Abstract
Predicting blood pressure (BP) response to antihypertensive therapy is challenging. The therapeutic intensity score (TIS) is a summary measure that accounts for the number of medications and the relative doses a patient received, but its relationship to BP change and its utility as a method to project dosing equivalence has not been reported. We conducted a prospective, single center, randomized controlled trial to compare the effects of Joint National Committee (JNC) 7 compliant treatment with more intensive (<120/80 mm Hg) BP goals on left ventricular structure and function in hypertensive patients with echocardiographically determined subclinical heart disease who were treated over a 12-month period. For this preplanned subanalysis, we sought to compare changes in BP over time with changes in TIS. Antihypertensive therapy was open label. TIS and BP were determined at 3-month intervals with titration of medication doses as needed to achieve targeted BP. Mixed linear models defined antihypertensive medication TIS as an independent variable and change in systolic BP as an outcome measure, while controlling for gender, age, baseline BP, and treatment group. A total of 123 patients (mean age 49.4 ± 8.2 years; 66% female; 95.1% African-American) were enrolled and 88 completed the protocol. For each single point increase in total antihypertensive TIS, a 14.5 (95% confidence interval: 11.5, 17.4) mm Hg decrease in systolic BP was noted (15.5 [95% confidence interval: 13.0, 18.0] mm Hg for those who completed the trial). Total TIS is a viable indicator of the anticipated BP-lowering effect associated with antihypertensive therapy.
Keywords: Antihypertensive therapy, hypertension, subclinical hypertensive heart disease
Introduction
Uncontrolled hypertension (HTN) is extremely prevalent in the United States (US).1–6 Poor blood pressure (BP) control among persons with HTN continues to be an ongoing challenge for patients and clinicians.1,2,7–10 Less than 50% of persons with HTN have achieved recommended targets for BP control.1 There is a clearly established positive association between persistent elevated BP and risk of adverse events such as stroke, myocardial infarction, chronic kidney disease, heart failure, cognitive decline, and mortality.1,11–13 Better BP control, perhaps involving even lower target levels than previously recommended, is vital to arrest the progression of BP-mediated organ damage and HTN-related morbidity.1,14,15
Evidence-based recommendations for BP targets and medical therapy for persons with HTN have long been based on the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7).16 Recent updates in the Eighth Report (JNC 8) call for up-titration of antihypertensive therapy in those without contraindications, with an increase in dose or addition of a second drug when BP remains uncontrolled at 1-month posttreatment initiation.17 Moreover, if goal BP cannot be achieved with two drugs, a third drug should be added and titrated.17
While there is pathophysiological evidence that duration of HTN and microvascular remodeling contribute to HTN resistance and poor BP control, low adherence to prescribed medical therapy1,18,19 is a significant behavioral factor in poor HTN control. Therapeutic inertia (failure of clinicians to intensify treatment when BP rises above therapeutic goals)20,21 is also a major behavioral factor precluding proper treatment of HTN in clinical practice. A number of system-and provider-level factors contribute to therapeutic inertia, but clinician uncertainty regarding an expected effect of anti-hypertensive therapy across medication classes and doses may be especially important. Currently, there is no commonly accepted approach to measure therapeutic intensification for HTN in clinical trials or in routine clinical practice. Many studies utilize number of BP medications as a surrogate measure of therapeutic intensity, but this does not account for the wide variability in dosing observed between patients. Furthermore, while various methods of measuring antihypertensive therapeutic intensity have been previously proposed, there has been limited implementation of such approaches in routine clinical practice.5,22
One proposed method to quantify treatment intensity is the therapeutic intensity score (TIS). While this metric has been previously used to compare treatment in longitudinal HTN control trials,1,23 it has not been examined separately as a research and clinical tool to project the BP-lowering effect of antihypertensive therapy. To that end, this study details the development of TIS; a summary measure that may have utility to predict the expected effect of a given individual antihypertensive dose or concurrent dosing of multiple medications on BP. Our primary objective in this analysis was to quantify the relationship between BP change and TIS over time in a recently completed randomized controlled trial that compared usual (JNC 7 compliant) care with more intensive BP goals (<120/80 mm Hg) for patients with uncontrolled HTN and subclinical hypertensive heart disease.24
Methods
Overview
This was a preplanned subanalysis of a prospective, randomized controlled trial in a predominantly African-American population with poorly controlled chronic HTN and echocardiographic evidence of subclinical hypertensive heart disease.25 The parent trial was designed to compare the effect of usual care (control), which targeted JNC 7 recommended BP goals (<140/90 mm Hg [<130/80 mm Hg if diabetes or chronic kidney disease was present]) with more intensive management (intervention) that had a goal BP of <120/80 mm Hg for all, on echocardiographic findings, and patient reported quality of life at 1 year. The trial was approved by a university-based Institutional Review Board, and all subjects provided written informed consent prior to enrollment.
