SUMMARY
SETTING
Patients who initiated treatment for multi-drug-resistant tuberculosis (MDR-TB) at 15 Programmatic Management of Drug-resistant Tuberculosis (PMDT) health facilities in the Philippines between July and December 2012.
OBJECTIVES
To describe patients’ views of current interventions, and suggest changes likely to reduce MDR-TB loss to follow-up.
METHODS
In-depth interviews were conducted between April and July 2014 with MDR-TB patients who were undergoing treatment, had finished treatment at the time of the interview (controls), or had been lost to follow-up (LTFU). Responses were thematically analyzed.
RESULTS
Interviews were conducted with 182 patients who were undergoing or had completed treatment and 91 LTFU patients. Views and suggestions could be thematically categorized as approaches to facilitate adherence or address barriers to adherence. The top themes were the need for transportation assistance or improvements to the current transportation assistance program, food assistance, and difficulties patients encountered related to their medications. These themes were addressed by respectively 63%, 60%, and 32% of the participants.
CONCLUSIONS
A more patient-centered approach is needed to improve MDR-TB treatment adherence. Programs should strive to provide assistance that considers patient preferences, is adequate to cover actual costs or needs, and is delivered in a timely, uninterrupted manner.
Keywords: adherence, incentives, enablers, patient-centered, qualitative study
The philippines has been designated a high burden country for multidrug-resistant tuberculosis (MDR-TB, defined as TB resistant to at least isoniazid and rifampin [RMP]) by the World Health Organization (WHO).1 In 2013, an estimated 2% of new and 21% of retreatment cases had MDR-TB.2 The number of patients who have initiated treatment through health facilities offering Programmatic Management of Drug-resistant Tuberculosis (PMDT) has steadily increased from its initiation in 1999.3 However, the proportion of loss to follow-up has also risen. In the 2010 cohort of patients treated under PMDT, 38% had a recorded outcome of lost to follow-up (LTFU).3
In an effort to improve MDR-TB treatment completion rates, in-depth interviews were conducted with patients who were enrolled on MDR-TB treatment. The aim of these interviews was two-fold. The first was to identify factors significantly associated with loss to follow-up from MDR-TB treatment. These findings have been reported elsewhere.4 The second aim was to describe patients’ views regarding the types and delivery of interventions that would have a positive impact on preventing or reducing MDR-TB treatment loss to follow-up. We report on data collected as part of this second aim.
METHODS
Setting/study population
We conducted a case-control study. The study source population included all adult MDR-TB patients and patients with RMP-resistant TB aged ≥18 years who initiated MDR-TB treatment according to WHO guidelines between 1 July and 31 December 2012 in the Philippines.4,5 Cases were defined as patients from the source population who were LTFU from MDR-TB treatment (i.e., patients whose treatment was interrupted for at least 2 consecutive months) at any time during treatment. Controls were defined as patients from the source population who were undergoing or had finished their MDR-TB treatment at the time of the interview.
We included patients from the source population who received care through the 15 PMDT treatment centers* that had at least three drug-resistant TB patients who were LTFU and not known to have died by 1 January 2014. Study staff attempted to find all eligible LTFU patients using patient registers, and invited them to participate in the study, which included an in-depth interview and consent to a review of their medical documentation. For each case enrolled, two controls from the same PMDT treatment facility, who had initiated treatment in the same quarter as the case, were randomly selected and invited to participate in the study.
The team that conducted the interviews and medical documentation review comprised six nurses and one pharmacist. Staff underwent an intensive 4-day training course, during which they were oriented to the protocol, the informed consent process, the interview process, and the interview questions. They were also taught how to pace the interview, ask probing questions, take field notes and document responses, and perform medical record abstraction.
Data collection
Interviews took place in participants’ homes, a treatment center, or a mutually agreed location. Interviews were conducted in a structured manner, using a standard interview form that had been field-tested. A funnel sequence of questions developed by the study investigators was used to solicit participant views on interventions to improve treatment adherence. Participants were first asked to think about their own experience and identify what the National Tuberculosis Program (NTP) could do to help patients adhere to treatment. This was followed by questions focused on specific interventions and implementation of these interventions. Finally, an open-ended question asked participants to consider the preceding discussion and identify the top ‘three things’ that would enable patients to remain adherent to treatment. We report participants’ responses and accompanying comments to this final question.
Interviews lasted approximately 90 min and were conducted in English, Tagalog, Bicolano, or Cebuano (the most common languages at the enrollment locations) by staff fluent in these languages. Responses were transcribed onto the interview forms, and translated into English by the same interviewer.
Data analysis
Data were entered into a Microsoft Access electronic database (Redmond, WA, USA). Responses to open-ended questions were exported to Microsoft Excel, and thematic analysis was performed.6 Themes were not imposed a priori; instead an initial list of codes was developed by two investigators based upon a preliminary review of the participants’ responses. Each participant’s response was then read independently by two of three staff, coded, code frequencies generated, and the results compared. Discrepancies were discussed and resolved.
