Abstract
Purpose
We aimed to determine the developmental potential of human reconstructed oocytes after polar body genome transfer (PBT) and to report the case of a woman with multiple cycles of severe embryo fragmentation.
Methods
Fresh and cryopreserved first polar bodies (PB1s) were transferred to enucleated metaphase II oocytes (PB1T), while fresh PB2s were removed from fertilized oocytes and used instead of the female pronucleus in donor zygotes. Reconstructed oocytes underwent intracytoplasmic sperm injection (ICSI) and were cultured to blastocyst. Biopsied trophectoderm cells of PBT-derived blastocysts were screened for chromosomes by next-generation sequencing (NGS). Then, cryopreserved PB1T was carried out in one woman with a history of several cycles of extensive embryo fragmentation, and the blastocysts derived from PB1T were screened for aneuploidy but not transferred to the patient.
Results
There were no significant differences in the rates of normal fertilization and blastocyst formation between fresh and cryopreserved PB1T and control oocytes. Of the three fresh and three cryopreserved PB1T-derived blastocysts, two and one blastocysts exhibited normal diploidy respectively. In contrast, 17 PB2 transfers yielded 16 two pronuclei (2PN) zygotes with one normal and one small-sized pronucleus each and no blastocyst formation. In the female patient, 18 oocytes were inseminated by ICSI in the fourth cycle and the PB1s were biopsied. Although the embryos developed from the patient’s own oocytes showed severe fragmentation, the oocytes reconstructed after PB1T produced three chromosomally normal blastocysts.
Conclusions
Normal blastocysts can develop from human reconstructed oocytes after PB1T. The application of the first PB transfers may be beneficial to patients with a history of poor embryo development and excessive fragmentation.
Keywords: Polar body transfer, Blastocyst, Embryo fragmentation, Assisted reproductive technique
Introduction
Polar bodies are formed during the process of oocyte meiotic division. They are considerably smaller than the oocytes and are dispensable for subsequent embryo development. The first polar body (PB1) degenerates within hours of formation, whereas the second polar body (PB2) remains intact and persists through the early blastocyst stage [1]. Chromosomes are the main contents of polar bodies. PB1 contains a subset of bivalent chromosomes, whereas PB2 contains a haploid set of chromatids. In addition to chromosomes, the polar body also contains cortical granules, ribosomes, Golgi complexes, mitochondria, and other cytoplasmic materials [2].
As the polar body contains chromosomes complementary to the oocyte, it has several benefits in assisted reproductive technology (ART). The application of polar bodies in preimplantation genetic diagnosis (PGD) is well known. PB1 biopsy was applied for PGD early in 1990 to detect genetic abnormalities in oocytes [3]. Another application of the polar body is polar body transfer (PBT), which involves removing PB1 from unfertilized oocytes and transferring it to an enucleated donor oocyte (PB1T) or removing PB2 after fertilization and substituting it for the female pronucleus in the donor zygote (PB2T). Both PB1T and PB2T have been proven to produce offspring in mice [4, 5]. A recent study showed that PBT could be used to prevent the transmission of inherited mitochondrial DNA (mtDNA) diseases [6]. Researchers have found that the reconstructed oocytes after PB1T and PB2T developed into blastocysts and living pups with high efficiency. Thus far, researchers have only researched PBT in mouse models, and its feasibility in human oocytes is unclear.
Currently, PBT has only been applied to prevent mtDNA diseases [7]. However, some conditions encountered in ART, including severe embryo fragmentation and delayed embryo development, may also benefit from nuclear replacement therapy. Only diseases caused by cytoplasmic defects can be treated by this therapy; however, the mechanisms of these diseases are complicated and remain elusive. Accordingly, several studies on ooplasmic transplantation have revealed that the developmental potential of patients experiencing multiple cycles of severe embryo fragmentation or slow cleavage can be restored by transfer of ooplasm from the donor oocyte [8–10].
Fragmentation is defined as the presence of anucleate structures derived from a blastomere [11]. It is a common phenomenon in human preimplantation embryos. The pathogenesis of embryo fragmentation is unknown, and a number of hypotheses have been proposed [12]. Although embryo fragmentation and sperm DNA oxidation have been shown to be correlated [13], many studies indicate that fragmentation is of oocyte origin [14, 15], and studies have focused mainly on apoptosis. Fragmented embryos show increased annexin V staining and terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling staining [16, 17]; moreover, embryo fragmentation is associated with apoptotic granulosa cells [18]. Another hypothesis considers fragmentation as a deviant form of cytokinesis, which is closely affected by the cytoskeleton [19]. The relationship between high-density lipoprotein metabolism and embryo fragmentation has also been reported [20]. It has been demonstrated that a high degree of fragmentation negatively correlates with the developmental potential of embryos [21, 22]. Severely fragmented embryos are less likely to be viable, despite microsurgical removal of the fragments [23].
