definition of a third VLR gene in hagfish. PNAS. 2013;110:15013–18. doi: 10.1073/pnas.1314540110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8:1255–65. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- Litman GW, Hinds K, Berger L, Murphy K, Litman R. Structure and organization of immunoglobulin VH genes in Heterodontus, a phylogenetically primitive shark. Dev Comp Immunol. 1985;9:749–58. doi: 10.1016/0145-305x(85)90040-0. [DOI] [PubMed] [Google Scholar]
- Lugo-Villarino G, Balla KM, Stachura DL, Banuelos K, Werneck MB, Traver D. Identification of dendritic antigen-presenting cells in the zebrafish. PNAS. 2010;107:15850–55. doi: 10.1073/pnas.1000494107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. PNAS. 2000;97:12694–99. doi: 10.1073/pnas.97.23.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magor KE, Miranzo Navarro D, Barber MR, Petkau K, Fleming-Canepa X, et al. Defense genes missing from the flight division. Dev Comp Immunol. 2013;41:377–88. doi: 10.1016/j.dci.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning MJ, Horton JD. Histogenesis of lymphoid organs in larvae of the South African clawed toad, Xenopus laevis (Daudin) J Embryol Exp Morphol. 1969;22:265–77. [PubMed] [Google Scholar]
- Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–16. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- Mescher AL, Wolf WL, Moseman EA, Hartman B, Harrison C, et al. Cells of cutaneous immunity in Xenopus: studies during larval development and limb regeneration. Dev Comp Immunol. 2007;31:383–93. doi: 10.1016/j.dci.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Mussmann R, Courtet M, Schwager J, Du Pasquier L. Microsites for immunoglobulin switch recombination breakpoints from Xenopus to mammals. Eur J Immunol. 1997;27:2610–19. doi: 10.1002/eji.1830271021. [DOI] [PubMed] [Google Scholar]
- Mussmann R, Du Pasquier L, Hsu E. Is Xenopus IgX an analog of IgA? Eur J Immunol. 1996;26:2823–30. doi: 10.1002/eji.1830261205. [DOI] [PubMed] [Google Scholar]
- Neely HR, Flajnik MF. CXCL13 responsiveness but not CXCR5 expression by late transitional B cells initiates splenic white pulp formation. J Immunol. 2015;194:2616–23. doi: 10.4049/jimmunol.1401905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo VN, Korner H, Gunn MD, Schmidt KN, Riminton DS, et al. Lymphotoxin alpha/beta and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J Exp Med. 1999;189:403–12. doi: 10.1084/jem.189.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta Y, Flajnik M. IgD, like IgM, is a primordial immunoglobulin class perpetuated in most jawed vertebrates. PNAS. 2006;103:10723–28. doi: 10.1073/pnas.0601407103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olah I, Nagy N. Retrospection to discovery of bursal function and recognition of avian dendritic cells; past and present. Dev Comp Immunol. 2013;41:310–15. doi: 10.1016/j.dci.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med. 1996;184:1397–411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchappan R, Muthukkaruppan V. Thymus-dependent lymphoid regions in the spleen of the lizard, Calotes versicolor. J Exp Zool. 1977;199:177–88. doi: 10.1002/jez.1401990203. [DOI] [PubMed] [Google Scholar]
- Roberts CW, Shutter JR, Korsmeyer SJ. Hox11 controls the genesis of the spleen. Nature. 1994;368:747–49. doi: 10.1038/368747a0. [DOI] [PubMed] [Google Scholar]
- Rumfelt LL, Diaz M, Lohr RL, Mochon E, Flajnik MF. Unprecedented multiplicity of Ig transmembrane and secretory mRNA forms in the cartilaginous fish. J Immunol. 2004;173:1129–39. doi: 10.4049/jimmunol.173.2.1129. [DOI] [PubMed] [Google Scholar]
- Rumfelt LL, McKinney EC, Taylor E, Flajnik MF. The development of primary and secondary lymphoid tissues in the nurse shark Ginglymostoma cirratum: B-cell zones precede dendritic cell immigration and T-cell zone formation during ontogeny of the spleen. Scand J Immunol. 2002;56:130–48. doi: 10.1046/j.1365-3083.2002.01116.x. [DOI] [PubMed] [Google Scholar]
