Abstract
Purpose
The aim of this study was to investigate whether infection of women by the hepatitis C virus (HCV) reduces the chance of conceiving after in vitro fertilization (IVF).
Methods
We performed a retrospective blind matched case-control study where IVF outcomes for the first 37 cycles of HCV sero-positive women were compared to those of 107 cycles of an uninfected control group. Our results were included in a systematic literature review.
Results
Out of five eligible studies, ours included, three observed an impact of HCV infection, though at various levels including response to stimulation, fertilization, implantation, and pregnancy rates. Two studies differentiated results for patients with confirmed active viral replication. Matching criteria and populations studied varied between studies.
Conclusions
More and larger studies with well-defined groups are needed to clarify the eventual impact of the HCV on IVF outcomes. Data concerning the infectious status of a patient as well as her health state should be systematically recorded. A multi-disciplinary approach as well as a thorough knowledge of the patient’s general health state might prove useful in the management and counseling of these patients in terms of success in conceiving.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-017-0892-8) contains supplementary material, which is available to authorized users.
Keywords: HCV, IVF, Implantation, Hepatitis C virus, In vitro fertilization, Systematic review
Introduction
Hepatitis C, an RNA-enveloped virus from the Flaviviridae family, is the first cause of chronic infectious hepatitis worldwide [1]. The World Health Organization estimates about 123 million people to be carriers of the virus [1]. Transmission occurs mainly through parenteral exposure. The virus has also been detected in other body secretions including semen, saliva, and breast milk though at lower titers. Sexual transmission is considered to be extremely low [2] but remains controversial. Vertical transmission is estimated at around 5% and doubles in case of co-infection by HIV or when HCV titers reach over 106 IU/ml [2]. Infection leads, in an average of 10 years, to 80% of patients developing chronic liver disease. Thereafter, 35 and 5%, respectively, will develop liver cirrhosis and hépato-carcinoma [3]. Treatment with peg-interferon Alfa and ribavirin is not efficient for all viral genotypes, is a long procedure, and can be accompanied by side effects [4]. Moreover, ribavirin has been shown to be teratogenic in animal studies. The development of new, efficient, and direct anti-viral drugs [5] is promising, though currently, not many people can afford this therapy [6]. Couples infected by HCV and who are unable to conceive naturally seek help through assisted reproductive techniques (ART). Several studies have addressed ART outcomes when the male partner is infected by HCV with some observing an adverse effect of viral infection on sperm parameters, hormonal levels, and pregnancy rates [7–10] while others saw no differences when comparing results to uninfected couples [11–13]. On the other hand, it seems that few studies have investigated ART outcomes for HCV-infected women. We therefore performed a systematic literature review where the impact of HCV infection on women treated by IVF was evaluated. We equally performed our own matched case-control study. Ovarian stimulation, embryology, and pregnancy results were compared between HCV sero-positive and sero-negative women for their first IVF attempt. A subgroup of HCV-infected women with active viral replication was also studied.
Material and methods
Systematic literature search
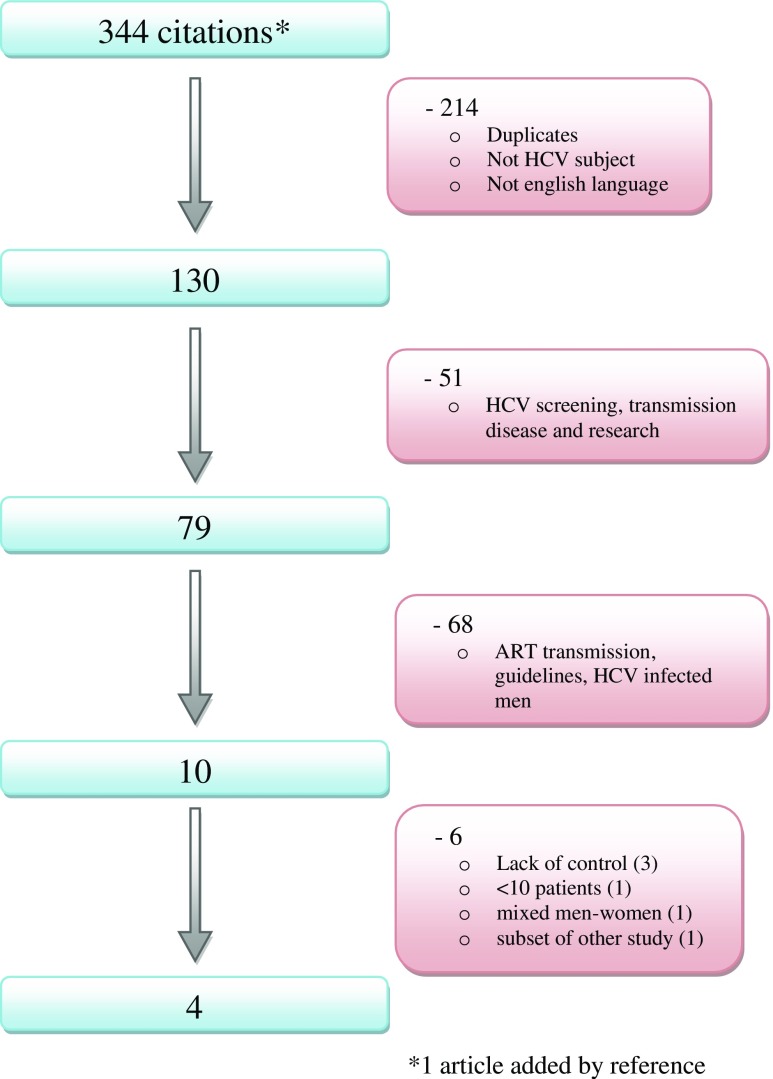
A systematic literature review was registered on 6/12/16 in the Prospero registry (number CRD42016052972). The databases “pubmed” and “science direct” were searched with the keywords “HCV and in vitro fertilization” as well as “HCV and assisted reproduction.” Full articles in English, where IVF outcomes for women infected by HCV were studied, were included. Studies for which there was no adequate control group or less than 10 cycles analyzed were excluded. Figure 1 shows the flowchart for article inclusion.