Variables
Outcomes for the parent study have been previously reported.24 The aim of this subanalysis was to evaluate the relationship between HTN therapeutic intensification and BP reduction using TIS as a summative representation of medication dosing. TIS is a proportional measure of prescribed to maximum US Food and Drug Administration recommended dosage that was calculated for each antihypertensive medication prescribed at study specified follow-up visits. Individual TIS scores of each current antihypertensive medication were calculated for each patient and added to yield a single, summative TIS score. To calculate TIS, the prescribed daily dose for each medication is set as the numerator value, and the corresponding maximum Food and Drug Administration–approved daily dose is set as the denominator value. As an example, if a study participant was prescribed three antihypertensive medications each at 25% of the maximum approved dosage, then the total TIS score for that participant would be calculated as 0.75 (0.25 + 0.25 + 0.25). Similarly, a study participant who is prescribed three antihypertensive medications, two at 50% maximum dosage and one at 75% maximum dosage, would have a total TIS of 1.75 (0.50 + 0.50 + 0.75). The maximum achievable TIS is patient specific and dependent on the total number of antihypertensive medications reported as prescribed at each visit. This algorithm was used to compute the TIS score for each study participant at each of five study specific follow-up visits (0, 3, 6, 9, and 12 months) over a 12-month period. Information on prerandomization (ie, visit month −0.5) antihypertensive therapy was also collected, and where possible, TIS was calculated; however, patients were often not aware of actual dosing making accurate determination of prerandomization treatment TIS difficult.
Study Setting and Sample Population
Study participants were recruited from a large, urban, emergency department (ED) at a tertiary, academic medical center, which treats more than 90,000 patients each year. Patients eligible for inclusion were identified in the ED using the facility’s electronic medical record (FirstNet by Cerner Corp.; Kansas City, MO, USA). Patients 35 years of age or older who had an initial and repeat (within 1 hour) BP >140/90 mm Hg and normal exertional tolerance (defined as class 1 on the Goldman Specific Activity Scale) were eligible for inclusion.25,26 Patients with acute illness requiring hospitalization, a prior history of cardiac disease, or any presentation with symptoms potentially attributable to hypertensive heart disease (eg, dyspnea, chest pain) were excluded. We also excluded patients with a self-reported stable source of primary care, as non-study HTN management could confound outcomes based on study directed antihypertensive therapy.
Eligible patients who completed written informed consent were scheduled for a post-ED screening visit in a dedicated HTN clinic. At this baseline visit (visit month −0.5), a detailed medical history was obtained, two-dimensional echocardiography with tissue Doppler imaging was performed, and the Short-Form 36 (SF-36; a validated patient reported measure of health status) was completed. Patients with subclinical hypertensive heart disease on echocardiogram (ie, left ventricular [LV] mass ≥ 48 g/m2.7 in males or ≥ 45 g/m2.7 in females), LV systolic dysfunction (ejection fraction < 50%), or LV diastolic dysfunction (based on standard criteria) were then randomized to either the control or intervention treatment arm.27
Study Protocol
Patients were followed for 1-year postenrollment in a single HTN clinic. Study-specific HTN care was delivered by a multidisciplinary team that included physician assistants, nurse practitioners, and physicians. Eligible participants in each arm had an initial randomization visit where baseline BP was established (visit month 0) and four subsequent follow-up visits at 3, 6, 9, and 12 months. At each follow-up visit, a trained research assistant, who was blinded to treatment groups, performed automated oscillometric brachial cuff BP measurements taken in participants’ right arm while seated. The average reading of three independent measurements was used to determine the BP reading recorded for that clinic visit. Using JNC 7 guidelines, the physician assistant or nurse practitioner titrated antihypertensive therapy at each visit in an open label fashion, per the BP goal predetermined by the patients’ random group assignment. To encourage patient adherence with prescribed treatment in this study, all antihypertensive therapy was provided to participants free of charge, with pills dispensed directly to them at study specified follow-up visits. Participants were also provided with transportation to and from appointments and received regular telephone reminders for scheduled visits. At each follow-up visit, BP readings, all study prescribed medications, including antihypertensive and nonantihypertensive therapy and additional survey data were recorded. Patients were instructed to take their medications prior to study directed follow-up visits, and visit timing was variable, depending on patient and clinic availability. Echocardiography (performed in the same laboratory, using the same approach with an independent reader who was blinded to the initial echocardiogram, the randomized study group, and all patient information other than age and gender) and other baseline study measures including the SF-36 were repeated at 1 year, upon completion of the study treatment protocol.
Sample Size
Using data from our HTN clinic to derive a between-measurement correlation of 0.65 and an intraperson systolic BP standard deviation of 10 mm Hg, repeated measure sample size calculations (baseline BP; and BP measurements at 3, 6, 9, and 12 months) were performed indicating that 45 patients per group (total N = 90) would be needed to achieve 90% power (beta error = 0.1) with a two-tail test and alpha error = 0.05 to demonstrate a clinically significant difference (6 mm Hg difference) in systolic BP change between groups. Accounting for a projected 15% attrition rate over the study period, the enrollment target was 104 patients. The actual patient attrition rate was 28.5% (n = 35); therefore, 19 additional subjects were recruited to maintain a stable sample.
Data Analysis
Means, standard deviations, 95% confidence intervals (CIs), and medians with interquartile ranges were determined for continuous data, while proportions and 95% CI were generated for categorical data. The distributions of continuous variables were examined for skewness/normality using Shapiro–Wilk statistic or Kolmogorov–Smirnov statistic with normalization using log-transformations prior to analysis for any continuous variable that was far from normality assumption. Continuous and categorical data were compared using t tests and chi-square analyses, between group differences and corresponding 95% CIs. Two-tailed alpha significance was set at 0.05, with multiple group comparisons. We developed repeated measures mixed linear models that adjusted for age, gender, baseline BP (systolic and diastolic BP were modeled as separate effects), treatment randomization group, and antihypertensive TIS. All data were analyzed with SAS (Cary, NC). Missing data were imputed using a “last observation carried forward” strategy. All group comparisons were conducted first by intention to treat (ITT) and subsequently using on-treatment (OT) analyses.