In this study, we investigated the developmental potential of human oocytes after PBT and reported PBT in one patient with severe embryo fragmentation who underwent four cycles of ART.
Materials and methods
Materials
This study was approved by the Ethics Committee of the Reproductive and Genetic Hospital of CITIC-Xiangya (reference LL-SC-SG-2015-004). Between September 2015 and April 2016, immature metaphase I (MI) oocytes from intracytoplasmic sperm injection (ICSI) cycles were collected for in vitro maturation. The immature oocytes had been donated by couples who had signed informed consent forms for PBT research. To investigate the developmental potential of embryos after PBT, sperm samples were obtained from the sperm bank of the Reproductive and Genetic Hospital of CITIC-Xiangya for use in the ICSI of in vitro matured oocytes.
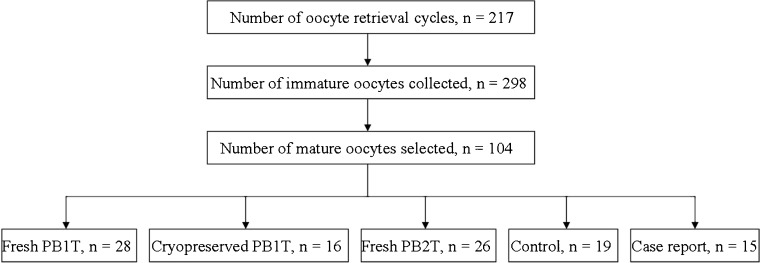
Oocytes in the MI stage were placed in maturation medium (G1.5 with 5% human serum albumin; Vitrolife, Göteborg, Sweden). Only those oocytes that reached metaphase II (MII) after 4–6 h of incubation and exhibited normal morphology (spherical structure enclosed by uniform zona pellucida, with uniform translucent cytoplasm free of inclusions, and a size-appropriate PB1) were selected. In this study, a total of 298 immature metaphase I oocytes from 217 oocyte retrieval cycles were collected, and 104 in vitro matured oocytes were selected for experiment. The allocation of the experimental oocytes into different groups are presented in Fig. 1.
Fig. 1.
Basic information of the experimental oocytes
PB1T
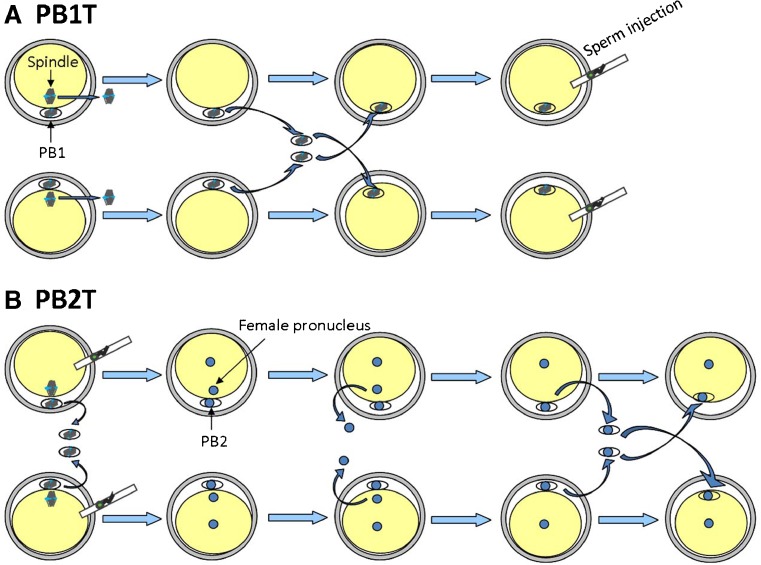
In this experiment, both fresh and cryopreserved PB1 transfers were carried out in matured MII oocytes. Fresh PB1T was performed when more than one MII oocytes were obtained on 1 day. For fresh PB1T (Fig. 2a), in vitro matured MII oocytes were placed into G-MOPS (Vitrolife) in an ICSI petri dish, and the PB1 of each oocyte was removed using a pipette (internal diameter 13–15 μm). The oocytes were immediately prepared for enucleation after PB1 biopsy, and enucleation was performed as described previously [24]. Briefly, oocytes were transferred to G-MOPS supplemented with 5 μg/mL of cytochalasin B for 5–10 min before removal of the meiotic spindle. The spindle was visualized through a Polescope Imaging System (Cambridge Research & Instrumentation Inc.). The zona pellucida was ablated at the 3 o’clock position by a Zilos TK laser (Hamilton Thorne, MA, USA), and the meiotic spindle was removed using an enucleation pipette (internal diameter 13–15 μm). Then, an enucleated oocyte was fused with the PB1 from another oocyte (n = 24). The PB1 was placed into inactivated SeV extract (HVJ-E) for approximately 10 s and was placed into the perivitelline space of the enucleated oocytes. After the PB1s and enucleated oocytes were fused, the reconstructed oocytes were inseminated by ICSI and cultured in sequential culture medium (Vitrolife) in a time-lapse incubator (EmbryoScope, Unisense Fertilitech, Aarhus, Denmark) at 37 °C in the presence of 6% CO2 and 5% O2 until they reached the blastocyst stage.