Fig. 1.
Flowchart diagram of the literature search
Our matched control study
Studied population
Patients presenting HCV antibodies between 1998 and 2015 and treated for their first IVF cycle were enrolled in our study. We excluded patients who had a co-infection for HBV or HIV as well as patients whose partners were infected by HCV, HBV, or HIV. We additionally excluded patients with a false positive result or cycles involving gamete donation. The same exclusion criteria were applied for the control group of patients who all had a negative HCV antibody. The first population of HCV cases that was analyzed comprised 37 patients presenting positive antibodies to the HCV. Among these patients, three had a negative RT-PCR, 17 had active viral replication, and for the remaining 17 patients, the RT-PCR result at the time of their first IVF attempt was not available. We therefore compared clinical outcomes for the subgroup of patients with a confirmed viral replication to those of the control group of uninfected patients. Since most sero-positive patients develop chronic hepatitis, it is possible that a majority of patients with an unknown RT-PCR result had an active infection; however, we cannot exclude the possibility that some patients had been treated and had cleared the viral infection.
Database and matching of controls
Data of patients treated by ART are systematically and prospectively encoded and checked on a regular basis. A specific computer program was conceived to match HCV cases to controls following determined criteria, though without access to trial results. The matching was therefore automatized and totally blinded to the trial results. When several controls were available, the one closest in time to the IVF cycle of the HCV case was selected. Matching was performed separately for the two main studied outcomes:
Matching for the analysis of ovarian response to stimulation: To study the response to ovarian stimulation for HCV-infected women, we matched each case with three controls for their first IVF cycle for the following criteria (100% matching): age (±two years), period of IVF (cycle ± 6 months), and main female infertility etiology (tubal, idiopathic, hormonal, endometriosis). We then matched patients when possible for two additional criteria: primary/secondary infertility (89% matching) and ethnical origin (55% matching). The matching process was performed for HCV sero-positive patients and then for a subgroup of RT-PCR-positive patients.
Matching for the analysis of embryology and pregnancy results: To study embryology and pregnancy results for HCV-infected women, we then matched each case who had an embryo transfer to one control for the following criteria (100% matching): age (±2 years), period of IVF cycle (±12 months), main female infertility etiology (tubal, idiopathic, hormonal, endometriosis), extreme male factor (sperm preparation by simple washing due to bad quality), and day of embryo transfer (days 2 and 3 versus 5). We then matched patients when possible for three additional criteria: primary/secondary infertility (96% matching), ethnical origin (48% matching), and male factor (92% matching). The matching process was performed for HCV sero-positive patients and then for a subgroup of RT-PCR-positive patients.
Ovarian stimulation and oocyte retrieval
Patients were monitored and managed according to standardized clinical protocols as previously reported [14]. Briefly, ovarian stimulation was performed with Humegon® (NV Organon, The Netherlands) or hMG (Menopur®; Ferring, Denmark), recombinant FSH (Puregon®; NV Organon, the Netherlands or Gonal-F®; Merck-Serono, Switzerland) or corifollitropine alfa (Elonva®; NV Organon, The Netherlands). The dose of gonadotropins was determined on an individual basis according to the woman’s age, day 3 serum FSH value, and antral follicle count. Pituitary inhibition was obtained by GnRH analog (long or short protocol) (Suprefact®; Senofi-Avantis, Germany or Decapeptyl®; Ipsen) or GnRH antagonist (Orgalutran®; NV Organon, the Netherlands or Cetrotide®; Merck-Serono, Switzerland). When three or more leading follicles reached 17 to 18 mm, ovulation was triggered with 5000–10,000 IU of hCG (Pregnyl®; NV Organon, The Netherlands). Oocyte retrieval was performed transvaginally and ultrasound guided 34–36 h after hCG injection.
Laboratory procedures
After oocyte retrieval, oocytes were either inseminated or injected by ICSI and cultured overnight. Normally fertilized oocytes were cultured to days 2 or 3. Even though day of transfer was one of the criteria selected for the matching process, after matching, we observed that all transfers occurred at the cleavage stage. The best quality embryos were selected for transfer (number depending on the Belgian law from year 2003 onwards) and supernumerary embryos of good quality were cryopreserved. A top-quality embryo on days two and three were, respectively, defined as embryos with four–five cells/<20% fragmentation and ≥eight cells/<20% fragmentation. Infected gametes or embryos were cultured in separate incubators and cryopreserved in separate tanks.
Studied outcomes and definitions
For ovarian response to stimulation, we studied the type of stimulation, the peak estrogen value during stimulation, the number of follicles ≥15 mm, the number of retrieved oocytes, and the percentage of abandoned cycles due to poor response. For pregnancy rates, the clinical pregnancy (number of positive βHCGs with a fetal sac divided by the number of embryo transfers), biochemical (number of positive βHCGs without a fetal sac divided by the number of embryo transfers), spontaneous abortion (number of early and late miscarriages divided by the number of embryo transfers), delivery (number of births divided by the number of embryo transfers), and implantation rates (number of gestational sacs divided by the number of transferred embryos) were investigated. Additional data were collected such as fertilization rates (number of embryos obtained divided by number of inseminated or injected oocytes), embryo quality at transfer, FSH levels, insemination methods, pelvic inflammatory disease (PID), and duration of infertility. Our study covered a long period; data such as BMI, smoking habits, total amounts of administered gonadotropines, antral follicular count, or anti-mullerian hormone were not systematically recorded during the first years of the database. Since a substantial amount of data was missing, they were therefore not considered in this study.