Results
A total of 149 patients met the screening eligibility requirements in the ED, and 123 (control n = 65, intervention n = 58—ITT cohort) were randomized; 71.5% (88 total: control n = 45, intervention n = 43—OT cohort) completed the 12-month study protocol. The sample population was predominantly self-identified African-American (95%), and female (66%), with a mean age of 49.5 ± 8.2 years. Most patients (82.9%) reported a known history of HTN that had been treated for an average of 8.8 ± 8.6 years. Additional demographic and clinical characteristics of the sample population are presented in Table 1. Patients who did not complete the study were similar to those who did with respect to baseline characteristics including SF-36 data on general and mental health (data not shown).
Table 1.
Baseline patient characteristics
| Control (n = 65) | Intervention (n = 58) | P Value | |
|---|---|---|---|
| Demographics | |||
| Gender, n (% male) | 21 (32.3) | 22 (37.9) | .51 |
| Race, n (% African-American) | 59 (90.8) | 58 (100.0) | .06 |
| Age, mean (SD) | 49.3 (8.1) | 49.6 (8.3) | .83 |
| Body mass index (kg/m2), mean (SD) | 31.7 (6.3) | 32.8 (5.8) | .30 |
| Screening SBP (mm Hg), mean (SD) | 181.4 (21.0) | 181.5 (22.8) | .98 |
| Screening DBP (mm Hg), mean (SD) | 105.3 (13.3) | 105.3 (12.1) | .99 |
| Initial SBP (mm Hg), mean (SD) | 153.2 (20.6) | 151.2 (25.1) | .63 |
| Initial DBP (mm Hg), mean (SD) | 100.6 (13.2) | 97.9 (15.5) | .31 |
| Medical conditions, n (%) | |||
| Hypertension | 50 (78.1) | 46 (82.1) | .60 |
| Hyperlipidemia | 6 (9.2) | 3 (5.3) | .70 |
| Stoke and/or TIA | 2 (3.1) | 2 (3.5) | .89 |
| Diabetes | 5 (7.7) | 3 (5.2) | .57 |
| Chronic kidney disease | 1 (1.5) | 0 (0.0) | .54 |
| Any liver disease | 1 (2.0) | 1 (2.6) | .54 |
| Social history, n (%) | |||
| Education of high school or greater | 49 (77.8) | 37 (67.3) | .32 |
| Any exercise | 32 (51.6) | 37 (64.9) | .14 |
| Tobacco use | 38 (60.3) | 34 (59.6) | .94 |
| Alcohol use | 35 (55.6) | 32 (56.1) | .63 |
| Illicit drug use | 19 (30.2) | 14 (24.6) | .49 |
| Hypertensive history | |||
| Duration of HTN in years, mean (SD) | 7.5 (9.9) | 8.1 (9.1) | .73 |
| Number of ED visits for HTN, mean (SD) | 1.4 (4.2) | 1.6 (2.8) | .75 |
| Number of HTN hospitalizations, mean (SD) | 0.1 (0.4) | 0.3 (0.8) | .06 |
| Medical therapy for HTN, n (%) | 13 (20.0) | 15 (25.9) | .44 |
| Duration of HTN therapy in years, mean (SD) | 10.1 (11.9) | 7.3 (9.5) | .42 |
| HTN medications missed per week, mean (SD) | 1.1 (2.0) | 0.6 (1.0) | .36 |
| Practice diet modification of HTN, n (%) | 17 (28.3) | 13 (25.0) | .53 |
| Consumes a no added or low-salt diet, n (%) | 15 (75.0) | 10 (66.7) | .59 |
| Attempted weight-loss diet, n (%)* | 4 (20.0) | 3 (25.0) | .74 |
| Antihypertensive medications | |||
| Number of HTN medications, mean (SD) | 1.8 (0.8) | 1.8 (1.2) | .89 |
| Beta blocker, n (%) | 3 (4.6) | 0 (0.0) | .10 |
| Calcium channel antagonists, n (%) | 1 (1.5) | 4 (6.9) | .13 |
| Sympatholytics, n (%) | 1 (1.5) | 1 (1.7) | .94 |
| Thiazide diuretics, n (%) | 10 (15.4) | 8 (13.8) | .80 |
| Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, n (%) | 3 (4.6) | 5 (8.6) | .37 |
| Others, n (%) | 0 (0.0) | 1 (1.7) | .29 |
| Social Functioning Questionnaire (mean, SD) | |||
| General health | 57.5 (19.9) | 68.0 (16.6) | .00 |
| Mental health | 69.8 (20.9) | 71.3 (21.5) | .69 |
| Vitality | 61.2 (21.5) | 64.8 (22.1) | .36 |
| Social function | 71.7 (26.4) | 76.3 (26.0) | .34 |
| Physical function | 76.2 (26.0) | 79.1 (22.2) | .50 |
| Bodily pain | 63.6 (29.4) | 68.3 (31.6) | .40 |
| Physical limitations | 71.1 (30.6) | 70.1 (27.7) | .85 |
| Emotional limitations | 74.7 (27.2) | 74.3 (27.5) | .92 |
| Health transition | 50.8 (20.7) | 50.4 (26.1) | .94 |
| Physical component summary | 67.1 (21.6) | 71.6 (19.0) | .23 |
| Mental health component summary | 69.4 (20.9) | 71.9 (20.5) | .50 |
DBP, diastolic blood pressure; ED, emergency department; HTN, hypertension; SBP, systolic blood pressure; SD, standard deviation; TIA, transient ischemic attack.
