Abstract
Purpose
We evaluated the relationship between meiotic spindle characteristics and in vitro fertilization cycle outcome.
Methods
Five hundred sixty-nine oocytes from 86 in vitro fertilization cycles were analyzed for fertilization and subsequent implantation rates. Oocytes were assessed for maturation status. The oocytes and embryos were cultured in sequential and nonsequential media (G Series, Vitrolife, Sweden) and incubated in 6% CO2, 5% O2 at 37 °C.
Two hours following oocyte decumulation (38–39 h post-hCG/GnRH administration) and prior to microinjection, the structure of the meiotic spindle was assessed using the Oosight Imaging System (CRI, UK).
Results
Four hundred fifty-six oocytes (80.5%) had a visible meiotic spindle, 82 (14.7%) had no meiotic spindle, and 31 (5.5%) were in telophase I. Oocytes exhibiting a meiotic spindle had a significantly higher fertilization rate and a lower rate of abnormal fertilization. Implantation data were obtained for 195 of the embryos transferred. The implantation rate for embryos derived from oocytes with a meiotic spindle was 32.9%, while in embryos originating from oocytes without a meiotic spindle and oocytes in telophase, this value dropped significantly (8.8 and 0%, respectively). To determine the correlation between retardance values and implantation rate for each oocyte, we established four groups, finding a range of retardance values with significantly higher implantation rates (27.5, 21, 29.3, and 53.8%, respectively).
Conclusion
Meiotic spindle imaging may be a valuable tool for prediction of oocyte quality, and retardance values of meiotic spindles, together with classical morphological classification, can be useful to select embryos with a higher implantation potential.
Keywords: Meiotic spindle, Oocyte quality, Embryo selection, Embryo implantation
Introduction
Recently, the trend in assisted reproduction has been to reduce the number of embryos transferred in order to avoid multiple pregnancies. Multiple pregnancies are of high risk, potentially leading to numerous complications for both the mother and offspring. Most common risks are preterm birth and low birth weight. In addition, an increased risk of perinatal morbidity and mortality was observed [1–3].
Pregnancy after transferring a single embryo depends on proper embryo selection. The cryopreservation of remaining good-quality embryos allows us to maintain the cumulative pregnancy rate per cycle of in vitro fertilization (IVF).
The use of vitrification as a cryopreservation method has facilitated routine laboratory procedures and improved embryo survival [4]. Furthermore, pregnancy rates following transfer of cryopreserved embryos resemble those of fresh embryos [5–8].
The issue of embryo selection is controversial. In the absence of a definitive selection criterion, the identification of reliable markers of embryo quality represents an important challenge in assisted reproductive technology.
Classical embryo selection systems are based primarily on morphology (e.g., cell number, fragmentation, and multinucleation). In an attempt to complement morphology-based selection systems, it has been postulated that preimplantation genetic screening (PGS) may be used in combination with morphology, adding objectivity to the process. PGS is an invasive approach that involves obtaining biopsy specimens of one or more cells of the embryo and requires a high-standard laboratory to successfully implement the technique. In addition, PGS is costly and an analysis of its cost-effectiveness is still missing. These aspects represent some of the limiting factors for an extensive application of PGS [9].
Numerous noninvasive approaches to embryo selection have been proposed, including metabolomics and proteomics [10], oxygen intake [11], and mitochondrial DNA quantification [12]. All offer promising results at the experimental and clinical levels, although they are difficult to apply in routine use in IVF laboratories and have yet to be clinically validated.
It has also been argued that morphokinetics holds potential for embryo selection. Many groups have described the benefits of time-lapse imaging in embryo selection, but they use different time intervals for each parameter. This lack of uniform criteria impedes widespread laboratory use, requiring interval adjustments for each laboratory based on their experience, which may be highly work-intensive in centers with a low volume of in vitro fertilization cycles [13].
Continuing the search for new parameters of embryo selection, some researchers have focused their efforts in oocyte quality. Oocyte quality is a key limiting factor in female fertility, reflecting the intrinsic developmental potential of an oocyte, and has a crucial role not only in fertilization, but also in subsequent development [14].
For this purpose, several authors tried to evaluate if any morphological feature of human oocytes has a predictive value for further development. Unfortunately, these criteria fall short in being able to accurately discern oocyte quality and tend not to be helpful to the majority of the population [15].
One marker that may be a highly useful parameter is the evaluation of the meiotic spindle in oocytes. The meiotic spindle is a microtubular dynamic structure that plays a key role during meiosis. The spindle is responsible for separating homologous chromosomes and sister chromatids during meiosis to produce haploid oocytes. An incorrect spindle assembly or chromosome alignment may result in aneuploidy and increase the likelihood of segregation errors [16, 17].
Classically, the meiotic spindle was visualized through fluorescence microscopy, which offered detailed information on microtubular structures. However, this technique requires oocyte fixation and staining, which are incompatible with the preservation of cell viability, and therefore inappropriate for clinical application [18]. In recent years, advances in polarized light microscopy have created the possibility of visualizing the meiotic spindle noninvasively, thereby preserving the cell and allowing for clinical use [19, 20].
When illuminated with polarized light, the meiotic spindle (MS) exhibits an intrinsic optical property called birefringence. Birefringence, also known as double refraction of light, is an optical phenomenon that occurs when light passes through an anisotropic structure (e.g., the spindle). The incident light splits into two light beams, which pass through the sample at different speeds. The phase difference between the two light rays is called retardance and is measured in nanometers. In the MS, birefringence is due to the orientation of the meiotic spindle microtubules. A high retardance value can be interpreted as a high degree of macromolecular density and structural organization, which is an indicator of well-aligned or organized structures. Such measurements could be translated into oocyte viability in such a way that the higher the mean retardance for the spindle, the greater potential for oocyte development should be expected.