Statistical analysis
To assess differences in continuous variables between cases and controls, the t test or Mann-Whitney test was used. To assess differences in categorical variables between cases and controls, the chi-square test or Fisher’s exact test was used. In a subgroup analysis, we restricted to HCV-infected women with active viral replication (positive RT-PCR). P values <0.05 were considered statistically significant. The analyses were performed using the SAS System, SAS Institute Inc., Cary, NC, USA.
Ethical statement and informed consent
All our protocols have been approved by the local Ethics Committee and all our patients have given their informed, written consent prior to treatment.
Results
Our matched control study
Ovarian response
The principal demographic characteristics of the HCV patients and their matched control group (three controls per case) are presented in Table 1. Most patients included in this study presented secondary tubal infertility. Response to ovarian stimulation was found to be similar for all criteria analyzed when comparing 37 HCV sero-positive and 111 HCV sero-negative patients for their first IVF attempt (Table 1). We then isolated a subgroup of 17 patients with confirmed active viral replication that were matched to 51 uninfected patients. Demographic data was similar to the sero-positive group (data not shown), and again no significant differences in terms of ovarian response to stimulation were found between cases and controls (table 7 Online Resource).
Table 1.
Principal demographic characteristics and response to ovarian stimulation for HCV patients and their matched control group (three controls per case)
| Controls N = 111 | HCV cases N = 37 | P value | |||
|---|---|---|---|---|---|
| Months between cycles | Median 1.9 (<0. 1 to max 5.97) | ||||
| Age mean ± std | 36.0 ± 4.3 | 36.2 ± 4.5 | 0.87 | ||
| Age median (min-max) | 36.3 (24.7 to 44.4) | 36.5 (25.4 to 44.1) | |||
| Ethnical origin (N) | 106 | 36 | |||
| Caucasian | 58 | 55% | 20 | 56% | 0.36 |
| Sub-Saharan African | 36 | 34% | 12 | 33% | |
| Maghrebian | 11 | 10% | 2 | 6% | |
| Asian | 1 | 1% | 2 | 6% | |
| Female infertility etiology | |||||
| Tubal | 57 | 51% | 19 | 51% | 1 |
| Hormonal | 18 | 16% | 6 | 16% | 1 |
| Idiopathic | 30 | 27% | 10 | 27% | 1 |
| Endometriosis | 9 | 8% | 3 | 8% | 1 |
| Tubal and hormonal | 3 | 3% | 1 | 3% | 1 |
| Secondary infertility (N) | 111 | 36 | |||
| 64 | 58% | 23 | 64% | 0.51 | |
| Length Infertility (N) | 108 | 29 | |||
| Median (min-max) (years) | 3 (<1 to 25) | 3 (1 to 14) | 0.86 | ||
| FSH day 3 (N) | 81 | 21 | |||
| Median (min-max) | 6.1 (1.0 to 92.0) | 7.4 (3.5 to 18.0) | 0.13 | ||
| PID (N) | 64 | 24 | |||
| 17 | 27% | 6 | 25% | 1 | |
| Type of stimulation (N) | 107 | 37 | |||
| Long | 69 | 64% | 24 | 65% | 0.41 |
| Short | 17 | 16% | 3 | 8% | |
| Cetrotide®/Orgalutran® | 21 | 20% | 10 | 27% | |
| E2 max median (min-max) | 1620 (5 to 15547) | 1428 (5 to 6912) | 0.27 | ||
| Oocytes≥15 at scan (N) | 109 | 35 | |||
| Median (min-max) | 5 (0 to 31) | 4 (0 to 15) | 0.21 | ||
| Oocytes at retrieval (N) | 86 | 30 | |||
| Median (min-max) | 6.5 (1 to 21) | 6.0 (1 to 15) | 0.43 | ||
| Cycle cancellation | 0.52 | ||||
| Medical (not poor response) | 5 | 19% | 3 | 38% | |
| Non-medical | 1 | 4% | – | – | |
| Poor response | 20 | 77% | 5 | 63% | |
Std standard deviation, N is provided when some data are missing, FSH follicle-stimulating hormone, PID pelvic inflammatory disease, E2 estradiol, E2 max the peak value during stimulation
Embryology and pregnancy results
Among the 37 HCV patients who initiated an IVF cycle, 25 went on to have an embryo transfer. For these patients, we then compared embryology and pregnancy results between HCV sero-positive patients and their matched negative controls for their first IVF cycle as well as for patients with active viral replication. The demographic data for sero-positive patients (table 8 Online Resource) and RT-PCR positive patients (data not shown) were similar to those presented in Table 1. For the additional criteria matched, 80% of the couples were diagnosed with male factor infertility with 8% presenting an extreme male factor etiology. All transfers occurred at the cleavage stage. We again looked at ovarian response (except for cycle cancellation) since we matched the HCV patients to a new set of control patients and reached the same conclusions as above (data not shown). Fertilization and implantation rates were found to be significantly reduced for sero-positive patients compared to their matched negative controls (Table 2). For patients with active viral replication, implantation rates were again shown to be significantly reduced and no live births occurred for HCV-infected patients (Table 3). Calculation of clinical pregnancy rates per transfer for all IVF attempts for sero-positive and RT-PCR-positive patients were 13 (9/67) and 9 (3/33) percent, respectively, with a total of seven live births (data not shown).