P < .05.
At initial enrollment in the ED, mean systolic BP was 182.5 ± 23.3 mm Hg with a mean diastolic BP 104.8 ± 12.3 mm Hg. At randomization (visit month 0), both average BP readings were lower with a mean systolic BP of 151.2 ± 24.1 mm Hg and a mean diastolic BP of 97.2 ± 15.8 mm Hg. Some patients were prescribed antihypertensives for the interval (n = 12 days, interquartile range: 7–19) between recruitment and randomization,2,28 at the discretion of the treating emergency physician and had a calculable TIS at visit month 0, but this was not uniform nor was initiation of antihypertensive therapy at ED discharge part of the study protocol. As shown in Figure 1, systolic BP continued to decrease steadily in both intervention and control patients through 3 months, with a relative plateau thereafter. There was no statistical difference in change in systolic BP between study groups at any time point. At the end of the study, 17.2% of patients in the intervention group (23.3% in the OT subgroup) and 32.3% of control patients (46.7% in the OT subgroup) achieved their respective BP goals.
Figure 1.
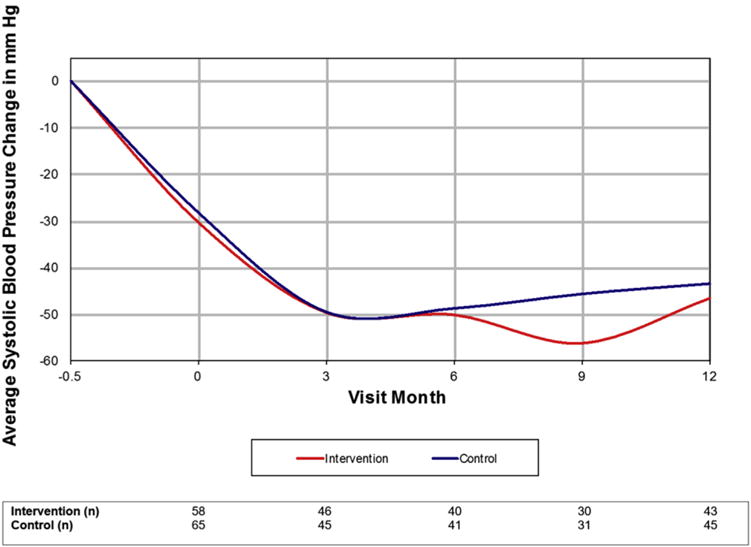
Change in systolic blood pressure over time by randomized study group.
Chlorthalidone, angiotensin-converting enzyme inhibitors, spironolactone, and calcium antagonists were the most commonly prescribed medications (Table 2). Total TIS increased in both groups over the first 3 months and continued to rise through month 6 in the intervention arm but not in the control arm (Figure 2). Beyond these time points, only minimal increases in TIS were noted. Similar patterns were noted for TIS when stratified by individual medication class in intervention and control groups (Figure 3A and B). While we recognize that even at comparative dosage, some antihypertensive medications have greater impact on BP than others, the study was not sufficiently powered to evaluate the impact of TIS on change in BP by discrete medication class.
Table 2.
Patient use of individual medication classes across the study period
| Medication Class | Baseline (%,Mean TIS) | 3-Month Visit (%, Mean TIS) |
6-Month Visit (%, Mean TIS) |
9-Month Visit (%, Mean TIS) |
12-Month Visit (%, Mean TIS) |
|---|---|---|---|---|---|
| Beta blocker | 5.2, 0.58 | 6.4, 0.23 | 6.7, 0.17 | 7.4, 0.16 | 4.9, 0.12 |
| Angiotensin receptor blocker | 1.7, 1.0 | 4.1, 0.61 | 3.1, 0.55 | 2.5, 0.58 | 2.9, 0.67 |
| Calcium antagonists (dihydropyridines) | 12.1, 0.64 | 5.8, 0.68 | 5.5, 0.69 | 5.0, 0.63 | 3.9, 0.56 |
| Calcium antagonists (diphenylalkylamines) | 1.7, 0.25 | 5.8, 0.63 | 6.1, 0.6 | 7.4, 0.53 | 9.7, 0.61 |
| Angiotensin-converting enzyme (ACE) inhibitors | 15.5, 0.26 | 20.3, 0.36 | 24.5, 0.37 | 21.5, 0.37 | 21.4, 0.38 |
| Chlorthalidone | 43.1, 0.54 | 40.1, 0.53 | 38.0, 0.53 | 38.0, 0.55 | 37.9, 0.56 |
| Spironolactone | N/A | 11.0, 0.36 | 12.3, 0.34 | 14.9, 0.34 | 12.6, 0.33 |
| Sympatholytic | 3.4, 0.41 | 1.7, 0.38 | N/A | N/A | N/A |
N/A, no study participants were receiving drugs from the specified class at the specified visit; TIS, therapeutic intensity score.