Numerous groups have attempted to demonstrate the utility of polarized light equipment in daily clinical practice, doing so by evaluating the presence and others features of MS and relating it to further development: fertilization rates [19, 21–27]; cleavage, embryo quality, blastocyst formation [19, 22, 24–26, 28, 29], and pregnancy and implantation rates [27, 28, 30]. The results of these studies are contradictory, thus calling into question the usefulness of polarized light microscopy in IVF laboratories. While most studies used polarized light imaging systems to visualize the spindle, they do not measure retardance and therefore do not acquire quantitative data. We reconsider the use of polarized light equipment as a reproducible and quantitative tool that can be used for egg grading and viable embryo selection.
In our study, we describe our experience using an integrated system for real-time spindle imaging in a routine clinical setting. In addition to the descriptive analysis of the oocyte population in our patients, we aim to evaluate the association between spindle observation in mature oocytes and its potential for predicting implantation.
Material and methods
Eighty-six infertile patients who underwent fertility treatment with intracytoplasmic sperm injection (ICSI) at Hospital Quiron A Coruña between 2012 and 2015 were included in the study. Patient age ranged from 28.8 to 42.5 years (mean 37.6 ± 3.3). Female and male infertility patients were included; couples with severe male infertility (semen sample from testicular biopsy or semen samples of very low quality in which it has been necessary to microinject with the help of pentoxifylline) and previous implantation failure were excluded from the study.
This was a retrospective study evaluating the data from patients who have been treated in our assisted reproduction center. The study was approved by the Hospital’s Medical Director.
A total of 86 ICSI cycles (from 86 patients) were included. All women were subjected to controlled ovarian stimulation before oocyte retrieval using gonadotropins and GnRH antagonist according to our protocol. Recombinant hCG or GnRH agonist were administered to trigger ovulation. Collected oocytes were denuded 37 h following hCG/GnRH administration. Cumulus cells were removed using 80 IU/ml hyaluronidase (HYASE, Origio, Denmark) and mechanical pipetting. Oocytes then were assessed for maturation status, determining each to be either at germinal vesicle stage (GV, oocyte with a germinal vesicle), metaphase I stage (MI, oocyte without a germinal vesicle or polar body), or metaphase II stage (MII, oocyte with fist polar body). The oocytes and embryos were cultured in sequential and nonsequential media (G Series, Vitrolife, Sweden) and incubated in 6% CO2, 5% O2 at 37 °C.
Two hours following oocyte decumulation (39 h post-hCG/GnRH administration), and prior to microinjection, the structure of the meiotic spindle was assessed using the Oosight Imaging System (CRI, UK). The oocytes were placed in 5-μL drops of MOPS buffer (G Series, Vitrolife, Sweden) in glass-bottomed culture dishes (Willco, Netherlands) on a heated microscope stage (Tokai Hit, Japan). ICSI micropipettes (Humagen, Origio, Denmark) were used to rotate the oocytes in order to allow appropriate observation of the meiotic spindle or until it could be confirmed that the spindle was not visible. The Oosight software provides a retardance value for each oocyte’s meiotic spindle. In some cases, even when the spindle was visible, the software was not able to provide a retardance value automatically. In these cases, the value was obtained from the manual capture tool, following the specifications provided by the manufacturer. All evaluations were performed by the same embryologist.
After microinjection, embryos were cultured until the embryo was transferred (day 3 or day 5). The luteal phase was supported by the administration of micronized progesterone (800 mg/day). In some cases where the levels of estradiol were higher (>2500 pg/mL), embryo transfer was deferred and all viable embryos were vitrified (day 3 or day 5). All procedures for embryo vitrification and warming were performed using Kitazato media (BioPharma, Japan) and heat-sealed straws (HSV, CBS, Cryo Bio System, France). Vitrified embryo transfers were performed subsequently in natural cycles. The luteal phase was supported by the administration of micronized progesterone (800 mg/day).