Ethics statement
Before initiating the study, approval was obtained from the Institutional Review Board (IRB) of the Tropical Disease Foundation (Makati City), the Lung Center of the Philippines’ Ethics Review Committee (ERC) (Quezon City), and the ERC of the Philippine Tuberculosis Society, Inc (Quezon City, the Philip-pines). The US Centers for Disease Control and Prevention (CDC; Atlanta, GA, USA) determined that CDC involvement did not constitute engagement in human subject research, and submission to the CDC IRB was not required.
RESULTS
Study population
A total of 91 cases and 182 controls from a group of 136 LFTU patients and 341 non-LTFU patients who were eligible for study inclusion were interviewed. As shown in Table 1, interview participants included 164 (60.1%) males. The mean age at treatment start was 39 ± 13 years (median 40, range 16–68). All had pulmonary TB; 97 (35%) had cavitary disease. Table 2 summarizes interventions to enable treatment adherence that were available to MDR-TB patients at the time of the study.
Table 1.
Participant demographics
| Characteristics | All participants (n = 273) n (%) |
Persons lost to follow-up (cases) (n = 91) n (%) |
Persons undergoing/completed treatment (controls) (n = 182) n (%) |
|---|---|---|---|
| Sex | |||
| Male | 164 (60.1) | 60 (65.9) | 104 (57.1) |
| Female | 109 (39.9) | 31 (34.1) | 78 (42.9) |
| Age, years | |||
| <20 | 12 (4.4) | 4 (4.4) | 8 (4.4) |
| 20–29 | 68 (24.9) | 18 (19.8) | 50 (27.5) |
| 30–39 | 55 (20.1) | 15 (16.5) | 40 (21.9) |
| 40–49 | 77 (28.2) | 31 (34.1) | 46 (25.3) |
| 50–59 | 42 (15.4) | 13 (14.3) | 29 (16.0) |
| ≥60 | 19 (7.0) | 10 (10.9) | 9 (4.9) |
| Civil status | |||
| Single, separated, widowed | 109 (39.9) | 42 (46.2) | 67 (36.8) |
| Married, living together | 164 (60.1) | 49 (53.8) | 115 (63.2) |
| Employment status | |||
| Unemployed | 96 (35.2) | 33 (46.5) | 63 (51.2) |
| Employed | 98 (35.9) | 38 (53.5) | 60 (48.8) |
| Other (retired, student, disabled, housewife) | 43 (15.8) | 12 (24) | 31 (34.1) |
| Unknown | 36 (13.1) | 8 (17.4) | 28 (31.8) |
| Education | |||
| No formal schooling/elementary/high school | 197 (72.2) | 67 (73.6) | 130 (71.4) |
| College/graduate school | 75 (27.5) | 24 (26.4) | 51 (28.0) |
| Unknown | 1 (0.3) | 0 | 1 (0.6) |
| Socio-economic class* | |||
| Category C: living at or below the poverty line | 250 (91.6) | 85 (93.4) | 165 (91.2) |
| Category A and B: living above the poverty line | 22 (8.0) | 6 (6.6) | 16 (8.8) |
| Unknown | 1 (0.4) | 0 | 1 (0.5) |
| Residence | |||
| Rural area | 40 (14.6) | 15 (16.5) | 25 (13.7) |
| Urban slum | 126 (46.2) | 49 (53.8) | 77 (42.3) |
| Urban area | 105 (38.5) | 26 (28.6) | 79 (43.4) |
| Unknown | 2 (0.7) | 1 (1.1) | 1 (0.6) |
| HIV test result | |||
| Missing (HIV test not done/missing) | 217 (79.5) | 86 (94.5) | 131 (72.0) |
| Positive | 2 (0.7) | 1 (1.1) | 1 (0.5) |
| Negative | 51 (18.7) | 2 (2.2) | 49 (27.0) |
| HIV test done, but result not recorded | 3 (1.1) | 2 (2.2) | 1 (0.5) |
| Site of disease | |||
| Pulmonary | 273 (100) | 91 (33.3) | 182 (66.7) |
| Extra-pulmonary | 0 (0) | 0 (0) | 0 (0) |
| Cavity on baseline chest X-ray reading | |||
| Yes | 97 (35.5) | 31 (34.1) | 66 (36.3) |
| No | 134 (49.1) | 39 (42.8) | 95 (52.2) |
| Unknown | 42 (15.4) | 21 (23.1) | 21 (11.5) |
Living at or below the poverty line is defined as per capita income of 16 841 Philippine Peso a year.7
HIV = human immunodeficiency virus.
Table 2.
Program services to enable treatment adherence available to MDR-TB patients at the time of this study
| Anti-tuberculosis medicines provided to patients at no cost |
| Medications to manage adverse events provided to patients at no cost (limited formulary) |
| TB diagnostic tests provided to patients at no cost |
| Fixed amount provided for transportation costs (funds provided retrospectively, based upon adherence to appointments) |
| Food basket incentives (limited availability) |
| Temporary housing located near PMDT treatment centers for patients from rural areas (limited availability) |
| 44 PMDT treatment centers located throughout the country to provide comprehensive MDR-TB services |
| Patient education (non-standardized) |
| Patient support groups (located within some facilities) |
| Small monetary bonus provided to patients at month 6, 12, and 18 months, if fully adherent during each 6-month period |
MDR-TB = multidrug-resistant tuberculosis; PMDT = programmatic management of drug-resistant tuberculosis.