Percentages are computed with a denominator equal to all patients receiving a prescription for antihypertensive medication at the specified visit. In most cases, this reflects the actual number of patients remaining in the study at that visit; however, in rare instances, subjects were not prescribed antihypertensive therapy at a specific visit.
Figure 2.
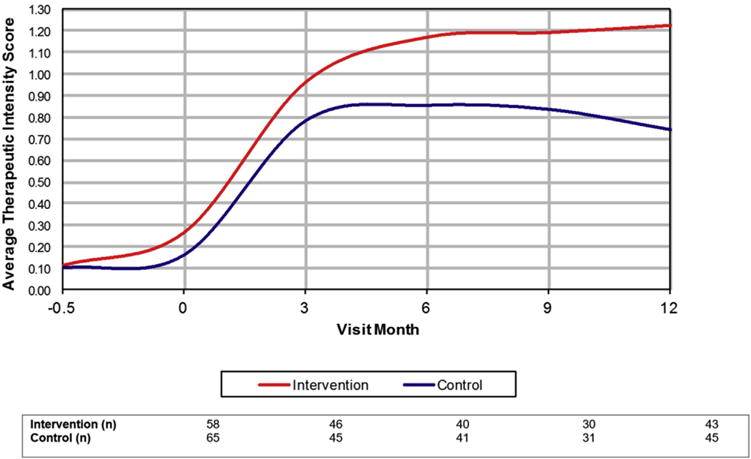
Average therapeutic intensity score over time by randomized study group.
Figure 3.
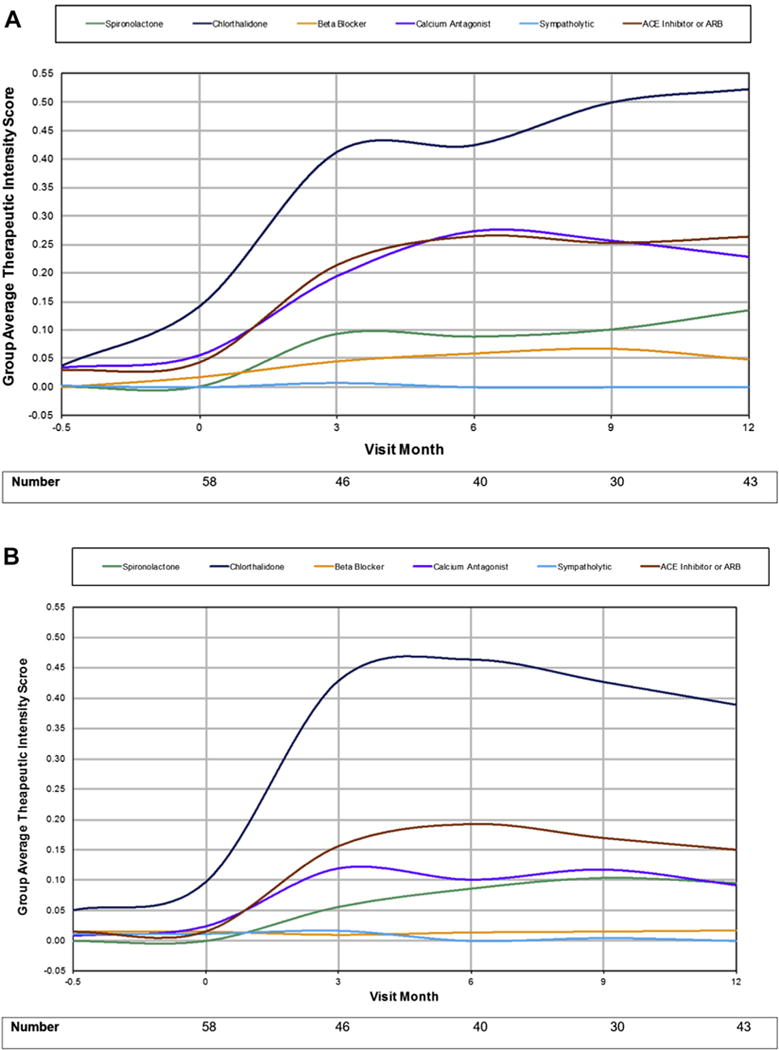
(A) Therapeutic intensity score by medication class for intervention group. (B) Therapeutic intensity score by medication class for control group. ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker.
On mixed linear modeling adjusting for baseline systolic and diastolic BP, regression coefficients indicated a 15.5 mm Hg (95% CI: 13.0, 18.0 mm Hg; P ≤ .0001) and a 16.6 mm Hg (95% CI: 14.0, 19.2 mm Hg; P ≤ .0001) reduction in systolic BP for each single point increase in antihypertensive TIS, respectively, were noted in the ITT cohort. Regression coefficients representing the magnitude of effect were similar in the OT cohort, corresponding to a decrease in systolic BP of 14.4 mm Hg (95% CI: 11.5, 17.3 mm Hg; P ≤ .0001) per TIS point when adjusting for baseline systolic BP and 16.0 mm Hg (95% CI: 13.0, 19.0 mm Hg; P ≤ .0001) when adjusting for baseline diastolic BP. This relationship was also stable when evaluated in the cohort of patients (n = 103) who completed at least one study visit postrandomization. For all models, a statistically significant interaction between TIS and baseline BP was noted, suggesting that antihypertensive therapy was driven in part by the degree of baseline HTN in addition to randomization group.