Table 2.
Comparison of embryology and pregnancy results between sero-positive HCV patients and their matched negative controls (one control per case)
| Controls N = 25 | HCV cases N = 25 | P value | |||
|---|---|---|---|---|---|
| N oocytes at retrieval median (min-max) | 7 (1 to 14) | 6 (1 to 15) | 0.95 | ||
| Insemination method | 0.38 | ||||
| ICSI only | 11 | 44% | 13 | 52% | |
| IVF only | 8 | 32% | 10 | 40% | |
| ICSI and IVF | 6 | 24% | 2 | 8% | |
| Fertilization rate median (min-max) | 86% (50 to 100%) | 67% (14 to 100%) | 0.04 | ||
| N TF embryosa median (min-max) | 2 (1 to 3) | 2 (1 to 3) | 0.96 | ||
| Quality of TF embryos | 0.13 | ||||
| At least one good quality | 12 | 48% | 14 | 56% | |
| No good quality, at least one average quality | 12 | 48% | 8 | 32% | |
| Only bad quality | 1 | 4% | 3 | 12% | |
| Implantation rate | 11/47 | 23% | 3/47 | 6% | 0.04 |
| Outcome | 0.19b | ||||
| No sac | 16 | 64% | 21 | 84% | |
| No sac, biochemical pregnancy | – | – | 1 | 4% | |
| Miscarriage | 3 | 12% | 1 | 4% | |
| Delivery | 6 | 24% | 2 | 8% | |
| Clinical pregnancy | 9 | 36% | 3 | 12% | 0.10c |
| Babies born | 7 | 2 | |||
Implantation rate: total N sacs/total N embryos transferred
TF transferred
aAll transfers took place on days 2/3
bOverall test, considering the four categories
cClinical pregnancy: yes vs no
Table 3.
Comparison of embryology and pregnancy results between HCV patients with a positive RT-PCR and their matched negative controls (one control per case)
| Controls N = 13 | HCV cases N = 13 | P value | |||
|---|---|---|---|---|---|
| N oocytes at retrieval median (min-max) | 7 (1 to 14) | 6 (1 to 15) | 0.78 | ||
| Insemination method | 1 | ||||
| ICSI only | 5 | 38% | 6 | 46% | |
| IVF only | 6 | 46% | 6 | 46% | |
| ICSI and IVF | 2 | 15% | 1 | 7% | |
| Fertilization rate median (min-max) | 100% (60 to 100%) | 67% (25 to 100%) | 0.12 | ||
| N TF embryosa median (min-max) | 2 (1 to 3) | 1 (1 to 3) | 0.72 | ||
| Quality of TF embryos | 0.13 | ||||
| At least one good quality | 6 | 46% | 8 | 62% | |
| No good quality, at least one average quality | 7 | 54% | 3 | 23% | |
| Only bad quality | – | – | 2 | 15% | |
| Implantation rate | 6/23 | 26% | 0/22 | 0% | 0.02 |
| Outcome | 0.15b | ||||
| No sac | 9 | 69% | 12 | 92% | |
| No sac, biochemical pregnancy | – | – | 1 | 8% | |
| Miscarriage | 1 | 8% | – | – | |
| Delivery | 3 | 23% | – | – | |
| Clinical pregnancy | 4 | 31% | – | – | 0.10c |
| Babies born | 4 | – | |||
Implantation rate: total N sacs/total N embryos transferred
TF transferred
aAll transfers took place on days 2/3
bOverall test, considering the four categories
cClinical pregnancy: yes vs no
Systematic literature review
Design and characteristics of the four eligible studies included after the systematic review are presented in Table 4 and compared to our own study. All five studies were retrospective matched controlled studies, though matching varied from two to ten criteria between studies. Three out of the five studies confirmed exclusion of co-infections. Only one study gave clear information on the health state of the patients included. Comparison between studies, for response to ovarian stimulation, embryology, and pregnancy results are presented in Table 5. Two studies gave results for patients with a positive RT-PCR (Table 6). Only the study from Hanafi and colleagues [15] compared IVF outcomes in relation to viral load. A significant negative correlation was found between the number of oocytes and viral load but not between pregnancy and viral load. None of the studies analyzed results in relation to the viral genotype. We then calculated when possible, outcomes combining the results of the different studies. For patients sero-positive to HCV, the percentage of cycle cancelations compared to control cycles was, respectively, 30% (48/159) versus 11% (27/235), the mean oocytes retrieved were 6.7 ± 2.1 (150 cycles) versus 8.9 ± 2.5 (243 cycles), and the clinical pregnancy rates per transfer were 32% (71/225) versus 52% (746/1436). The same calculations were done for the two studies evaluating outcomes for RT-PCR-positive patients (Table 6). The percentage of cycle cancelations for RT-PCR positive patients compared to control cycles was, respectively, 40% (23/57) versus 13% (12/91), the mean oocytes retrieved were 5.3 ± 1.8 (34 cycles) versus 9.2 ± 4.0 (71 cycles), and the clinical pregnancy rates per transfer were 6% (2/31) versus 45% (23/51). Fertilization and implantation rates could not be combined since only percentages were available.
Table 4.