Fig. 2.
Schematic drawing showing approaches of polar body (PB) transfer. a PB1T: oocytes are enucleated and the first PBs (PB1s) are biopsied. Then, the PB1s are interchanged and fused with the enucleated oocytes. The reconstituted oocytes are inseminated by ICSI. b PB2T: oocytes are inseminated by ICSI and the PB1s are removed simultaneously. After fertilization, the female pronuclei are removed, and then the PB2s are biopsied and fused with the enucleated zygotes
For cryopreserved PB1T, biopsied PB1s were placed into the empty zona pellucida from discarded embryos (unfertilized or poor-quality embryos, obtained with signed informed consent). The zona pellucida containing PB1s was vitrified using a Kitazato vitrification kit (Kitazato Biopharma, Shizuoka, Japan) in combination with closed High Security Vitrification Straws (Cryo Bio System, France). Vitrification was performed according to the manufacturer’s protocols. The PB1s were stored in liquid nitrogen until transfer to a fresh matured oocyte. After enucleation of the fresh oocytes, previously stored PB1s were warmed using commercially available warming solution (Kitazato Biopharma). The warmed, equilibrated PB1s (n = 9) were fused with fresh enucleated oocytes through HVJ-E mediation, and then the reconstructed oocytes were inseminated by ICSI and cultured to the blastocyst stage in an EmbryoScope.
PB2T
Fresh PB2T was performed when more than one MII oocyte was obtained on 1 day (Fig. 2b). The oocytes were inseminated by ICSI, the PB1s were simultaneously removed, and the inseminated oocytes were cultured in an EmbryoScope. The pronuclei of fertilized oocytes were carefully observed under an inverted microscope every 30 min after extrusion of PB2. As long as the pronuclei could be clearly identified, the female pronucleus was removed by an ICSI needle (Humagen, USA). The pronucleus that was the nearest to the PB2 was considered the female pronucleus, and the movement trajectory of the female pronucleus from the meiotic spindle could be observed by time-lapse monitoring, which helped identify its position. Then, an enucleated zygote was fused with the PB2 from another zygote. The PB2s were biopsied and fused with the enucleated zygotes through HVJ-E mediation, and the reconstructed zygotes were cultured to the blastocyst stage in the EmbryoScope.
Case report
A 32-year-old female patient with idiopathic infertility agreed to experimental treatment. Before she first sought infertility treatment at our center, she had undergone one in vitro fertilization (IVF) and one ICSI cycle in another center, and 18 and 12 oocytes were retrieved from those cycles, respectively. Both cycles exhibited normal fertilization but poor embryo quality, and no embryos were transferred. Both the patient and her husband approved of all the treatments and analyses carried out in this study by signing an informed consent.