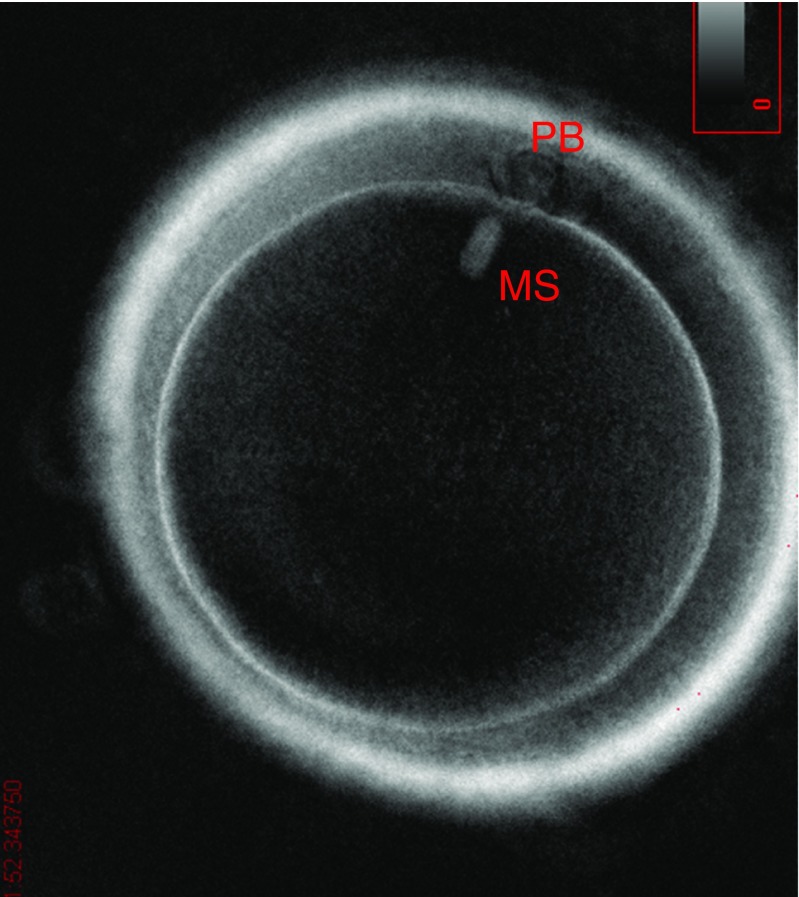
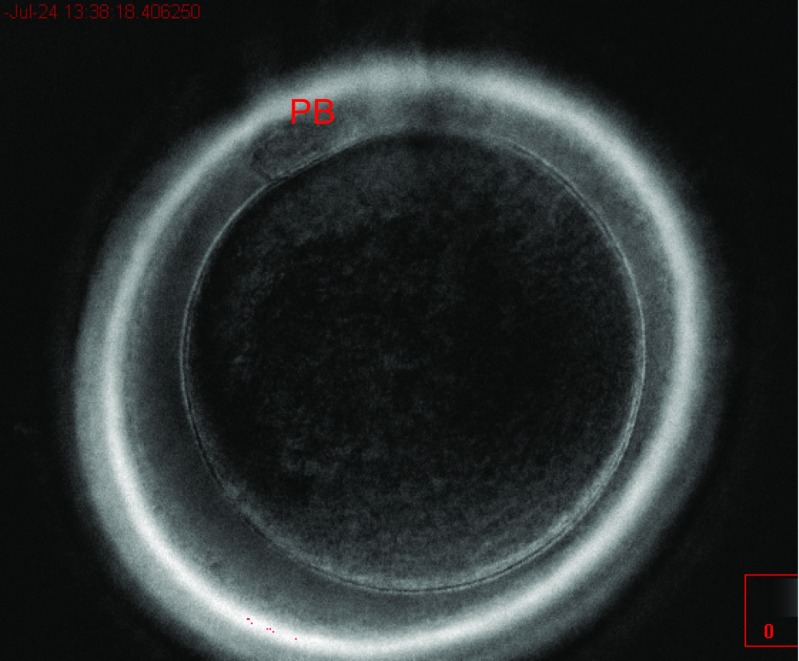
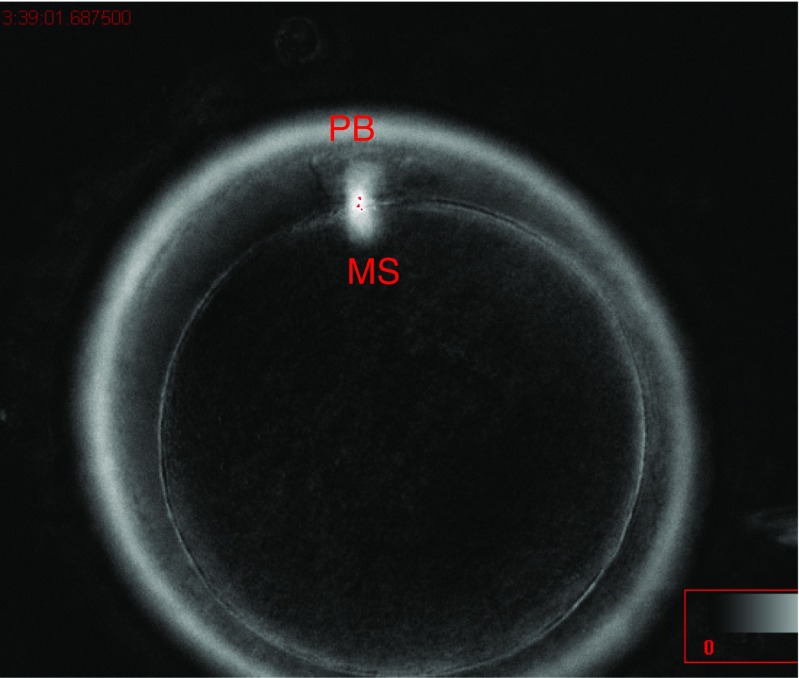
For the descriptive study of the oocyte population, we used all the MII oocytes obtained (n = 569). The oocytes were divided into three categories: oocytes with a meiotic spindle (group I), oocytes without a meiotic spindle (group II), and oocytes with a first polar body and meiotic spindle but still in telophase I (group III) (Figs. 1, 2, and 3). The rate of normal fertilization, abnormal fertilization, and implantation was calculated for each group.
Fig. 1.
Group I oocyte (with meiotic spindle)
Fig. 2.
Group II oocyte (without meiotic spindle)
Fig. 3.
Group III oocyte (in telophase)
In order to establish the association between retardance values and implantation rate, we analyzed embryo transfer results for the cycles included in the study, only taking into account those embryos with known implantation data: all single-embryo transfers were included in the analysis (n = 53), while double-embryo transfers (n = 71) were only considered when the βHCG result was negative or positive with two gestational sacs. βHCG was determined 14 days after the embryo transfer, and clinical pregnancy was confirmed when a gestational sac with fetal heart beat was visible by ultrasound examination at 7 weeks of gestation. The number of embryos with known implantation data was 195. We included fresh embryo transfers (n = 78) and vitrified/warmed transfers (n = 46).
To calculate the probability of implantation, retardance values were converted into categorical variables by dividing these values by quartile. In using this procedure, we avoided potential bias due to differences in the total number of embryos in each category. The implantation rates (IRs) were calculated for the different categories.