Impact of HCV on IVF outcomes: comparison of study designs and matching of controls in the literature
| Author and year of study | Shaw-Jackson et al. 2016a | Hanafi et al. [15]b | Englert et al. [16] | Yang et al. [18] | Prisant et al. [17] |
|---|---|---|---|---|---|
| N HCV patients/N control patients | 37/111 | 40–40/40 | Not specified | 90/1256 | 22/42 |
| N HCV cycles/N control cycles | 37/111 | 40–40/40 | 42/84 | 90/1256 | 45/45 |
| Rank of trial | 1st IVF attempt | Previous IVF attempts | Previous IVF attempts | Not specified | Previous IVF attempts |
| Design | Retrospective blind matched controlled | Retrospective matched controlled | Retrospective matched controlled | Retrospective matched controlled | Retrospective matched controlled |
| Matching criteria | Age | Age | Age | Age | Age |
| Rank of trial | – | Rank of trial | – | Rank of trial | |
| Date of trial | – | – | – | – | |
| F infertility | Tubal condition | Tubal condition | – | F infertility | |
| EMF | ICSI | ICSI/IVF | – | ICSI/IVF | |
| M infertility | M infertility | M infertility | – | M infertility | |
| – | – | – | Stimulation | – | |
| Day of transfer | – | – | – | – | |
| Ethnical origin | – | – | – | – | |
| 1ary infertility | – | – | – | – | |
| Closest trial date chosen |
Closest trial date chosen |
Closest trial date chosen |
– | – | |
| Exclusions | Couple co-infections Donor cycles |
Not specified | Couple co-infections | Liver disease Chronic hepatitis Antiviral treatment |
Couple co-infections |
F infertility female etiology of infertility; M infertility male etiology of infertility; EMF extreme male factor
a For response to ovarian stimulation and pregnancy rates, patients were matched and outcomes were studied independently (see Material and methods). Ethnical origin, primary infertility, and male factor infertility etiology were matched when possible.
bTwo study groups of patients were included (sero-positive and RT-PCR positive) each with 40 cases and compared to 40 controls.
Table 5.
Comparison of ovarian response and laboratory and pregnancy results between studies in the literature for women sero-positive to HCV
| Authors and year of study | Shaw-Jackson et al.2016b | Hanafi et al. [15]c | Englert et al. [16]d | Yang et al. [18]e | Prisant et al. [17]f | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients sero-positive for HCV with an unknown or negative RT-PCR resulta | |||||||||||||||
| Ovarian response | |||||||||||||||
| Cycles | 37 | 111 | P | 40 | 40 | P | 42 | 84 | P | 90 | 1256 | P | 45 | 45 | P |
| HCV | controls | value | HCV | controls | value | HCV | controls | value | HCV | controls | value | HCV | controls | value | |
| Cycle cancelation | 63% | 77% | 0.52 | 30% | 5% | 0.0001 | 10 | 5 | <0.01 | – | – | – | – | – | – |
| Dose FSH | – | – | – | 28.4 ± 4.6 | 26.1 ± 6.54 | NS | 3610 ± 1563 | 2968 ± 1379 | 0.03 | 2025 | 2625 | 0.269 | – | – | – |
| Length stim | – | – | – | 12.5 ± 1.1 | 12.0 ± 1.5 | ≤0.05 | 11.7 ± 2.4 | 11.5 ± 2.5 | ≥0.05 | 10 | 11 | 0.181 | – | – | – |
| Estradiol max | 1428 | 1620 | 0.27 | 2890 ± 1586 | 4670 ± 1693 | ≤0.05 | 2496 ± 1221 | 2929 ± 1300 | ≥0.05 | – | – | – | – | – | – |
| Oocytes collected | 6.5 | 6.0 | 0.43 | 9.4 ± 4.2 | 12.0 ± 5.2 | ≤0.05 | 9.4 ± 4.7 | 11.3 ± 6.7 | ≥0.05 | 84% | 85% | 0.205 | 5.6 ± 4.9 | 6.0 ± 4.4 | >0.05 |
| P significance | <0.05 | ≤0.05 | – | <0.05 | NS | ||||||||||
| Laboratory and pregnancy results | |||||||||||||||
| Cycles | 25 | 25 | P | 28 | 38 | P | 31 | 78 | P | 90 | 1256 | P | 37 | 42 | P |
| HCV | controls | value | HCV | controls | value | HCV | controls | value | HCV | controls | value | HCV | controls | value | |
| Fertilization rate | 67% | 86% | 0.04 | 32% | 67% | 0.0035 | 56% | 59% | ≥0.05 | 77% | 78% | 0.413 | 71% | 70% | >0.05 |
| Embryo quality | 56% | 48% | 0.36 | 62% | 61% | >0.05 | 2.9 ± 1.3 | 3.1 ± 1.0 | ≥0.05 | 67% | 71% | 0.649 | – | – | – |
| Implantation rate | 6% | 23% | 0.04 | 45% | 52% | NS | 19% | 19% | ≥0.05 | – | – | – | 5% | 10% | >0.05 |
| Pregnancy rate | 12% | 36% | 0.10 | 33% | 48% | >0.05 | 19% | 26% | ≥0.05 | 46% | 55% | 0.138 | 11% | 13% | >0.05 |
| Live births | 2 | 7 | – | 11 | 15 | NS | – | – | – | – | – | – | 2 | 4 | >0.05 |
| P significance | <0.05 | ≤0.05 | <0.05 | NS | ≤0.05 | ||||||||||
Results are presented as percentages, values or mean values ± standard deviation or otherwise specified. P values are provided from individual studies with their level of significance. Length stim length of stimulation (days); Estradiol max the peak value during stimulation; NS not specified.
a All studies were for sero-positive patients with an unknown status for viral replication except for the study of Hanafi et al., where RT-PCR was negative. For the study of Yang et al., the majority of patients were also confirmed to be RT-PCR negative (personal communication).