In the patient’s first cycle at our center, the patient was stimulated with an ultra-long downregulation protocol [25]. The oocytes were inseminated with her husband’s sperm, which was characterized by normal count and progressive motility. Embryos were checked for the appearance of pronuclei 17 h after insemination and then cultured to day 5. Before the second cycle, to investigate the paternal effect on embryo quality, the husband’s sperm was used to inseminate donor in vitro matured oocytes and the developmental potential of embryos was observed. The GnRH antagonist protocol was applied in the second cycle. The oocytes were inseminated by ICSI and the PB1s were biopsied. PB1s were transferred into empty zona pellucida (one PB1 per zona pellucida) and vitrified. After verifying fertilization, the fertilized oocytes were cultured in a Primo Vision time-lapse system (Vitrolife) and the best quality embryos were chosen for transfer. When in vitro matured oocytes were provided, the corresponding amount of cryopreserved PB1s were warmed for PB1T to the in vitro matured oocytes (Fig. 3). The reconstructed oocytes were inseminated with the husband’s sperm and cultured to blastocyst stage in the EmbryoScope. The blastocysts that developed from reconstructed oocytes were not transferred to the patient.
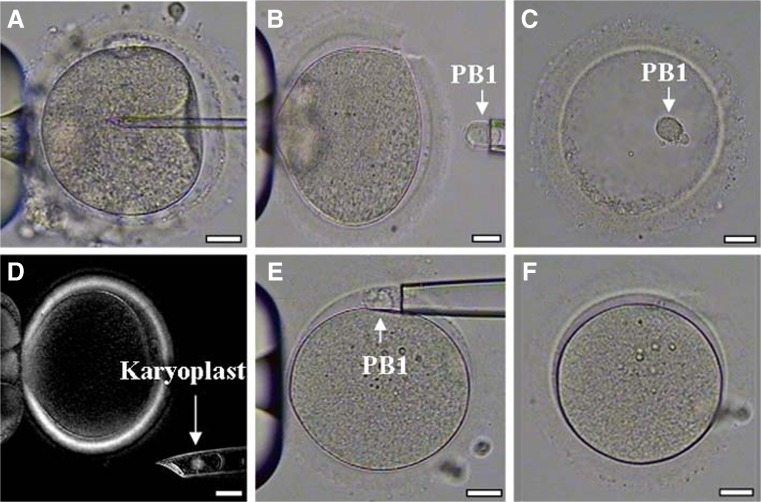
Fig. 3.
Polar body 1 transfer (PB1T) procedure. a Intracytoplasmic sperm injection (ICSI) of a patient’s oocyte. b The first polar body (PB1) was retrieved from the patient’s oocyte. c PB1 was placed into an empty zona pellucida for subsequent cryopreservation. d The spindle of a donor oocyte was visualized and removed under a polarized microscope. e Warmed PB1 derived from patient’s oocyte was transferred to the perivitelline space of the enucleated donor oocyte. f PB1 was fused with the cytoplast of the donor oocyte. Scale bars, 25 μm for all panels
Chromosome analysis of blastocysts
Trophectoderm biopsy was performed on the reconstructed blastocysts, and the biopsied trophectoderm cells were screened for chromosomes by next-generation sequencing (NGS). Both trophectoderm biopsy and NGS were performed as previously described [26, 27].
Statistical analysis
Categorical variables were presented as percentages and compared using chi-square or Fisher’s exact tests. Maternal age was presented as mean ± SD and compared using one-way ANOVA. Analyses were performed using the statistical package SPSS, version 19.0 (SPSS, Chicago, IL, USA).
Results
Efficiency of PB1T
A total of 28 intact PB1s were retrieved from 28 in vitro matured oocytes for fresh PB1T transfer. Of these, 24 (85.7%) oocytes survived enucleation, 22 (91.7%) PB1s fused with enucleated oocytes, and 21 (95.5%) reconstructed oocytes survived ICSI. After fertilization, 13 (61.9%) zygotes exhibited two pronuclei (2PN), resulting in 4 (30.8%) blastocysts (Table 1).
Table 1.