In order to determine the real predictive value of meiotic spindle birefringence, we evaluated the morphology of the transferred embryos using the following categories: Category 1 (high-quality embryos), which corresponds to groups A and B categories for the previously described Asociación para el Estudio de la Biología de la Reproducción (ASEBIR) classification [31]; category 2 (medium-quality embryos), which corresponds to ASEBIR category C; and category 3 (low-quality embryos), which corresponds to ASEBIR category D.
Statistical analysis was performed. Categorical variables were compared using the Fisher’s exact test. Differences were considered to be statistically significant at P ≤ 0.05.
Results
We have evaluated 86 ICSI cycles in 86 patients (ages between 28.8 and 42.5 years, own oocytes). A total of 711 oocytes were obtained, 569 of which were MII and were used for the study. A total of 124 transfers were performed, 53 single embryo transfer, 71 double embryo transfer. Of the 569 MII oocytes analyzed, 456 had a visible meiotic spindle, 82 had no spindle, and 31 were considered to be in telophase I although they had extruded the first polar body. The normal fertilization (visualization of two pronuclei 16–20 h after intracytoplasmic sperm injection) rate after ICSI was higher in the group of oocytes with a visible meiotic spindle than the others groups (oocytes without a spindle and oocytes that were in telophase I). The rate of abnormal fertilization (characterized by the visualization of one pronuclei, three or more pronuclei or cleavage without observation of pronuclei) observed after ICSI was similar in the three groups (Table 1).
Table 1.
Presence/absence of meiotic spindle in human oocytes
| Group I | Group II | P value (Fisher’s test), groups I–II | Group III | P value (Fisher’s test), groups I–III | |
|---|---|---|---|---|---|
| Meiotic spindle (%) | No spindle (%) | Telophase (%) | |||
| MII oocytes | 456 (80.5) | 82 (14.7) | 31 (5.5) | ||
| Fertilization | 355 (77.8) | 55 (67.1) | 0.04 | 18 (58) | 0.01 |
| Abnormal fertilization | 18 (3.9) | 5 (6.1) | >0.05 | 2 (6.5) | >0.05 |
Relationship with normal fertilization and aneuploidy
Group I oocytes had a significantly higher fertilization rate compared to all other groups (group I versus II, P = 0.04; group I versus III, P = 0.01). No significant difference between groups was observed for the abnormal fertilization rate; however, in the group I, the abnormal fertilization rate was slightly lower compared to groups II and III.
Of the total number of embryos transferred, 195 had known implantation data.
The implantation rate for embryos derived from group I oocytes was 32.9%. For the groups II and III, this value dropped to 8.8 and 0%, respectively (Table 2).
Table 2.
Meiotic spindle observation
| Group I | Group II | P value (Fisher’s test), groups I–II | Group III | P value (Fisher’s test), groups I–III | |
|---|---|---|---|---|---|
| Meiotic spindle (%) | No spindle (%) | Telophase (%) | |||
| Implant | 52 | 3 | 0 | ||
| No implant | 106 | 31 | 3 | ||
| Implantation rate (%) | 32.9 | 8.8 | <0.01 | 0 | >0.05 |
Relationship with human embryo implantation
Group I oocytes had a significantly higher implantation rate compared with group II (group I versus II, P < 0.01). There was no statistical difference when comparing the implantation rates for group I and group III (P > 0.05).
To relate the values of retardance for each oocyte with the IR, we divided the sample by quartiles. We have ordered the values of the meiotic spindle from lowest to highest, and we have calculated the values that divide the sample into four equal parts or almost, since there are repeated values (we have not taken into account for the establishment of quartiles oocytes without spindle or in telophase). Then, in our data, the three quartiles are as follows: Q1 (1.12), Q2 (1.41), and Q3 (1.61). We established a cutoff point (Q2) indicating values above which implantation rates were higher. Differences observed were statistically significant. The implantation rate for embryos with spindle retardance values lower than the cutoff was 24.4%, while the rate for embryos with higher values than the established cutoff was 41.2% (P = 0.04) (Table 3).
Table 3.
Retardance values
| Spindle retardance | Implantation rate (%) | P value (Fisher’s exact test) |
|---|---|---|
| Values < Q2 | 19/78 (24.4) | 0.04 |
| Values > Q2 | 33/80 (41.2) |
Relationship with embryo implantation
Later, we divided the sample in four groups based in the quartiles: QI (0.1–1.12), QII (1.13–1.41), QIII (1.42–1.61), and QIV (≥1.62). QIV had a significantly higher implantation rate than QIII (QIV versus QIII, P = 0.04), QII (QIV versus QII, P < 0.01), and QI (QIV versus QI, P = 0.03) (Table 4).
Table 4.
Retardance values
| Spindle retardance | Implant | No implant | Implantation rate (%) | P value (Fisher’s test)* | |
|---|---|---|---|---|---|
| QI | 0.1–1.12 | 11 | 28 | 27.5 | 0.03 |
| QII | 1.13–1.41 | 8 | 30 | 21 | <0.01 |
| QIII | 1.42–1.61 | 12 | 29 | 29.3 | 0.04 |
| QIV | >1.62 | 21 | 18 | 53.8 | |
Relationship with embryo implantation
*P value: each group with respect to QIV
In order to determine the real predictive value of meiotic spindle birefringence, we classified the 195 embryos with known implantation data into several categories according to women’s age, the embryo transfer (fresh or vitrified/warmed), and the day of the embryo transfer. Differences observed between groups of transfers were not statistically significant (Table 5).
Table 5.