bCycle cancelation is calculated as the percentage of cancelations due to poor response. Estradiol max, oocytes collected, and fertilization rates are presented as median values without min-max due to lack of space. Embryo quality is assessed for transferred embryos. Clinical pregnancy rates are calculated per transfer
cCycle cancelation is calculated for women with no mature oocytes at retrieval. FSH dose is calculated as the number of ampoules. Clinical pregnancy rates are calculated per cycle
dIn this study, 42 cycles are analyzed without the number of patients being specified
eFSH dose and stimulation length are provided as median values without min-max due to lack of space. Oocytes collected are calculated as percentage of oocytes retrieved. Clinical pregnancy rate is the ratio between the number of pregnancies and the number of periodicities
f22 HCV positive women and 42 control women with 45 oocyte retrievals in both cases were included. Clinical pregnancy rates are calculated per transfer
Table 6.
Comparison of ovarian response and laboratory and pregnancy results between studies in the literature for women RT-PCR positive to HCV
| Authors and year of study | Shaw-Jackson et al. 2016a | Hanafi et al. [15]b | ||||
|---|---|---|---|---|---|---|
| Patients sero-positive for HCV with confirmed viral replication by RT-PCR | ||||||
| Ovarian response | ||||||
| Cycles | 17 HCV | 51 controls | P value | 40 HCV | 40 controls | P value |
| Cycle cancelation | 67% | 83% | 0.52 | 53% | 5% | 0.0001 |
| Dose FSH | – | – | – | 31.6 ± 8.3 | 26.1 ± 6.54 | 0.015 |
| Length Stim | – | – | – | 13.6 ± 1.6 | 12.0 ± 1.5 | 0.311 |
| Estradiol max | 1771 | 1439 | 0.77 | 1280 ± 1317 | 4670 ± 1693 | 0.012 |
| Oocytes collected | 5.5 | 5.5 | 0.93 | 4 ± 4.2 | 12.0 ± 5.2 | 0.001 |
| P significance | <0.05 | ≤0.05 | ||||
| Laboratory and pregnancy results | ||||||
| Cycles | 13 HCV | 13 controls | P-Value | 19 HCV | 38 controls | P value |
| Fertilization rate | 67% | 100% | 0.12 | 28% | 67% | 0.0035 |
| Embryo quality | 62% | 46% | 0.13 | 60% | 61% | >0.05 |
| Implantation rate | 0% | 26% | 0.02 | 33% | 52% | 0.038 |
| Pregnancy rate | 0% | 31% | 0.10 | 5% | 48% | 0.001 |
| Live births | 0 | 4 | – | 2 | 15 | 0.012 |
| P significance | <0.05 | ≤0.05 | ||||
Results are presented as percentages, values or mean values ± standard deviation or otherwise specified. P values are provided from individual studies with their levels of significance. Length stim length of stimulation (days) Estradiol max the peak value during stimulation
aCycle cancelation is calculated as the percentage of cancelations due to poor response. Estradiol max, oocytes collected, and fertilization rates are presented as median values without min-max due to lack of space. Embryo quality is assessed for transferred embryos. Clinical pregnancy rates are calculated per transfer
bCycle cancelation is calculated for women with no mature oocytes at retrieval. FSH dose is calculated as the number of ampoules. Clinical pregnancy rates are calculated per cycle
Discussion
We investigated the possibility that infection of women by the HCV might have a negative impact on IVF outcomes. Only five studies, ours included, addressed this issue, with three indeed observing an adverse effect of HCV infection at different levels including response to stimulation, fertilization, implantation, and pregnancy results. Our own matched controlled study showed significantly reduced fertilization and implantation rates for HCV-infected women compared to matched controls for their first IVF attempt. On the other hand, no adverse effects of the virus on ovarian response to stimulation or on the quality of transferred embryos were observed. The first population of HCV cases that was analyzed comprised patients presenting antibodies to the HCV. We observed a similar result when we isolated a subgroup of patients known to have a positive RT-PCR result at the time of their IVF attempt. Moreover, in this subgroup of patients with active viral replication, no live birth occurred. In the study by Englert and colleagues [16], 45 cycles were included with patients having a history of 3.3 previous cycles. This study equally covered a long period though with matching to control cycles at the closest calendar date. HCV sero-positive patients were shown to have reduced ovarian response to stimulation compared to sero-negative controls [16]. Higher doses of gonadotropins were required to obtain similar peak levels of estradiol and more canceled cycles due to poor response occurred when compared to cycles of uninfected women. These authors did not observe adverse effects of infection though, on fertilization, implantation, or pregnancy rates. In the second study [17], no impact of the HCV was found on IVF outcomes when comparing cycles of sero-positive patients to those of matched control patients. However, clinical pregnancy and implantation rates were surprisingly low for the control group (11 and 10%, respectively). In both these studies, the HCV-infected women were all sero-positive. Results were not analyzed specifically for patients with viral replication. A third and larger study from China [18] considered patients sero-positive to HCV without liver pathology or chronic hepatitis. Matching of patients and controls was limited to age and type of stimulation. No differences were found for ovarian response or pregnancy rates per cycle compared to an uninfected control population. The last study, by Hanafi and colleagues [15], found reduced response to ovarian stimulation and reduced fertilization, implantation, and pregnancy rates for RT-PCR-positive women compared to sero-negative controls. For patients with HCV antibodies without viral replication, a higher number of canceled cycles due to poor ovarian response and lower fertilization rates were observed, though no deleterious effect on pregnancy rates was seen when compared to uninfected controls. High cycle cancellation rates were found for both sero-positive (30%) and RT-PCR-positive (53%) patients in this study.