- Salinas I. The mucosal immune system of teleost fish. Biology. 2015;4:525–39. doi: 10.3390/biology4030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders HL, Oko AL, Scott AN, Fan CW, Magor BG. The cellular context of AID expressing cells in fish lymphoid tissues. Dev Comp Immunol. 2010;34:669–76. doi: 10.1016/j.dci.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Sepahi A, Salinas I. The evolution of nasal immune systems in vertebrates. Mol Immunol. 2016;69:131–38. doi: 10.1016/j.molimm.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SL, Sim RB, Flajnik MF. Immunobiology of the Shark. Boca Raton, FL: CRC Press; 2015. [Google Scholar]
- Steiniger B, Ulfig N, Risse M, Barth PJ. Fetal and early post-natal development of the human spleen: from primordial arterial B cell lobules to a non-segmented organ. Histochem Cell Biol. 2007;128:205–15. doi: 10.1007/s00418-007-0296-4. [DOI] [PubMed] [Google Scholar]
- Steiniger BS. Human spleen microanatomy: why mice do not suffice. Immunology. 2015;145:334–46. doi: 10.1111/imm.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- Sugimura M, Hashimoto Y, Nakanishi YH. Thymus- and bursa-dependent areas in duck lymph nodes. Jpn J Vet Res. 1977;25:7–16. [PubMed] [Google Scholar]
- Sun Y, Wei Z, Li N, Zhao Y. A comparative overview of immunoglobulin genes and the generation of their diversity in tetrapods. Dev Comp Immunol. 2013;39:103–9. doi: 10.1016/j.dci.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Kawamoto S, Maruya M, Fagarasan S. GALT: organization and dynamics leading to IgA synthesis. Adv Immunol. 2010;107:153–85. doi: 10.1016/B978-0-12-381300-8.00006-X. [DOI] [PubMed] [Google Scholar]
- Tangye SG. Staying alive: regulation of plasma cell survival. Trends Immunol. 2011;32:595–602. doi: 10.1016/j.it.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983;302:575–81. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Turpen JB, Smith PB. Analysis of hemopoietic lineage of accessory cells in the developing thymus of Xenopus laevis. J Immunol. 1986;136:412–21. [PubMed] [Google Scholar]
- Vondenhoff MF, Desanti GE, Cupedo T, Bertrand JY, Cumano A, et al. Separation of splenic red and white pulp occurs before birth in a LTαβ-independent manner. J Leukoc Biol. 2008;84:152–61. doi: 10.1189/jlb.0907659. [DOI] [PubMed] [Google Scholar]
- Wittamer V, Bertrand JY, Gutschow PW, Traver D. Characterization of the mononuclear phagocyte system in zebrafish. Blood. 2011;117:7126–35. doi: 10.1182/blood-2010-11-321448. [DOI] [PubMed] [Google Scholar]
- Yasuda M, Kajiwara E, Ekino S, Taura Y, Hirota Y, et al. Immunobiology of chicken germinal center. I Changes in surface Ig class expression in the chicken splenic germinal center after antigenic stimulation. Dev Comp Immunol. 2003;27:159–66. doi: 10.1016/s0145-305x(02)00066-6. [DOI] [PubMed] [Google Scholar]
- Yasuda M, Taura Y, Yokomizo Y, Ekino S. A comparative study of germinal center: fowls and mammals. Comp Immunol Microbiol Infect Dis. 1998;21:179–89. doi: 10.1016/s0147-9571(98)00007-1. [DOI] [PubMed] [Google Scholar]
- Yu P, Wang Y, Chin RK, Martinez-Pomares L, Gordon S, et al. B cells control the migration of a subset of dendritic cells into B cell follicles via CXC chemokine ligand 13 in a lymphotoxin-dependent fashion. J Immunol. 2002;168:5117–23. doi: 10.4049/jimmunol.168.10.5117. [DOI] [PubMed] [Google Scholar]
- Zapata AG, Torroba M, Sacedón R, Varas A, Vicente A. Structure of the lymphoid organs of elasmobranchs. J Exp Zool. 1996;275:125–43. [Google Scholar]
- Zhu C, Lee V, Finn A, Senger K, Zarrin AA, et al. Origin of immunoglobulin isotype switching. Curr Biol. 2012;22:872–80. doi: 10.1016/j.cub.2012.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]