Relationship between implantation rates and woman age, kind of embryo, and day of embryo transfer
| <40 | >40 | P value (Fisher’s exact test) | VW–ET | F–ET | P value (Fisher’s exact test) | D3–ET | D5–ET | P value (Fisher’s exact test) | |
|---|---|---|---|---|---|---|---|---|---|
| All embryos (%) | 40/132 (30.3) | 15/63 (23.8) | >0.05 | 16/75 (21.3) | 39/120 (32.5) | >0.05 | 44/170 (25.8) | 11/25 (44) | >0.05 |
| MS embryos (%) | 3 7/108 (34.3) | 15/50 (30) | >0.05 | 16/59 (27.1) | 36/99 (36.3) | >0.05 | 41/134 (30.6) | 11/24 (45.8) | >0.05 |
| No MS embryos (%) | 3/21 (14.3) | 0/3 | >0.05 | 0/14 | 3/20 (15) | >0.05 | 3/33 (9.1) | 0/1 | >0.05 |
| TEL embryos (%) | 0/13 | 0/0 | >0.05 | 0/2 | 0/1 | >0.05 | 0/3 | 0 | >0.05 |
<40 young women transfers <40 years, >40 older women transfers >40 years, F–ET fresh embryo transfers, VW–ET) vitrified/warmed embryo transfers and, D3–ET day 3 embryo transfers, D5–ET day 5 embryo transfers
Moreover, we also classified the 195 embryos with known implantation data into the morphological categories used routinely in our laboratory (previously described).
Overall results suggest a relationship between implantation rates and embryo morphology. But if we classified the embryos into the categories differentiating their origins (embryos derived from oocytes with meiotic spindle, without meiotic spindle or in telophase), we can observe that implantation rates depends on the presence of the meiotic spindle instead of other factors (Table 6).
Table 6.
Relationship between implantation rates and embryo morphology
| HQE | MQE/LQE | P value (Fisher’s exact test) | |
|---|---|---|---|
| All embryos (%) | 42/127 (33.1) | 12/68 (17.6) | 0.02 |
| MS embryos (%) | 39/110 (35.5) | 12/48 (25) | >0.05 |
| No MS embryos (%) | 3/16 (18.7) | 0/18 | >0.05 |
| TEL embryos (%) | 0/1 | 0/2 | >0.05 |
HQE high-quality embryos, MQE medium-quality embryos, LQE low-quality embryos
Discussion
The ability to objectively and noninvasively screen embryos to select those with the highest implantation potential is a priority for IVF laboratories. We expect that by evaluating oocytes with polarized light microscopy, we will be able to use this quantitative parameter to choose the most suitable embryos for transfer.
Spindle presence/absence
Of the total metaphase II oocytes obtained during oocyte retrieval, a low percentage had no meiotic spindle or one that was not visible. The percentage of absent or invisible spindles found by different authors varies from 9 to 22% [19, 26, 29, 32], though some authors found higher percentages [24]. Correlations have been described between the presence and absence of the meiotic spindle and fertilization rates [19, 21–24, 26]. Most authors [19, 23, 24] report that oocytes without a meiotic spindle have poor reproductive outcomes.
In our study, 14.7% of the oocytes had no visible meiotic spindle. We found lower fertilization rates and lower implantation rates in this group of oocytes, and our data are in agreement with previous findings.
In our study, we evaluated another category previously described, that is, those oocytes which seem to be in metaphase II because they have extruded the first polar body but, when analyzed under polarized light, reveal they have not yet completed meiosis I and remain in telophase. Some authors [33, 34] have observed that telophase oocytes remain unable to fertilize or have three pronuclei. We also have evaluated the outcome of telophase oocytes and found that they have a similar reproductive prognosis to oocytes without a meiotic spindle, both in terms of fertilization and implantation rate.
During the transition to metaphase I to metaphase II, the meiotic spindle is a highly dynamic structure. The meiotic spindle disappear during the transition from telophase I to metaphase II and appear again later in the process [35]. Studying the dynamics of oocyte maturation, Montag et al. [36] observed that after the extrusion of the first polar body, the meiotic spindle remains anchored to the oocyte and in telophase stage, although the oocyte is morphologically in metaphase II. The meiotic spindle remains visible for 75 to 90 min before it is disassembled. During the following 40 to 60 min, the meiotic spindle disappears, and then finally, after 115 to 150 min, the spindle becomes visible again in the oocyte. With this evidence, we can establish two groups of oocytes without a meiotic spindle: those that are still in the process of maturing and those that are aberrant (the meiotic spindle may be absent, defective, or depolymerized with advancing age) [35]. Furthermore, suboptimal culture conditions (e.g., changes in temperature, pH, and stress) can cause depolymerization of the meiotic spindle [19, 35, 37]). The evaluation of our oocytes was performed on a heated plate, keeping the temperature stable at 37 °C. Approximately 2 to 2.5 h after polar body extrusion, the meiotic spindle should be visible in the oocytes. We decumulated the oocytes 37 h post-hCG, and in most cases, these oocytes were metaphase II. Two hours later, evaluation and microinjection were performed, so at that time, we should have been able to observe the spindle in those oocytes that contained one, although some of them still remained in telophase. We were not able to determine whether they were aberrant oocytes without a spindle or whether they were maturing oocytes (telophase spindle or temporarily disappeared); in any case (spindle absence or delayed oocyte maturation), it seems logical that these had a poorer reproductive prognosis, as the absence of a spindle prevents the correct distribution of chromosomes during fertilization [37].
A previous study analyzed a pool of oocytes by polarized light microscopy and later by confocal microscopy, observe a high percentage of abnormal meiotic chromosomes, chaotic formations, and alignments in those oocytes without a meiotic spindle (analyzed by polarized light microscopy) as compared to oocytes with a visible meiotic spindle (analyzed by polarized light microscopy) [34]. Before them, other researchers [38] observed that 71% of oocytes with a visible spindle had normal chromosome alignment, while most oocytes without spindles had anomalous alignment.