Discussion
Evidence-based research has repeatedly demonstrated that increases in therapeutic intensity of antihypertensive medication throughout the course of treatment can result in better BP control.12,13,29 In this subanalysis of a prospective, randomized, controlled trial, we found that systolic BP decreased by a statistically significant 14–16 mm Hg for every 1-point increase in TIS, even when accounting for age, gender, and baseline BP. Furthermore, systolic BP appeared to demonstrate an inverse relationship with TIS, decreasing as TIS increased, and leveling off as TIS plateaued suggesting that TIS is an important indicator of antihypertensive therapy effect. To minimize the potential influence of nonadherence, we provided all antihypertensive therapy to study patients free of charge. While this does not equate with directly observed therapy, perhaps the only way to ensure 100% adherence, it does increase the likelihood that our findings do accurately reflect antihypertensive drug effects.
Failure to up-titrate antihypertensive medication when needed makes HTN control difficult. As previously described, one likely contributing factor to widespread therapeutic inertia is the challenge physicians face in standardizing the expected effects of antihypertensive dosing across medication classes, and in comparing antihypertensive doses between groups, or individual patients in routine clinical practice. A second likely contributing factor, borne out by this trial, is clinician uncertainty or reluctance to continue to treatment beyond usual care targets (JNC 7 compliant) to achieve more stringent BP control, particularly when managing BP with multiple antihypertensive classes and in patients with cardiovascular comorbidities. Thus, there is utility in the TIS, as it serves to delineate the impact of therapeutic intensification for BP control in routine clinical practice. TIS may also be a useful guide for medication titration itself, particularly when noncompliance is suspected in patients for whom the expected BP effect has not been achieved.
Such findings suggest an opportunity to impact clinician behavior through education and use of tools aimed at improving understanding of treatment effects and their relationship to clinical outcomes and quality of care. TIS provides a reasonable metric by which physicians can better assess their antihypertensive dosing practices and project expected treatment effects. The formula for calculating TIS is objective, easy to use, and may help combat therapeutic inertia by providing clinicians with an objective system that can support decision-making relative to desired antihypertensive treatment effects on BP control. Further, the computation of TIS, as described, serves as a superior metric of antihypertensive dosing intensity compared to less complex and less nuanced measures such as number and/or class of drug prescribed or number of dosage increases over time. We do note, however, that the computation of the relationship between TIS and BP assumes both a comparable dose response on BP across multiple drug classes and that incremental dosing increases and decreases in each discrete drug prescribed has a linear dose response on BP.
While we recognize that dosage response varies across medication classes, the extent to which synergistic effects across drug classes impacted BP reduction was not considered in this study. Instead, the goal was to demonstrate temporal trends in medication intensification and their impact on BP reduction. Moreover, a singular TIS value could represent multiple combinations, and our study does not provide insight into which is better—lower dosages of several medications or a higher single drug dose. Although a single pill may be preferred for supporting patient compliance,16 a meta-analysis by Law et al30 suggests a similar BP-lowering effect for multiple drug classes at “standard doses” with increased efficacy and reduced adverse effects using combination, “low-dose” drug treatments. To address such considerations, we encourage other investigators to explore the relationship between TIS and individual medication effects.
This is not the first trial designed to address the need for a standardized measure of antihypertensive therapeutic intensity beyond a simple summation of the number of antihypertensive drugs prescribed. Most recently, Rose et al22 compared two different scoring systems: a norm-based method, first described by Berlowitz,9 and a standard-based method (SBM), first described by Okonofua.31 The norm-based method is an observed versus expected scoring system, while the SBM is a simpler method that counts the number of dosage increases documented over several visits when BP > 140 mm Hg. Although Rose et al22 concluded that of the two methods, only the SBM is helpful in the measurement of treatment intensity for HTN. SBM does not provide a quantitative measure that adequately describes and critically assesses the amount or number of dosage increases, changes in prescription regimen, and changes made to multiple medications. More importantly, both measures provide only macrolevel insight into physician treatment practices and focus on therapeutic inertia, tracking the association between outcome (ie, BP change) and an action (ie, medication titration) rather specific effects of the titrated medications themselves. The TIS proposed in this study is best appreciated as a complementary measure which builds on previous work by quantifying the expected effect of incremental medication adjustment on change in BP. We believe our approach offers an advantage to clinicians, particularly those who want a numerical basis for their treatment decisions. Further, we have previously shown that increases in TIS are well tolerated by patients with no adverse effect on perceived health status,24 lending further support to TIS as a measure with clinical applicability.