Participant-recommended interventions to facilitate treatment adherence
When invited to identify the top three things the NTP could do to help patients, 87 cases and 181 controls identified one or more factors they perceived as most important. Participant responses were separated into two major themes, with a total of 19 subthemes.
The first theme, related to approaches for improving adherence, contained the following 14 subthemes: 1) transportation assistance; 2) food allowance or assistance; 3) housing; 4) treatment centers closer to the patient’s home; 5) general financial assistance for daily expenses; 6) reduced treatment duration; 7) directly observed therapy (DOT) at the patient’s home; 8) patient and community education; 9) psychological or spiritual counseling; 10) assistance with medication side effects; 11) free medication (type of medication not specified); 12) text message reminders; 13) improved efficiency in treatment centers; and 14) development of a ‘livelihood’ program so patients could earn money or educational assistance for their children.
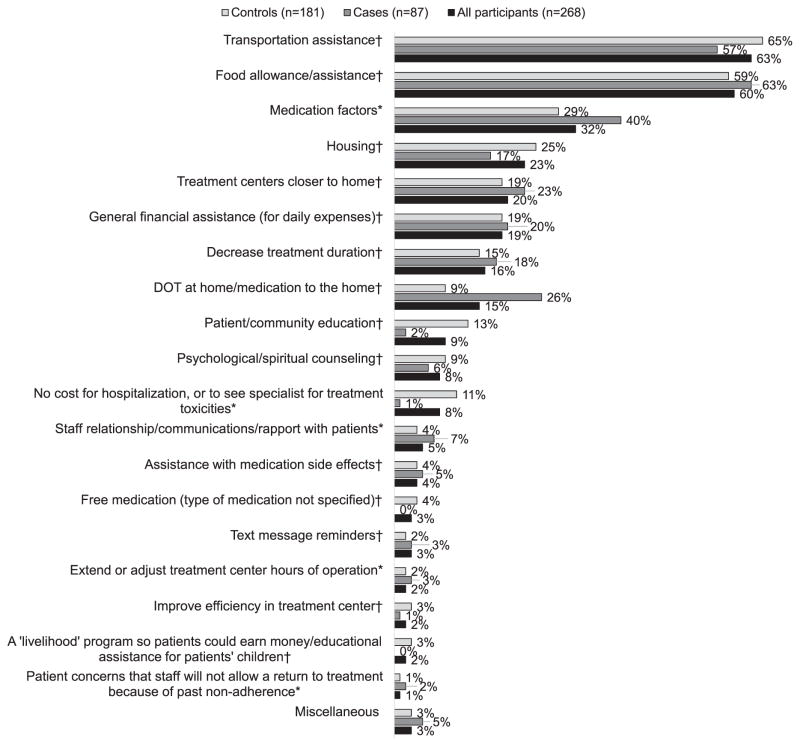
The second theme, which addressed barriers to treatment adherence, contained the following five subthemes: 1) medication factors; 2) costs associated with hospitalization or care for treatment toxicities; 3) staff relationships, communications, or rapport with patients; 4) opening hours of treatment facilities; and 5) treatment facility staff not allowing patients to continue or return to treatment due to past non-adherence. Figure 1 gives the percentage of participants who provided a response that reflected a particular theme. This figure also illustrates responses provided by cases and controls.
Figure 1.
Adherence barriers and enablers to improve treatment adherence as suggested by study participants (n =268). *A barrier to treatment adherence. †An enabler to improve treatment adherence. DOT = directly observed therapy.
Among the factors to improve treatment adherence, transportation assistance was mentioned by the majority of participants (n = 168, 63%). Table 3 shows the subanalysis of participants’ responses related to transportation assistance. Most participants who discussed transportation assistance provided general comments in which they simply stated the need for this type of assistance (110/168, 65%). Forty-six (27%) noted a need to increase the amount of assistance provided to cover the full cost of transportation to and from the treatment facility each day. In addition, 26 (15%) participants discussed difficulties encountered when provision of transportation assistance is delayed or interrupted. Fourteen (8%) participants acknowledged that they, or other patients they knew, had used the transportation assistance to purchase food rather than transportation. Four (2%) participants expressed hope that the NTP would be able to pick patients up at their homes, take them to the treatment facility, and then drive them home. Another three (2%) expressed a desire to obtain enough transportation assistance to allow a companion to accompany them to the treatment facility. These participants explained that they had become debilitated and were unable to travel on their own.
Table 3.