Meiotic spindle deviation
Another widely studied parameter is meiotic spindle displacement relative to polar body position. Fertilization rates and embryo quality are independent of the position of the meiotic spindle and the polar body [39]. However, other studies have shown that large degrees of deviation between the two increase the risk of abnormal fertilization [32]. In our patients, we have rarely observed oocytes with displaced meiotic spindles, and hence do not have sufficient data to evaluate the importance of this indicator. It is believed that, rather than a pathological feature of the oocyte, meiotic spindle deviation is caused by the mechanical stress to which the eggs are subjected to during denudation [40].
Meiotic spindle retardance and reproductive outcome
There are few studies correlating spindle characteristics with pregnancy rates [24, 27, 38].
Two studies focused more on implantation rate. One of them [28] compared the implantation rate between embryos with or without meiotic spindle and failed to find any differences. They found that differences in implantation rates depended on embryo morphology, a finding that challenges the predictive value of spindle visualization. Meanwhile , Chamayou et al. [24] compared implantation rates in groups. Not all these studies focused on the implantation rate per embryo, and this was our objective.
In our study, we tried to avoid biases, showing that despite transferring embryos on different days of development, transferring fresh and previously frozen embryos, transferring embryos in women of different age groups, and transferring embryos of different embryo quality, we only found significant differences in implantation rates when we selected embryos according to the presence or absence of a meiotic spindle. In addition to assessing the presence or absence of the spindle, we observed that higher retardance values correlated with increased rates of implantation.
Despite our findings, some authors have questioned the usefulness of polarized light. Montag et al. [41] considers that meiotic spindle retardance values are dependent on orientation and time observation. In our study, all images were taken at the same time with respect to the hCG trigger time and obtained by the same person, who used the same criteria for placement of the oocyte. Others authors [42, 43] reported that meiotic spindle retardance may reflect changes associated with physiological events. The existence of external factors, such as temperature and age, can influence microtubule dynamics and then to alter retardance values [43, 44]. Changes associated with physiological events or external factors, although possibly influencing meiotic spindle retardance, consequently also influence the reproductive outcome of the oocyte. Our experience evaluating oocytes is being successful despite some authors’ reasons to exclude polarized light as a method for oocyte evaluation.
As shown in our results, it can be expected that oocytes with a visible meiotic spindle will have better fertilization rates and will result in higher rates of embryo implantation. Furthermore, our data indicate that those oocytes at telophase or without a visible meiotic spindle have a higher abnormal fertilization rate.
In conclusion, meiotic spindle imaging may be an important tool for prediction of oocyte quality. It allows us to select the best-quality oocytes, excluding those which do not have a meiotic spindle or are in telophase. This may be useful for patients who do not want to fertilize more than a certain number of oocytes (for various reasons) or in case of oocyte vitrification, where technical considerations require us to choose only those with the best reproductive prognosis.
To the best of our knowledge, this is the first study to quantitatively and qualitatively evaluate retardance in oocyte meiotic spindles, correlating this parameter with embryo implantation rates. We found that implantation rates were clearly higher in cases having high retardance, thus suggesting predictive value for embryo selection. We therefore believe that classical morphological classification in combination with retardance values of spindles can be helpful in selecting embryos with higher implantation potential.
References
- 1.Grady R, Alavi N, Vale R, Khandwala M, McDonald SD. Elective single embryo transfer and perinatal outcomes: a systematic review and meta-analysis. Fertil Steril. 2012;97:324–331. doi: 10.1016/j.fertnstert.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 2.Pinborg A. IVF/ICSI twin pregnancies: risks and prevention. Hum Reprod Update. 2005;11:575–593. doi: 10.1093/humupd/dmi027. [DOI] [PubMed] [Google Scholar]
- 3.Workshop Group TEC Multiple gestation pregnancy. Hum Reprod. 2000;15:1856–1864. doi: 10.1093/humrep/15.8.1856. [DOI] [PubMed] [Google Scholar]
- 4.Balaban B, Urman B, Ata B, Isiklar a, Larman MG, Hamilton R, et al. A randomized controlled study of human day 3 embryo cryopreservation by slow freezing or vitrification: vitrification is associated with higher survival, metabolism and blastocyst formation. Hum Reprod. 2008;23:1976–1982. doi: 10.1093/humrep/den222. [DOI] [PubMed] [Google Scholar]