Developmental potential of reconstructed oocytes after PB1T and PB2T
| Treatment | No. oocytes | Average maternal age (years) | Survived enucleation (%) | Fusion (%) | Survived ICSI (%) | Normal fertilization (%) | Blastocyst formation (%) |
|---|---|---|---|---|---|---|---|
| Fresh PB1T | 28 | 29.7 ± 3.9c | 24 (85.7)c | 22 (91.7)c | 21 (95.5)c | 13 (61.9)c | 4 (30.8)c |
| Cryopreserved PB1T | 10 | 30.7 ± 3.3c | 9 (90.0)c | 8 (88.9)c | 8 (100.0)c | 6 (75.0)c | 3 (50.0)c |
| Fresh PB2T | 18b | 31.0 ± 3.4c | 17 (94.4)c | 17 (100.0)c | – | – | 0d |
| Controla | 19 | 31.4 ± 4.2c | – | – | 19 (100.0)c | 16 (84.2)c | 7 (43.8)c |
aIn vitro matured oocytes inseminated by intracytoplasmic sperm injection (ICSI) without polar body transfer (PBT) were set as the control
bA total of 26 oocytes were used for fresh PB2T, and 18 zygotes presented with two pronuclei 7–8 h after ICSI
c,dValues with different superscripts (c and d) in the same column are significantly different (P < 0.05)
A total of 16 in vitro matured oocytes were used for cryopreserved PB1T; among them, 6 oocytes were only used for PB1 collection, and 10 oocytes were used for both PB1 collection (vitrified for the next round of experiment) and oocyte enucleation. Nine out of 10 (90.0%) fresh in vitro matured oocytes were successfully enucleated. Of the 16 PB1s that were vitrified, 10 were selected for warming and 9 (90.0%) survived. Then, 8 (88.9%) warmed PB1s were successfully fused with the enucleated oocytes, and all the reconstructed oocytes survived ICSI. There were no significant differences in the rates of normal fertilization (61.9, 75.0, and 84.2%, respectively) and blastocyst formation (30.8, 50.0, and 43.8%, respectively) between fresh or cryopreserved PB1Ts and control oocytes (Table 1).
Efficiency of PB2T
A total of 26 in vitro matured oocytes were used for fresh PB2-pronucleus exchange. After ICSI, 18 (69.2%) zygotes clearly presented with two small pronuclei 7–8 h later. The median time interval between extrusion of the PB2 and emergence of pronuclei was 3.3 (range 2.3–5.2) h. A total of 17 (94.4%) zygotes survived after the female pronucleus was removed. All 17 PB2s were successfully biopsied and fused with enucleated zygotes. About 10 h later, 16 (94.1%) zygotes exhibited 2PN, but all of the 2PN zygotes possessed a normal size pronucleus and a much smaller pronucleus (Fig. 4 (a)), and no blastocyst formation was observed (Table 1).
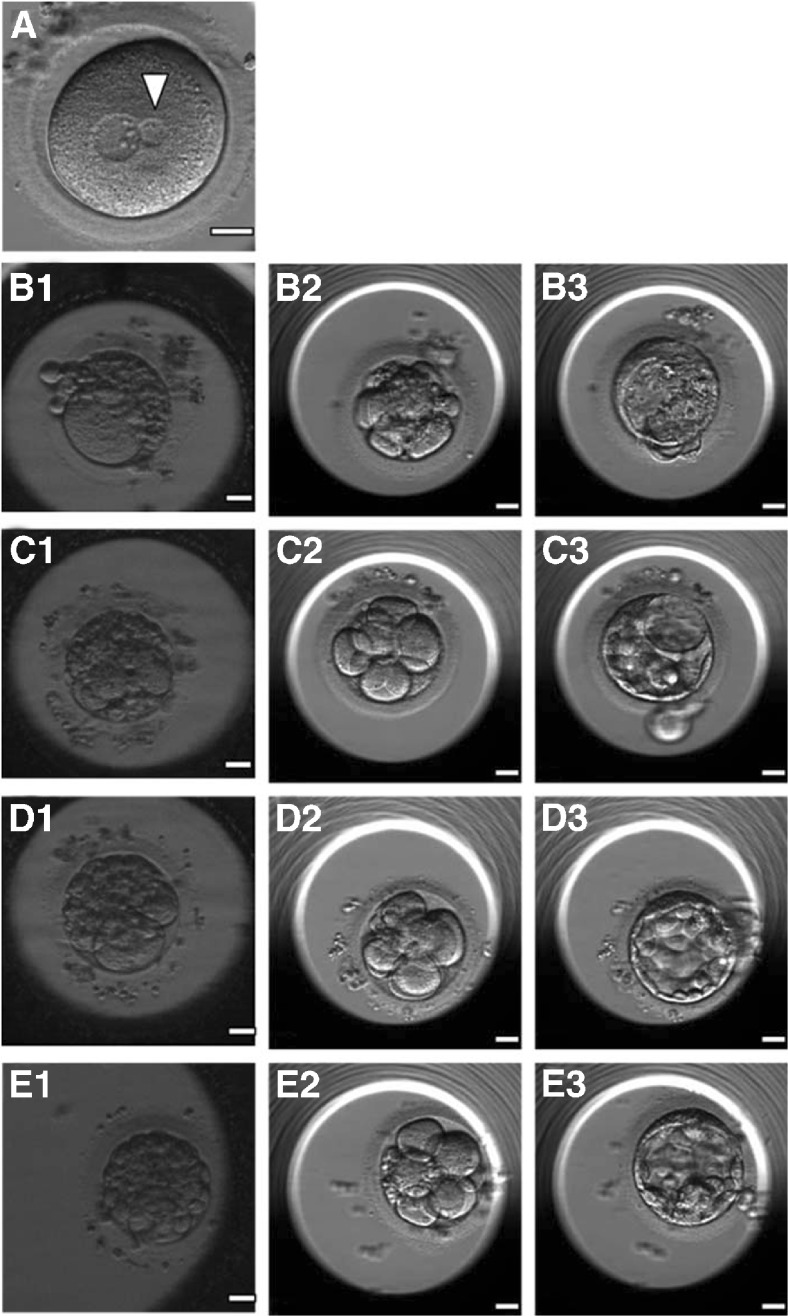
Fig. 4.