Heterogeneity of IVF outcomes for HCV-infected women could be explained by various factors. Matching criteria is one possibility, since important variation was found between studies. For example, we matched our patients differently according to the studied outcome and analyzed the impact of viral infection on ovarian response and pregnancy results independently. For analysis of ovarian response, apart from age, date of cycle, ethnical origin, and duration of infertility, only female etiology of sterility was considered since male factor has no influence. On the other hand, for pregnancy outcomes, day of embryo transfer [19] as well as extreme male factor was considered as criteria that might have a strong impact on results. Indeed, we hypothesize that simply matching by male etiology of infertility or by insemination method (IVF versus ICSI) as done in most studies may not be sufficient to distinguish between couples with moderate OAT infertility and extreme male factor. Nevertheless, although we matched patients for the most important criteria, one cannot exclude the possibility that other variables could have been overlooked. For example, over 50% of our studied population was diagnosed with tubal infertility. We did not separate the various tubal etiologies even though this clearly can result in different chances of success. Prevalence of pelvic inflammatory disease was however similar between infected and uninfected patients for whom the data were available.
The type of population studied is another important variable. The general health state of each patient is probably a factor that should be considered for succeeding in conceiving. Studied HCV-infected patients could have positive antibodies with a negative RT-PCR, involving patients who spontaneously cleared the virus or treated patients. For instance, in the study by Yang and colleagues [18], no impact of viral infection on clinical outcomes was observed. Most patients were RT-PCR negative (author’s personal communication) or at early stages of infection, since none of them had chronic infection or liver disease. Patients, on the other hand, could have a positive RT-PCR with various symptoms.
Infection by HCV is indeed known to be accompanied by multiple extrahepatic clinical manifestations including cryoglobulinemia, diabetes, thyroid dysfunction, fatigue, depression, and many other symptoms [20]. Treatment of women prior to IVF with antiviral therapy might therefore improve their general state of health and enhance their chances of pregnancy by clearing hepatic as well as certain extrahepatic manifestations.
Two studies reported reduced ovarian response to stimulation [15, 16]. Altered ovarian function could vary depending on the degree of liver pathology. Modification of the function of granulosa cells is also thought to be one of the mechanisms involved in ovarian dysfunction. The latter cells carry the LDL receptor for the virus though it is not known whether the virus effectively enters and replicates in this cell type [21]. Ovary response to stimulation and possibly oocyte quality and fertilization could therefore be altered in these circumstances.
Chronic inflammation is an important factor in several extrahepatic symptoms. The study of Hanafi and colleagues [15] and ours, in which viral replication was confirmed, both observed reduced implantation despite the transfer of good quality embryos. Recent data in the literature suggest that implantation failure in IVF patients may be hindered by a deregulation in the immune profile [22].
For implantation to occur, tolerance of the fetal semi-allograft is shown to be accompanied by a local switch from the adaptive Th1 to the innate Th2 type. In patients with recurrent implantation failure, an overactivation or a low immune profile was detected [22]. Treatment to restore immune balance has shown encouraging results. Indeed, natural killer cells in the uterine environment promote angiogenesis and placental growth. Modification of the Th1/Th2 ratio and cytokine profile can however result in natural killer cells becoming cytotoxic with ensuing embryo rejection and endometrium destruction [22]. Interestingly, different species of helminthes for example were found to be associated with contrasting effects on fecundity in humans, with one species enhancing fecundity possibly by inducing an immune profile similar to the one found during implantation [23]. In the case of HCV, it would be interesting to study the uterine immune profile in patients with reduced implantation to see if they could possibly benefit from protocols involving cytokine regulation.
Our matched controlled study is hampered by some limitations. Sample size was small since we limited the study to the first IVF attempt for each patient to avoid a methodology problem during matching to controls in subsequent cycles. Additionally, we excluded patients or patients whose partners had a co-infection. Our study was equally retrospective and covered a long period. Nevertheless, we studied sero-positive as well as RT-PCR-positive patients, and matching to controls was performed carefully.
The systematic literature review shows that three out of five studies describe reduced IVF clinical outcomes for HCV-infected women, though at various levels. More and larger studies with well-defined groups are needed to clarify the eventual impact of the HCV on women treated for infertility. Data concerning the infectious status of a patient as well as her health state should be systematically recorded. A thorough knowledge of a patient’s medical history as well as a multi-disciplinary approach could help to decide which patients might benefit from treatment prior to starting ART
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 176 kb)
(PDF 175 kb)
Acknowledgments
The authors are grateful to Jean-Pierre Mulkay (gastro-enterologist) for participating in scientific discussions.
Authors’ contributions
C. Shaw-Jackson wrote the manuscript, participated in patient matching, clinical work, and in data analysis. M. Capraro participated in patient matching, data extraction, and analysis. L. Ameye performed the statistical analysis. J. Vandromme designed the program for patient matching. C. Autin participated in clinical work. S. Rozenberg and Y. Manigart participated in data extraction and analysis. All authors contributed to scientific discussions and revised the manuscript.
Compliance with ethical standards
All our protocols have been approved by the local Ethics Committee and all our patients have given their informed, written consent prior to treatment.
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-017-0892-8) contains supplementary material, which is available to authorized users.