Thematic analysis of participants’ responses focusing on transportation and food assistance as enablers, and medication factors as barriers to treatment adherence
| Topic and response themes | Percentage of participants whose responses reflected each theme
|
||
|---|---|---|---|
| All participants (n = 168) % |
Cases (n = 50) % |
Controls (n = 118) % |
|
| Transportation assistance | |||
| Participant made a general comment regarding the need for transportation allowance | 65 | 70 | 64 |
| Need to increase the current transportation allowance, or provide sufficient amount to cover transportation costs noted | 27 | 16 | 32 |
| Delays receiving transportation allowance or interruptions in the provision of the transportation allowance reported | 15 | 12 | 17 |
| Participant acknowledged that they, or someone they knew, used the transportation allowance to purchase food | 8 | 10 | 8 |
| TB program-sponsored transportation that would pick up and return patients to their homes suggested | 2 | 6 | 1 |
| Request for expanded transportation allowance so that a companion could accompany a patient during treatment | 2 | 2 | 2 |
| Food assistance | (n = 161) | (n = 55) | (n = 106) |
| Participant made a general comment regarding the need for food assistance | 50 | 53 | 49 |
| Request for food to be provided at the time of DOT | 23 | 18 | 25 |
| Need for food assistance to lessen medication side effects noted | 15 | 16 | 14 |
| Need for food assistance to strengthen patients or enable patients to regain weight or health noted | 9 | 16 | 6 |
| Request for an allowance to purchase food | 3 | 4 | 3 |
| Medication factors | (n = 87) | (n = 35) | (n = 52) |
| Request for better medication with fewer side effects (some patients noted that fewer side effects could be achieved by taking fewer drugs) | 55 | 51 | 58 |
| Request for less or fewer drugs, or changes in the medications (i.e., flavor, change liquid to a pill), to make it easier to take each dose | 47 | 34 | 56 |
| Request for ‘more effective’ drugs to achieve cure in less time | 18 | 20 | 17 |
| Request to change or eliminate ‘PASER’ (para-aminosalicylic acid) | 10 | 14 | 8 |
TB = tuberculosis; DOT = directly observed therapy.
An allowance for food, or food assistance, was the second most common intervention suggested by participants (n = 161, 60%) as a means to improve adherence. Similar to the general comments made regarding transportation assistance, Table 3 shows that most participants who discussed the need for food assistance simply stated this need and did not expand their answers further (n = 81, 50%). Others provided specific suggestions or a rationale for their response. Thirty-seven (23%) participants suggested that food be provided at the treatment centers when patients are undergoing DOT. Some participants stated that food assistance would help lessen medication side effects (n = 24, 15%), while some noted that food assistance would help patients regain their health or weight (n =15, 9%). Five (3%) participants requested that patients be provided an allowance to purchase food.
The third topic addressed most often by participants was medication factors (n = 87, 32%). As shown in Table 3, participants’ comments tended to focus on reasons why patients did not want to take the medication. The majority of participant responses (n = 48, 55%) focused on medication side effects, or a desire for medications with fewer side effects. Some patients commented that fewer side effects could be achieved by taking fewer drugs. Furthermore, 41 (47%) participants specifically requested that fewer drugs be given, or that the medication be changed so that it would be easier for patients to take each dose. Participants who asked for fewer drugs noted that the amount of medication to be ingested was overwhelming at times. Those requesting medication changes asked for flavoring in liquid medication, the use of pills rather than liquids, injections rather than pills, or the elimination of injectable medication because of injection pain. Sixteen (18%) participants noted the need for ‘more effective drugs’ that would achieve a cure in less time than the current treatment available. Finally, nine (10%) participants specifically requested that PASER® (para-aminosalicylic acid, now considered an add-on agent8) be eliminated from the treatment regimen or changed into another formulation, citing the medicine’s side effects and taste as reasons.
Table 4 gives representative quotes related to these top three factors and other frequently proposed interventions, including halfway houses (temporary housing for patients from remote areas), treatment centers closer to patients’ homes, DOT at home, patient and community education, counseling, free hospitalization or access to specialists for adverse drug reactions, and improved patient-provider relationships. These quotes reflect concerns commonly expressed by participants. When examined alongside the list of proposed interventions and self-reported reasons from cases for stopping treatment (Figure 2), these quotes provide context for patients’ decision-making, and insights into program improvements.
Table 4.