The potential utility of TIS extends beyond clinical practice to the research setting, where it could permit projection of expected BP effects with differential antihypertensive dosing across medication classes for therapeutic trials and enable more accurate adjustment of antihypertensive therapy across study groups. For the latter, TIS would be analogous to the Charlson Comorbidity Index,32 and other methods to standardize the influence of risk-enhancing conditions on outcomes. In addition, a well-described process for scoring therapeutic intensity could be useful as a tool for comparative clinical effectiveness research involving new or existing antihypertensive agents. With broader adoption, in clinical practice, TIS may also have applications in health services research, serving as an objective measure for value determination of more or less aggressive antihypertensive treatment and related population-based outcomes.33
While our prospective design and inclusion of subjects with uncontrolled BP who, for the most part, were not receiving antihypertensive therapy at baseline are strengths, there are several limitations that warrant consideration. Among these is the fact our study was conducted at a single center, with a relatively limited study sample that was comprised of predominantly African-Americans and females seeking services in an under-resourced clinical setting. Moreover, our study patient population was preselected based on the presence of subclinical hypertensive heart disease. Accordingly, the relationship between TIS and BP we report may not be generalizable to the broader hypertensive population and the magnitude of effect not be similar, depending on factors such as race, gender, and underlying cardiac structure/function. Nonetheless, our study includes a representative sample from a high-risk, understudied population where subclinical hypertensive heart disease is exceedingly common,25 and our results have clear applicability when interpreted within such a context. We acknowledge that objective and accurate measures of patient medication adherence at baseline and over the follow-up period as well as a 24-hour ambulatory BP monitoring protocol would strengthen the study findings and implications. We also note that while a variety of evidence-based antihypertensive medications classes were utilized in our study population, the sample size was too small to model with any degree of stability the association between TIS and BP change for individual drugs or classes. However, as shown in a meta-analysis by Law et al30 that included data from 354 randomized trials involving more than 50,000 patients, similar reductions in BP can be expected across the range of medication classes. Finally, our study did suffer from a relatively high rate of patient attrition with 28.5% of randomized subjects not completing the full study protocol. While there were no baseline differences between those who did and did not complete the study, it does serve to highlight the unique challenges inherent to recruiting and retaining patients from the ED. Nonetheless, it is possible that attrition was not random and that subjects who dropped out did so because they experienced different, perhaps unpleasant effects from treatment, related or unrelated to BP change. As a consequence, this and other potential sources of missing data may have biased apparent effects on BP. To address this, we did impute missing BP values using a conservative, last observation carried forward approach. While multiple imputation may have provided a more robust estimate of BP effects, there was consistency on ITT and OT analyses suggesting that the magnitude of BP reduction we report is a stable finding.
Perspectives
This study provides new insight into the impact that therapeutic intensification can have on BP reduction among patients with uncontrolled HTN and subclinical hypertensive heart disease. In addition, it describes an underutilized measure TIS that can help to quantify the projected impact of a given dosing regimen on BP. Our analysis indicated that for every one-point increase in total antihypertensive TIS, there was an associated 14–16 mm Hg reduction in systolic BP over a 12-month follow-up period. While this is not the first study to address the development of a standardized measure of antihypertensive therapeutic intensification, clinicians will appreciate the ease of computation along with the ability to assess their antihypertensive dosing practices and respective project expected treatment effects in similar populations. Although the TIS bears further refinement, the measure holds significant potential for helping to combat therapeutic inertia by providing an objective benchmark that clinicians can use to evaluate therapeutic regimens against expected outcomes both in clinical practice and in clinical research. It also provides a useful tool for use in clinician education, providing a way to demonstrate the broad effects of antihypertensive therapy on BP control and a measure to help standardize development of an antihypertensive therapeutic plan.
Conclusions
Each one-point increase in total antihypertensive TIS was associated with a 14–16 mm Hg reduction in systolic BP. While future studies are needed to address the validity of TIS in more diverse populations with and without underlying cardiovascular disease, this measure holds promise for use in both clinical practice and research, providing an objective tool to evaluate expected treatment effects with modulation of therapeutic intensity across different antihypertensive regimens.
Acknowledgments
The authors thank all students and research technicians who were involved with the data collection and retention of participants enrolled in the study.
This research project was funded by a grant provided by the Robert Wood Johnson Foundation Physician Faculty Scholars Program.
Footnotes
Conflict of interest: No conflicts of interest were reported.