References
- 1.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5(9):558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 2.Ghosn J, Leruez-Ville M, Chaix ML. Transmission sexuelle du virus de l’hépatite C. Presse Med. 2005;34(14):1034–1038. doi: 10.1016/S0755-4982(05)84106-5. [DOI] [PubMed] [Google Scholar]
- 3.Ghany MG, Strader DB, Thomas DL, Seeff LB. American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gara N, Ghany MG. What the infectious disease physician needs to know about pegylated interferon and ribavirin. Clin Infect Dis. 2013;56(11):1629–1636. doi: 10.1093/cid/cit074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feld JJ, Jacobson IM, Hézode C, et al. ASTRAL-1 investigators. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015;373(27):2599–2607. doi: 10.1056/NEJMoa1512610. [DOI] [PubMed] [Google Scholar]
- 6.Rosenthal ES. Graham CS Price and affordability of direct-acting antiviral regimens for hepatitis C virus in the United States. Infect Agent Cancer. 2016;11:24. doi: 10.1186/s13027-016-0071-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durazzo M, Premoli A, Di Bisceglie C, et al. Alterations of seminal and hormonal parameters: an extrahepatic manifestation of HCV infection? World J Gastroenterol. 2006;12(19):3073–3076. doi: 10.3748/wjg.v12.i19.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofny ER, Ali ME, Taha EA, et al. Semen and hormonal parameters in men with chronic hepatitis C infection. Fertil Steril. 2011;95(8):2557–2559. doi: 10.1016/j.fertnstert.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Lorusso F, Palmisano M, Chironna M, et al. Impact of chronic viral diseases on semen parameters. Andrologia. 2010;42(2):121–126. doi: 10.1111/j.1439-0272.2009.00970.x. [DOI] [PubMed] [Google Scholar]
- 10.Moretti E, Federico MG, Giannerini V, Collodel G. Sperm ultrastructure and meiotic segregation in a group of patients with chronic hepatitis B and C. Andrologia. 2008;40(3):173–178. doi: 10.1111/j.1439-0272.2007.00818.x. [DOI] [PubMed] [Google Scholar]
- 11.Pasquier C, Daudin M, Righi L, et al. Sperm washing and virus nucleic acid detection to reduce HIV and hepatitis C virus transmission in serodiscordant couples wishing to have children. AIDS. 2000;14(14):2093–2099. doi: 10.1097/00002030-200009290-00004. [DOI] [PubMed] [Google Scholar]
- 12.Bourlet T, Levy R, Maertens A, et al. Detection and characterization of hepatitis C virus RNA in seminal plasma and spermatozoon fractions of semen from patients attempting medically assisted conception. J Clin Microbiol. 2002;40(9):3252–3255. doi: 10.1128/JCM.40.9.3252-3255.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levy R, Tardy JC, Bourlet T, et al. Transmission risk of hepatitis C virus in assisted reproductive techniques. Hum Reprod. 2000;15(4):810–816. doi: 10.1093/humrep/15.4.810. [DOI] [PubMed] [Google Scholar]
- 14.Shaw-Jackson C, Bertrand E, Becker B, et al. Vitrification of blastocysts derived from fair to poor quality cleavage stage embryos can produce high pregnancy rates after warming. J Assist Reprod Genet. 2013;30(8):1035–1042. doi: 10.1007/s10815-013-0037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanafi NF, Abo Ali AH. Abo el kheir HF. ICSI outcome in women who have positive PCR result for hepatitis C virus. Hum Reprod. 2011;26(1):143–7. doi:10.1093/humrep/deq317. [DOI] [PubMed]
- 16.Englert Y, Moens E, Vannin AS, Liesnard C, Emiliani S, Delbaere A, et al. Impaired ovarian stimulation during in vitro fertilization in women who are seropositive for hepatitis C virus and seronegative for human immunodeficiency virus. Fertil Steril. 2007;88(3):607–11. [DOI] [PubMed]
- 17.Prisant N, Tubiana R, Lefebvre G, Lebray P, Marcelin AG, Thibault V, et al. HIV-1 or hepatitis C chronic infection in serodiscordant infertile couples has no impact on infertility treatment outcome. Fertil Steril. 2010;93(3):1020–3. doi:10.1016/j.fertnstert.2009.07.1663. [DOI] [PubMed]
- 18.Yang L, Zhao R, Zheng Y, Song X. Effect of hepatitis C virus infection on the outcomes of in vitro fertilization. Int J Clin Exp Med. 2015;8(4):6230–5. [PMC free article] [PubMed]
- 19.Blake DA, Farquhar CM, Johnson N, Proctor M. Cleavage stage versus blastocyst stage embryo transfer in assisted conception. Cochrane Database Syst Rev. 2011; Oct 17;(4):CD002118. Review.Update in: Cochrane Database Syst Rev. 2012;7:CD002118. [DOI] [PubMed]
- 20.Gill K, Ghazinian H, Manch R, Gish R. Hepatitis C virus as a systemic disease: reaching beyond the liver. Hepatol Int. 2016;10(3):415–423. doi: 10.1007/s12072-015-9684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devaux A, Soula V, Sifer C, et al. Hepatitis C virus detection in follicular fluid and culture media from HCV+ women, and viral risk during IVF procedures. Hum Reprod. 2003;18(11):2342–2349. doi: 10.1093/humrep/deg431. [DOI] [PubMed] [Google Scholar]
- 22.Lédée N, Petitbarat M, Chevrier L, et al. The uterine immune profile may help women with repeated unexplained embryo implantation failure after in vitro fertilization. Am J Reprod Immunol. 2016;75(3):388–401. doi: 10.1111/aji.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blackwell AD, Tamayo MA, Beheim B, et al. Helminth infection, fecundity, and age of first pregnancy in women. Science. 2015;350(6263):970–972. doi: 10.1126/science.aac7902. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 176 kb)
(PDF 175 kb)