Representative participant comments
| Transportation assistance |
| I am receiving 50 pesos per day allowance from the center, but it is not enough and sometimes delayed. I am spending 100 pesos every day for my tricycle fare, so I always fill in the balance of 50 pesos. Sometimes there is a delay in depositing the money. That is why sometimes I borrow from my neighbors. If they let me borrow, I go to the center. If not, I just stay home. (Male, 47 years old, LTFU) |
| Another big help for me is transportation assistance. Though I am already receiving it, still it does not help a lot because it is less than my actual transportation fare. Maybe it would help if they adjust the amount to the actual fare. If this is so, I will not have any more reason not to go to the center and take my medication. (Female, 52 years old, undergoing treatment at time of interview) |
| Food assistance |
| It would be good if there is food in the center because sometimes we don’t have food at home. It will be a big help, not only for me, but for other patients as well. With this, I don’t need to worry about what to eat, or if there is something to eat. Sometimes also, I felt less side effects when my stomach is not empty. This is also the one thing to encourage the patients to go to the center. Besides, we don’t have to spend the transportation allowance to buy food. (Female, 18 years old, undergoing treatment at time of interview) |
| I would like to receive, if it is not too much to ask, food so that it will not be too difficult to take medication. Besides, it will lessen the adverse effects, especially stomach upset. Also, if there is food daily, I am assured that I have something to eat before or after taking my medication. (Male, 23 years old, LTFU) |
| Medication factors/treatment duration |
| Though I know that the minimum duration of treatment is 18 months, I still wish that it can be shortened. I am so eager to work so that my family may have something to eat, but I cannot do it if I am still on treatment. I cannot afford to work [and] at the same time, take medications because of so many side effects. So I have to choose treatment over work because I am sick. But it troubles me a lot to see my mother doing everything for us to survive the day. I wish that I am already done with my treatment so that I could help her. (Male, 35 years old, undergoing treatment at time of interview) |
| Treatment of MDR-TB is very complicated. The medications are also complicated. It has to be a combination of drugs and injections and there is nothing I can do but take them. But if I have a choice, I choose not to drink PASER (para-aminosalicylic acid). The mere sight of PASER made me vomit. There were times that I was walking and I thought of not going to the center instead. I would have my body ran [over] by a bus. I wanted to commit suicide. I just thought of my family. That’s why I did not do it. (Male, 57 years old, undergoing treatment at time of interview) |
| Housing |
| Another thing that would help is the provision of halfway house. I have already availed this benefit and I can testify that this is a big factor why I am able to finish my intensive phase…. If I were not in-housed, I might not be able to finish my treatment. There are many side effects that I had experienced. There was a time when I was not able to get up and walk due to joint pain. The people there helped me get through and I was thankful that I didn’t need to travel. Being in-housed was great, but the only thing that I want to suggest is provide food, enough food for the patient. (Male, 29 years old, undergoing treatment at time of interview) |
| Another thing that would help is provide a halfway house near the treatment center. This will be more convenient for us who live far from the center … and I can take my medication even if it’s raining. When it rains it floods in our community because the area is very low, and this caused me to be absent. (Female, 29 years old, undergoing treatment at time of interview) |
| If the government will open half-way home for the patient. This will protect the family of the patient to be infected. Then, if the patient [is sputum smear] negative, he has to leave the shelter so that new patients can live there also. (Male, 44 years old, undergoing treatment at time of interview) |
| Treatment centers closer to home |
| I had difficulty going to the center every day because of lack of money for transportation. Also, I need to wake up earlier because I have to travel 1 to 1½ hours to get there. I started having absences and eventually stopped. If there is a treatment center near my house, there will be no problem going there. I should have finished my treatment if there [was] one near my place. (Male, 25 years old, LTFU) |
| I would like to have a treatment center near my house. There are so many patients who need treatment but there is no treatment facility in the area. If the center is near my house, I don’t have to travel far. I am so weak and can’t travel anymore. I know that there [are] lots of patients out there like me. Another benefit is that aside from the short distance, I don’t need to spend a lot for transportation. (Male, 31 years old, LTFU) |
| Direct observed therapy at home |
| Though there is a health center near my house, I still want my medication to be taken at home. If ever there are adverse events, especially vomiting, I can easily go to my room and vomit there. At least I’m already at the comfort of my home and avoid embarrassment. (Male, 44 years old, undergoing treatment at time of interview) |
| I can’t see anymore. I need to have a companion to be able to go to the center and I can’t rely on my children to go with me because they have jobs … It would be best if somebody will bring my medication to my house. I won’t have to go out and bother my children or my son-in-law bringing and fetching me to and from the treatment center. (Female, 60 years old, LTFU) |
| Patient/community education |
| My number one [suggestion] is health education because I think it is what every individual should receive. It is very important that I know my sickness, what should I do to be cured, who should I talk to, and where can I go to get the right treatment. (Male, 30 years old, undergoing treatment at time of interview) |
| Psychological or spiritual counseling |
| Psychological counseling is very important because we, patients, are experiencing dilemma and anxiety. We are torn between the treatment and coping with the side effects of the medication, besides, facing the future with uncertainty. For me, I would always ask myself, what happens next? … Sometimes, I would think of quitting, especially when I experienced the pain due to uric acid elevation. I could no longer walk. But then again, I would think of my disease and my family. Talking to a counselor is a great help because I am able to release my unexpressed thoughts and get enlightenment. (Male, 28 years old, undergoing treatment at time of interview) |
| Losing a husband while I am sick is very devastating. I started experiencing depression and self-pity which made me feel more ill. This is the main reason why I quit treatment, because I felt that my life is already worthless so I wanted to die also. But as the days pass by, I realized I should not be selfish. I should consider my children, though they are already married, and my grandchildren whom I am really fond of. I wanted to become strong for them, but how? That is why I really need to talk to a professional counselor, because I know that he could help me through my depression. (Female, 48 years old, LTFU) |
| Free hospitalization or access to specialists for adverse drug reactions |
| Another thing that would help is the provision of free hospitalization and surgery. Sometimes, I don’t tell [the doctors and nurses] what I really feel because I am afraid that I will be hospitalized. It is very expensive and I don’t have money for it. But if it is provided, I will not need to hide what I really feel [medication side effects] because I know that I won’t have to pay for the hospitalization should there be a need. (Male, 24 years old, undergoing treatment at time of interview) |
| There are government hospitals which accommodate indigent patient[s]. The problem is they do not have medicines. They just give us prescriptions so that we may buy them [prescribed medications] from the outside. (Female, 52 years old, undergoing treatment at time of interview) |
| Improved staff relationships, communications, or rapport with patients |
| Train health staff more when it comes to knowledge, especially psychologically. Some staff are not accommodating and not approachable. (Male, 47 years old, undergoing treatment at time of interview) |
| Make sure that staff will be trained so that they will not despise patients. Patients might get fed up if this continues. (Male, 46 years old, undergoing treatment at time of interview) |
| Treating patients better will alleviate patients feeling of depression. (Female, 19 years old, undergoing treatment at time of interview) |
| The nurses are not accommodating. They scold the patients and hurt our feelings. (Female, 39 years old, LTFU) |
LTFU = lost to follow-up; MDR-TB = multidrug-resistant tuberculosis.