- 5.Ku PY, Lee RKK, Lin SY, Lin MH, Hwu YM. Comparison of the clinical outcomes between fresh blastocyst and vitrified-thawed blastocyst transfer. J Assist Reprod Genet. 2012;29:1353–1356. doi: 10.1007/s10815-012-9874-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roque M, Lattes K, Serra S, Sola I, Geber S, Carreras R, et al. Fresh embryo transfer versus frozen embryo transfer in in vitro fertilization cycles: a systematic review and meta-analysis. Fertil Steril. 2013;99:156–162. doi: 10.1016/j.fertnstert.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Basirat Z, Rad HA, Esmailzadeh S, Jorsaraei SGA, Hajian-Tilaki K, Pasha H, et al. Comparison of pregnancy rate between fresh embryo transfers and frozen-thawed embryo transfers following icsi treatment. Iran J Reprod Med. 2016;14:39–46. [PMC free article] [PubMed] [Google Scholar]
- 8.Gomaa H, Baydoun R, Sachak S, Lapana I, Soliman S. Elective single embryo transfer: is frozen better than fresh? J Bras Reprod Assist. 2016;20:3–7. doi: 10.5935/1518-0557.20160002. [DOI] [PubMed] [Google Scholar]
- 9.Vaiarelli A, Cimadomo D, Capalbo A, Orlando G, Sapienza F, Colamaria S, et al. Pre-implantation genetic testing in ART: who will benefit and what is the evidence? J Assist Reprod Genet. 2016;33:1273–1278. doi: 10.1007/s10815-016-0785-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krisher RL, Schoolcraft WB, Katz-Jaffe MG. Omics as a window to view embryo viability. Fertil Steril. 2015;103:333–341. doi: 10.1016/j.fertnstert.2014.12.116. [DOI] [PubMed] [Google Scholar]
- 11.Montag M, Toth B, Strowitzki T. New approaches to embryo selection. Reprod BioMed Online. 2013;27:539–546. doi: 10.1016/j.rbmo.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Murakoshi Y, Sueoka K, Takahashi K, Sato S, Sakurai T, Tajima H, et al. Embryo developmental capability and pregnancy outcome are related to the mitochondrial DNA copy number and ooplasmic volume. J Assist Reprod Genet. 2013;30:1367–1375. doi: 10.1007/s10815-013-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kovacs P. Embryo selection: the role of time-lapse monitoring. Reprod Biol Endocrinol. 2014;12:124. doi: 10.1186/1477-7827-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update. 2008;14:159–177. doi: 10.1093/humupd/dmm040. [DOI] [PubMed] [Google Scholar]
- 15.Rienzi L, Vajta G, Ubaldi F. Predictive value of oocyte morphology in human IVF: a systematic review of the literature. Hum Reprod Update. 2011;17:34–45. doi: 10.1093/humupd/dmq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howe K, Fitz HG. Recent insights into spindle function in mammalian oocytes and early embryos. Biol Reprod. 2013;89:71. doi: 10.1095/biolreprod.113.112151. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z-B, Schatten H, Sun Q-Y. Why is chromosome segregation error in oocytes increased with maternal aging? Physiology. 2011;26:314–325. doi: 10.1152/physiol.00020.2011. [DOI] [PubMed] [Google Scholar]
- 18.Aman RR, Parks JE. Effects of cooling and rewarming on the meiotic spindle and chromosomes of in vitro-matured bovine oocytes. Biol Reprod. 1994;50:103–110. doi: 10.1095/biolreprod50.1.103. [DOI] [PubMed] [Google Scholar]
- 19.Wang WH, Meng L, Hackett RJ, Keefe DL. Developmental ability of human oocytes with or without birefringent spindles imaged by Polscope before insemination. Hum Reprod. 2001;16:1464–1468. doi: 10.1093/humrep/16.7.1464. [DOI] [PubMed] [Google Scholar]
- 20.Wang WH, Meng L, Hackett RJ, Odenbourg R, Keefe DL. The spindle observation and its relationship with fertilization after intracytoplasmic sperm injection in living human oocytes. Fertil Steril. 2001;75:348–353. doi: 10.1016/S0015-0282(00)01692-7. [DOI] [PubMed] [Google Scholar]
- 21.Braga DPDAF, Figueira RDCS, Rodrigues D, Madaschi C, Pasqualotto FF, Iaconelli A, et al. Prognostic value of meiotic spindle imaging on fertilization rate and embryo development in in vitro-matured human oocytes. Fertil Steril. 2008;90:429–433. doi: 10.1016/j.fertnstert.2007.06.088. [DOI] [PubMed] [Google Scholar]
- 22.Cohen Y, Malcov M, Schwartz T, Mey-Raz N, Carmon A, Cohen T, et al. Spindle imaging: a new marker for optimal timing of ICSI? Hum Reprod. 2004;19:649–654. doi: 10.1093/humrep/deh113. [DOI] [PubMed] [Google Scholar]
- 23.Konc J, Kanyó K, Cseh S. Visualization and examination of the meiotic spindle in human oocytes with polscope. J Assist Reprod Genet. 2004;21:349–353. doi: 10.1023/B:JARG.0000046202.00570.1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madaschi C, de Souza Bonetti TC, de Almeida Ferreira Braga DP, Pasqualotto FF, Iaconelli A, Borges E. Spindle imaging: a marker for embryo development and implantation. Fertil Steril. 2008;90:194–198. doi: 10.1016/j.fertnstert.2007.05.071. [DOI] [PubMed] [Google Scholar]
- 25.Moon JH, Hyun CS, Lee SW, Son WY, Yoon SH, Lim JH. Visualization of the metaphase II meiotic spindle in living human oocytes using the polscope enables the prediction of embryonic developmental competence after ICSI. Hum Reprod. 2003;18:817–820. doi: 10.1093/humrep/deg165. [DOI] [PubMed] [Google Scholar]
- 26.Rama Raju G a, Prakash GJ, Krishna KM, Madan K. Meiotic spindle and zona pellucida characteristics as predictors of embryonic development: a preliminary study using PolScope imaging. Reprod BioMed Online. 2007;14:166–174. doi: 10.1016/S1472-6483(10)60784-5. [DOI] [PubMed] [Google Scholar]