References
- 1.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303(20):2043–50. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 2.Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension. 2007;49(1):69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- 3.Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension. 2008;52(5):818–27. doi: 10.1161/HYPERTENSIONAHA.108.113357. [DOI] [PubMed] [Google Scholar]
- 4.Burt VL, Cutler JA, Higgins M, Horan MJ, Labarthe D, Whelton P, et al. Trends in the prevalence, awareness, treatment, and control of hypertension in the adult US population. Data from the health examination surveys, 1960 to 1991. Hypertension. 1995;26(1):60–9. doi: 10.1161/01.hyp.26.1.60. [DOI] [PubMed] [Google Scholar]
- 5.Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension. 2004;44(4):398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- 6.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290(2):199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 7.Gu Q, Burt VL, Dillon CF, Yoon S. Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension: The National Health and Nutrition Examination Survey, 2001 to 2010. Circulation. 2012;126(17):2105–14. doi: 10.1161/CIRCULATIONAHA.112.096156. [DOI] [PubMed] [Google Scholar]
- 8.Bennett H, Laird K, Margolius D, Ngo V, Thom DH, Bodenheimer T. The effectiveness of health coaching, home blood pressure monitoring, and home-titration in controlling hypertension among low-income patients: protocol for a randomized controlled trial. BMC Public Health. 2009;9:456. doi: 10.1186/1471-2458-9-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berlowitz DR, Ash AS, Hickey EC, Friedman RH, Glickman M, Kader B, et al. Inadequate management of blood pressure in a hypertensive population. N Engl J Med. 1998;339(27):1957–63. doi: 10.1056/NEJM199812313392701. [DOI] [PubMed] [Google Scholar]
- 10.Nelson SA, Dresser GK, Vandervoort MK, Wong CJ, Feagan BG, Mahon JL, et al. Barriers to blood pressure control: a STITCH substudy. J Clin Hypertens (Greenwich) 2011;13(2):73–80. doi: 10.1111/j.1751-7176.2010.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pepine CJ, Kowey PR, Kupfer S, Kolloch RE, Benetos A, Mancia G, et al. INVEST Investigators Predictors of adverse outcome among patients with hypertension and coronary artery disease. J Am Coll Cardiol. 2006;47(3):547–51. doi: 10.1016/j.jacc.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 12.Appel LJ, Wright JT, Jr, Greene T, Agodoa LY, Astor BC, Bakris GL, et al. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010;363(10):918–29. doi: 10.1056/NEJMoa0910975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The ACCORD Study Group. Cushman WC, Evans GW, Byington RP, Goff DC, Jr, Grimm RH, Jr, Cutler JA, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575–85. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957–67. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 15.SPRINT Research Group. Wright JT, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–16. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 17.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 18.Lewis LM, Schoenthaler AM, Ogedegbe G. Patient factors, but not provider and health care system factors, predict medication adherence in hypertensive black men. J Clin Hypertens (Greenwich) 2012;14(4):250–5. doi: 10.1111/j.1751-7176.2012.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swain MA, Steckel SB. Influencing adherence among hypertensives. Res Nurs Health. 1981;4(1):213–22. doi: 10.1002/nur.4770040107. [DOI] [PubMed] [Google Scholar]
- 20.Nasser SA, Lai Z, O’Connor S, Liu X, Flack JM. Does earlier attainment of blood pressure goal translate into fewer cardiovascular events? Curr Hypertens Rep. 2008;10(5):398–404. doi: 10.1007/s11906-008-0074-2. [DOI] [PubMed] [Google Scholar]
- 21.Gil-Guillén V, Orozco-Beltrán D, Pérez RP, Alfonso JL, Redón J, Pertusa-Martínez S, et al. Clinical inertia in diagnosis and treatment of hypertension in primary care: quantification and associated factors. Blood Press. 2010;19(1):3–10. doi: 10.3109/08037050903350762. [DOI] [PubMed] [Google Scholar]
- 22.Rose AJ, Berlowitz DR, Manze M, Orner MB, Kressin NR. Comparing methods of measuring treatment intensification in hypertension care. Circ Cardiovasc Qual Outcomes. 2009;2(4):385–91. doi: 10.1161/CIRCOUTCOMES.108.838649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dresser GK, Nelson SA, Mahon JL, Zou G, Vandervoort MK, Wong CJ, et al. Simplified therapeutic intervention to control hypertension and hypercholesterolemia: a cluster randomized controlled trial (STITCH2) J Hypertens. 2013;31(8):1702–13. doi: 10.1097/HJH.0b013e3283619d6a. [DOI] [PubMed] [Google Scholar]
- 24.Burla MJ, Brody AM, Ference BA, Flack JM, Mahn JJ, Marinica AL, et al. Blood pressure control and perceived health status in African Americans with subclinical hypertensive heart disease. J Am Soc Hypertens. 2014;8(5):321–9. doi: 10.1016/j.jash.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Levy P, Ye H, Compton S, Zalenski R, Byrnes T, Flack JM, et al. Subclinical hypertensive heart disease in black patients with elevated blood pressure in an inner-city emergency department. Ann Emerg Med. 2012;60(4):467–74 e1. doi: 10.1016/j.annemergmed.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 26.Lawrence WF, Fryback DG, Martin PA, Klein R, Klein BE. Health status and hypertension: a population-based study. J Clin Epidemiol. 1996;49(11):1239–45. doi: 10.1016/s0895-4356(96)00220-x. [DOI] [PubMed] [Google Scholar]
- 27.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22(2):107–33. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 28.Brody A, Rahman T, Reed B, Millis S, Ference B, Flack JM, et al. Safety and efficacy of antihypertensive prescription at emergency department discharge. Acad Emerg Med. 2015;22(5):632–5. doi: 10.1111/acem.12660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho PM, Magid DJ, Shetterly SM, Olson KL, Peterson PN, Masoudi FA, et al. Importance of therapy intensification and medication nonadherence for blood pressure control in patients with coronary disease. Arch Intern Med. 2008;168(3):271–6. doi: 10.1001/archinternmed.2007.72. [DOI] [PubMed] [Google Scholar]
- 30.Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326(7404):1427. doi: 10.1136/bmj.326.7404.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okonofua EC, Simpson KN, Jesri A, Rehman SU, Durkalski VL, Egan BM. Therapeutic inertia is an impediment to achieving the Healthy People 2010 blood pressure control goals. Hypertension. 2006;47(3):345–51. doi: 10.1161/01.HYP.0000200702.76436.4b. [DOI] [PubMed] [Google Scholar]
- 32.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 33.Toussaint J, Milstein A, Shortell S. How the Pioneer ACO Model needs to change: lessons from its best-performing ACO. JAMA. 2013;310(13):1341–2. doi: 10.1001/jama.2013.279149. [DOI] [PubMed] [Google Scholar]