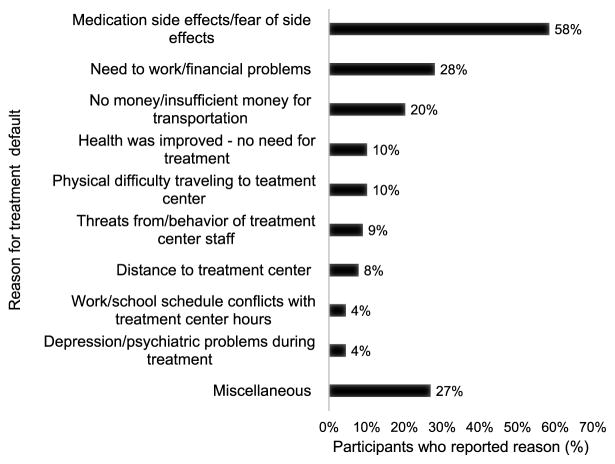
Figure 2.
Self-reported reasons for stopping treatment among persons lost to follow-up (n = 89*). Figure summarizes the results of qualitative analysis of answer on the question: ‘All of the questions I have asked address reasons why many people stop taking treatment before being advised to stop by their doctors. What were the main reasons you stopped taking your treatment early?’ *Of 91 cases, 1 person did not provide an answer and 1 person was initially categorized as a control but was a case. He was interviewed using a ‘control’ form, which did not contain this question, totaling 89 patients who responded to this question.
DISCUSSION
Interviews with both MDR-TB patients who remained adherent to treatment and those LTFU illustrate that assistance with the cost of transportation, food, and housing is essential for treatment adherence. The provision of food and financial assistance for transportation to and from a treatment center may be viewed less as an incentive used to coax a patient or a reward for those who remain on treatment, and more as an enabler that facilitates adherence during a treatment course that otherwise depletes patients and family members physically, financially, and mentally.9–12 Moreover, if the application process for financial assistance is long and difficult, if this assistance is not given in a timely manner, or if delivery of assistance is interrupted, patients are likely to abandon their efforts to adhere to treatment.
The time between the start of treatment and the provision of initial assistance requires careful consideration. A patient’s lack of resources can hamper treatment adherence immediately. Reports in the literature indicate that some TB patients expend their limited resources while pursuing a diagnosis.13,14 Furthermore, missed doses early in the course of treatment can become self-defeating for the patient15 and create an unfavorable impression of the patient among care givers. An efficient process is therefore needed to register patients into an assistance program, deliver initial help, and institute safeguards to avoid exploitation of available assistance.16–19
A little over a third of participants (32%) noted medication factors as barriers to adherence. While the quantity and format in which medications are delivered (e.g., injection or tablets) are not easily modified, the provision of care and treatment to help patients cope with medication side effects is within the purview of PMDT. This can reduce the number of patients compelled to make a conscious decision to miss doses or abandon treatment because they are unable to engage in instrumental activities of daily living (e.g., work, use of public transportation, housekeeping, meal preparation), or withhold reporting on side effects they are experiencing due to concerns regarding the cost of care or treatment for these problems.
As reported elsewhere, incentives and enablers are not a substitute for a strong patient-provider relationship based upon trust, mutual respect, and effective communication.20 Our findings reinforce this perspective given the participants’ endorsement of interventions centered on counseling, patient or community education, and staff relationships.21–24 Provider interventions focused on establishing patient trust and respect may address active listening, respectful feedback, empathy, health literacy, message-framing, and motivational interviewing.25–27
Finally, current LTFU rates in the Philippines and the results of this study highlight the need to assess the benefits and drawbacks of clinic-based DOT vs. home-based DOT.28,29 A number of cases indicated that home-based DOT would have been helpful. Furthermore, some study participants directly attributed their default from treatment to the costs associated with going to the clinic each day. Given that the majority of participants live below the poverty level, it is unlikely they overstated their situation, nor is their situation unique. In a study of unaccounted costs of anti-tuberculosis treatment, Guzman-Montes et al. reported that clinic-based DOT contributed disproportionately to the costs incurred by patients.30 The programmatic costs of home-based DOT thus need to be weighed against the costs incurred if the incidence of MDR-TB continues to increase in the Philippines.