- 27.Shen Y, Stalf T, Mehnert C, De Santis L, Cino I, Tinneberg H-R, et al. Light retardance by human oocyte spindle is positively related to pronuclear score after ICSI. Reprod BioMed Online. 2006;12:737–751. doi: 10.1016/S1472-6483(10)61086-3. [DOI] [PubMed] [Google Scholar]
- 28.Chamayou S, Ragolia C, Alecci C, Storaci G, Maglia E, Russo E, et al. Meiotic spindle presence and oocyte morphology do not predict clinical ICSI outcomes: a study of 967 transferred embryos. Reprod BioMed Online. 2006;13:661–667. doi: 10.1016/S1472-6483(10)60656-6. [DOI] [PubMed] [Google Scholar]
- 29.Fang C, Tang M, Li T, Peng WL, Zhou CQ, Zhuang GL, et al. Visualization of meiotic spindle and subsequent embryonic development in in vitro and in vivo matured human oocytes. J Assist Reprod Genet. 2007;24:547–551. doi: 10.1007/s10815-007-9171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilani S, Cooke S, Kan A, Chapman M. Are there non-invasive markers in human oocytes that can predict pregnancy outcome? Reprod BioMed Online. 2009;18:674–680. doi: 10.1016/S1472-6483(10)60013-2. [DOI] [PubMed] [Google Scholar]
- 31.Jose delos Santos M, Arroyo G, Busquet A, Calderon G, Cuadros J, Hurtado de Mendoza MV, et al. A multicenter prospective study to assess the effect of early cleavage on embryo quality, implantation, and live-birth rate. Fertil Steril. 2014;101:981–987. doi: 10.1016/j.fertnstert.2013.12.043. [DOI] [PubMed] [Google Scholar]
- 32.Rienzi L, Ubaldi F, Martinez F, Iacobelli M, Minasi MG, Ferrero S, et al. Relationship between meiotic spindle location with regard to the polar body position and oocyte developmental potential after ICSI. Hum Reprod. 2003;18:1289–1293. doi: 10.1093/humrep/deg274. [DOI] [PubMed] [Google Scholar]
- 33.Montag M, van der Ven H. Symposium: innovative techniques in human embryo viability assessment. Oocyte assessment and embryo viability prediction: birefringence imaging. Reprod BioMed Online. 2008;17:454–460. doi: 10.1016/S1472-6483(10)60231-3. [DOI] [PubMed] [Google Scholar]
- 34.Heindryckx B, De Gheselle S, Lierman S, Gerris J, De Sutter P. Efficiency of polarized microscopy as a predictive tool for human oocyte quality. Hum Reprod. 2011;26:535–544. doi: 10.1093/humrep/deq376. [DOI] [PubMed] [Google Scholar]
- 35.Shen Y, Betzendahl I, Tinneberg HR, Eichenlaub-Ritter U. Enhanced polarizing microscopy as a new tool in aneuploidy research in oocytes. Mutat Res - Genet Toxicol Environ Mutagen. 2008;651:131–140. doi: 10.1016/j.mrgentox.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 36.Montag M, Schimming T, van der Ven H. Spindle imaging in human oocytes: the impact of the meiotic cell cycle. Reprod BioMed Online. 2006;12:442–446. doi: 10.1016/S1472-6483(10)61996-7. [DOI] [PubMed] [Google Scholar]
- 37.Rienzi L, Ubaldi F, Iacobelli M, Minasi MG, Romano S, Greco E. Meiotic spindle visualization in living human oocytes. Reprod BioMed Online. 2005;10:192–198. doi: 10.1016/S1472-6483(10)60940-6. [DOI] [PubMed] [Google Scholar]
- 38.Wang WH, Keefe DL. Prediction of chromosome misalignment among in vitro matured human oocytes by spindle imaging with the PolScope. Fertil Steril. 2002;78:1077–1081. doi: 10.1016/S0015-0282(02)04196-1. [DOI] [PubMed] [Google Scholar]
- 39.Woodward BJ, Montgomery SJ, Hartshorne GM, Campbell KH, Kennedy R. Spindle position assessment prior to ICSI does not benefit fertilization or early embryo quality. Reprod BioMed Online. 2008;16:232–238. doi: 10.1016/S1472-6483(10)60579-2. [DOI] [PubMed] [Google Scholar]
- 40.Taylor TH, Chang C, Elliott T, Colturato LF, Kort HI, Peter Z. Effect of denuding on polar body position in in- vitro matured oocytes. Reprod BioMed Online. 2008;17:515–519. doi: 10.1016/S1472-6483(10)60238-6. [DOI] [PubMed] [Google Scholar]
- 41.Korkmaz C, Sakinci M, Bayoglu Tekin Y, Ercan CM. Do quantitative birefringence characteristics of meiotic spindle and zona pellucida have an impact on implantation in single embryo transfer cycles? Arch Gynecol Obstet. 2014;289:433–438. doi: 10.1007/s00404-013-2999-1. [DOI] [PubMed] [Google Scholar]
- 42.Montag M, Köster M, van der Ven K, van der Ven H. Gamete competence assessment by polarizing optics in assisted reproduction. Hum Reprod Update. 2011;17:654–666. doi: 10.1093/humupd/dmr016. [DOI] [PubMed] [Google Scholar]
- 43.Liu L, Trimarchi JR, Oldenbourg R, Keefe DL. Increased birefringence in the meiotic spindle provides a new marker for the onset of activation in living oocytes. Biol Reprod. 2000;63:251–258. doi: 10.1095/biolreprod63.1.251. [DOI] [PubMed] [Google Scholar]
- 44.Navarro P, Liu L, Trimarchi J, Ferriani R, Keefe D. Noninvasive imaging of spindle dynamics during mammalian oocyte activation. Fertil Steril. 2005;83:1197–1205. doi: 10.1016/j.fertnstert.2004.07.983. [DOI] [PubMed] [Google Scholar]
- 45.Sun X-F, Zhang W-H, Chen X-J, Xiao G-H, Mai W-Y, Wang W-H. Spindle dynamics in living mouse oocytes during meiotic maturation, ageing, cooling and overheating: a study by polarized light microscopy. Zygote. 2004;12:241–249. doi: 10.1017/S0967199404002850. [DOI] [PubMed] [Google Scholar]