Our study is subject to several limitations. First, some cases and eligible controls could not be located; these patients’ responses could be substantially different from those of persons who were less mobile and easily located. Participants’ responses may reflect socially desirable answers rather than their true thoughts and experiences. The quality of the data collected depended on the interviewers’ skills, and was subject to the use of field notes rather than audio recordings to capture responses as well as interviewer bias. To minimize this, interviewers received training in taking and transcribing field notes, bias, the influence of verbal and non-verbal communications, and how best to obtain candid responses. Furthermore, the analysis of participant responses can be interpretative; for this reason, themes and coding were performed independently by two researchers, then compared and reconciled. Finally, the retrospective design of this study is subject to recall bias.
Despite these limitations, this study provides important data for designing interventions to reduce loss to follow-up during MDR-TB treatment in the Philippines. Further evaluation is needed to determine if interventions developed based upon these data positively influence treatment completion. Evaluation of these interventions must assess fidelity to the intervention design. A cost analysis of these interventions would also be beneficial.
CONCLUSIONS
A revision of the PMDT strategy should address the identified barriers to MDR-TB treatment completion. Our findings highlight the need for a more patient-centered approach, including the provision of sufficient and timely assistance to patients, optimized care for treatment side effects, cultivation of patient-provider relationships through improved communication, and community-based DOT.
Acknowledgments
The authors would like to acknowledge the dedication and incredible work of the following staff from the Tropical Disease Foundation, Inc: J P S L Aliazas, J A Ambunan, M B Anonas, M T Baylon, M A A Gabatin, P D S Mallari, F E Romano, and M A G Pereña.
We also thank the following staff of the Division of TB Elimination, the US Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA: P Cegielski, A R Khan, and W Walton for their support and assistance; the officers and staff of the treatment centers, satellite treatment centers and health centers who graciously assisted with this project; and all the MDR-TB patients who kindly agreed to participate in this study.
This work was supported by the US Agency for International Development (USAID) Philippines (Manila, The Philippines) through a cooperative agreement with Philippine Business for Social Progress for the Innovations and Multisectoral Partnership to Achieve Control of Tuberculosis (IMPACT) project.
Footnotes
At the time of this study, there were 44 PMDT centers in the country.
Conflicts of interest: none declared.
Disclosure: references in this manuscript to any specific commercial products, process, service, manufacturer, or company does not constitute its endorsement or recommendation by the US Government, USAID or CDC. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the CDC or the USAID.
References
- 1.World Health Organization. Global tuberculosis report, 2013. Geneva, Switzerland: WHO; 2013. WHO/HTM/TB/2013.13. [Google Scholar]
- 2.World Health Organization. Global tuberculosis report, 2014. Geneva, Switzerland: WHO; 2014. WHO/HTM/TB/2014.08. [Google Scholar]
- 3.The Philippines Department of Health. Reports of accomplishments and targets of the Programmatic Management for Drug-resistant Tuberculosis in the Philippines. Manila, The Philippines: DoH; 2014. [Google Scholar]
- 4.Tupasi TE, Garfin AM, Kurbatova EV, et al. Factors associated with loss to follow-up during treatment for multidrug-resistant tuberculosis, the Philippines, 2012–2014. Emerg Infect Dis. 2016;22:491–502. doi: 10.3201/eid2203.151788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Guidelines for the programmatic management of drug-resistant tuberculosis, 2011 update. Geneva, Switzerland: WHO; 2011. WHO/HTM/TB/2011.6. [PubMed] [Google Scholar]
- 6.Boyatzis RE. Qualitative information: thematic analysis and code development. Thousand Oaks, CA, USA: Sage Publications; 1998. [Google Scholar]
- 7.Dela Cruz LJR. 2009 Philippine Poverty. Manila, The Philippines: Ateneo de Manila University; 2011. [Accessed September 2016]. http://www.slideshare.net/ldelacruz/poverty-situationer-2011-8294418. [Google Scholar]
- 8.World Health Organization. WHO treatment guidelines for drug-resistant tuberculosis, 2016 update. Geneva, Switzerland: WHO; 2016. [Accessed September 2016]. WHO/HTM/TB. 2016.04. http://www.who.int/tb/MDRTBguidelines2016.pdf. [Google Scholar]
- 9.Pedrazzoli D, Houben RM, Grede N, de Pee S, Boccia D. Food assistance to tuberculosis patients: lessons from Afghanistan. Public Health Action. 2016;6:147–153. doi: 10.5588/pha.15.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanimura T, Jaramillo E, Weil D, Raviglione M, Lönnroth K. Financial burden for tuberculosis patients in low-and middle-income countries: a systematic review. Eur Respir J. 2014;43:1763–1775. doi: 10.1183/09031936.00193413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isaakidis P, Rangan S, Pradhan A, Ladomirska J, Reid T, Kielmann K. ‘I cry every day’: experiences of patients co-infected with HIV and multidrug-resistant tuberculosis. Trop Med Int Health. 2013;18:1128–1133. doi: 10.1111/tmi.12146. [DOI] [PubMed] [Google Scholar]
- 12.Toczek A, Cox H, du Cros P, Cooke G, Ford N. Strategies for reducing treatment default in drug-resistant tuberculosis: systematic review and meta-analysis. Int J Tuberc Lung Dis. 2013;17:299–307. doi: 10.5588/ijtld.12.0537. [DOI] [PubMed] [Google Scholar]
- 13.Mauch V, Bonsu F, Gyapong M, et al. Free tuberculosis diagnosis and treatment are not enough: patient cost evidence from three continents. Int J Tuberc Lung Dis. 2013;17:381–387. doi: 10.5588/ijtld.12.0368. [DOI] [PubMed] [Google Scholar]
- 14.Laokri S, Amoussouhui A, Ouendo EM, et al. A care pathway analysis of tuberculosis patients in Benin: highlights on direct costs and critical stages for an evidence-based decision-making. PLOS ONE. 2014;9:e96912. doi: 10.1371/journal.pone.0096912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bandura A. Self-efficacy. In: Ramachaudran VS, editor. Encyclopedia of human behavior. Vol. 4. New York, NY, USA: Academic Press; 1994. pp. 71–81. (Reprinted in Friedman H, ed. Encyclopedia of mental health. San Diego, CA, USA: Academic Press, 1998.) [Google Scholar]
- 16.Boccia D, Pedrazzoli D, Wingfield T, et al. Towards cash transfer interventions for tuberculosis prevention, care and control: key operational challenges and research priorities. BMC Infect Dis. 2016;16:307. doi: 10.1186/s12879-016-1529-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boccia D, Hargreaves JR, Lönnroth K, et al. Cash transfer and microfinance interventions for tuberculosis control: review of the impact evidence and policy implications. Int J Tuberc Lung Dis. 2011;15(Suppl 2):S37–S49. doi: 10.5588/ijtld.10.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lutge E, Lewin S, Volmink J, Friedman I, Lombard C. Economic support to improve tuberculosis treatment outcomes in South Africa: a pragmatic cluster-randomized controlled trial. Trials. 2013;14:154. doi: 10.1186/1745-6215-14-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lutge E, Lewin S, Volmink J. Economic support to improve tuberculosis treatment outcomes in South Africa: a qualitative process evaluation of a cluster randomized controlled trial. Trials. 2014;15:236. doi: 10.1186/1745-6215-15-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. Module 9: Patient adherence to tuberculosis treatment. Atlanta, GA, USA: CDC; 1999. Self-study modules on tuberculosis. [Google Scholar]
- 21.van Hoorn R, Jaramillo E, Collins D, Gebhard A, van den Hof S. The effects of psycho-emotional and socio-economic support for tuberculosis patients on treatment adherence and treatment outcomes: a systematic review and meta-analysis. PLOS ONE. 2016;11:e0154095. doi: 10.1371/journal.pone.0154095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munro SA, Lewin SA, Smith HJ, Engel ME, Fretheim A, Volmink J. Patient adherence to tuberculosis treatment: a systematic review of qualitative research. PLOS MED. 2007;4:e238. doi: 10.1371/journal.pmed.0040238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansson E, Winkvist A. Trust and transparency in human encounters in tuberculosis control: lessons learned from Vietnam. Qual Health Res. 2002;12:473–491. doi: 10.1177/104973202129120025. [DOI] [PubMed] [Google Scholar]
- 24.M’imunya JM, Kredo T, Volmink J. Patient education and counselling for promoting adherence to treatment for tuberculosis. Cochrane Database Syst Rev. 2012;(5):CD006591. doi: 10.1002/14651858.CD006591.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thom DH. Physician behaviors that predict patient trust. J Fam Pract. 2001;50:323–328. [PubMed] [Google Scholar]
- 26.Thom DH, Bloch DA, Segal E. An intervention to increase patient trust in the physician. Acad Med. 1999;74:195–198. doi: 10.1097/00001888-199902000-00019. [DOI] [PubMed] [Google Scholar]
- 27.Thom DH. Training physicians to increase patient trust. J Eval Clin Pract. 2000;6:245–253. doi: 10.1046/j.1365-2753.2000.00249.x. [DOI] [PubMed] [Google Scholar]
- 28.Yin J, Yuan J, Hu Y, Wei X. Association between directly observed therapy and treatment outcomes in multidrug-resistant tuberculosis: a systematic review and meta-analysis. PLOS ONE. 2016;11:e0150511. doi: 10.1371/journal.pone.0150511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dobler CC, Korver S, Batbayar O, et al. Success of community-based directly observed anti-tuberculosis treatment in Mongolia. Int J Tuberc Lung Dis. 2015;19:657–662. doi: 10.5588/ijtld.14.0927. [DOI] [PubMed] [Google Scholar]
- 30.Guzmán-Montes GY, Ovalles RH, Laniado-Laborín R. Indirect patient expenses for antituberculosis treatment in Tijuana, Mexico: is treatment really free? J Infect Dev Ctries. 2009;3:778–782. doi: 10.3855/jidc.489. [DOI] [PubMed] [Google Scholar]