a A 2PN zygote with a normal-sized pronucleus and a much smaller pronucleus (arrowhead) after polar body 2 transfer (PB2T). (b1, c1, d1, and e1) Day 3 embryos developing from the patient’s oocytes showed massive fragmentation, and the embryos reconstructed after PB1T of the four oocytes showed slight fragmentation on day 3 (b2, c2, d2, and e2) and developed to blastocyst stage (b3, c3, d3, and e3)
Case report
A total of 18 oocytes were retrieved in the first cycle at our center, and all 18 oocytes exhibited 2PN. On day 3, all the embryos possessed few cells and exhibited severe fragmentation. The embryos were cultured to day 5: no blastocyst formation occurred and none of the embryos were transferred. Whereas, the insemination of five control donor oocytes with husband sperm resulted in three blastocysts with little fragmentation observed.
A total of 24 oocytes were retrieved in the second cycle, of which 18 MII oocytes were inseminated by ICSI. Their PB1s were biopsied and cryopreserved. Sixteen oocytes revealed 2PN at fertilization check, while the other two oocytes failed to exhibit PN. At the first cleavage, 15 (93.8%) fertilized oocytes presented a series of erratic cytoplasmic and membrane movements during or after cell division, and exhibited massive fragmentation. Two embryos were transferred on day 3, but no pregnancy was established. The other embryos were cultured to day 5 and no blastocysts formed. For cryopreserved PB1T, 15 (83.3%) cryopreserved PB1s survived after warming. Fifteen in vitro matured oocytes were donated by 15 couples (average age 29.5 ± 3.7 years). The donor oocytes were enucleated and fused with the warmed PB1s. After ICSI with the husband’s sperm, seven reconstructed oocytes exhibited 2PN, and four (57.1%) of the zygotes showed normal cytoplasmic movement at the first cleavage, resulting in four blastocysts (Fig. 4 (b–e)). When compared with the patient’s own embryos (Table 2), the PB1T-derived embryos had a lower fragmentation rate and a higher blastocyst formation rate (P < 0.05).
Table 2.
Embryo developmental potential of patient’s oocytes and PB1T-derived oocytes
| Patient’s oocytes | PB1T-derived oocytes | ||||||
|---|---|---|---|---|---|---|---|
| Oocyte/PB1 numbera | Fertilizationb | Day 3 embryo fragmentationc | Blastocyst formation | Fertilizationd | Day 3 embryo fragmentation | Blastocyst formatione | Blastocyst karyotype |
| 1 | 0PN/PB2 | C | No | 3PN/PB2 | D | No | |
| 2 | 2PN/PB2 | D | No | – | |||
| 3 | 2PN/PB2 | D | No | 2PN/PB2 | B | Yes (3CC) | 44,XY,-2,-15 |
| 4 | 2PN/PB2 | C | – | 2PN/PB2 | A | Yes (3BB) | 46,XY |
| 5 | 2PN/PB2 | C | No | Degenerated | – | ||
| 6 | 2PN/PB2 | C | No | 1PN/no PB2 | D | No | |
| 7 | 2PN/PB2 | D | No | 3PN/no PB2 | A | Yes (3CC) | Not determined |
| 8 | 2PN/PB2 | C | No | – | |||
| 9 | 2PN/PB2 | C | No | 2PN/PB2 | A | Yes (3BB) | 46,XX |
| 10 | 2PN/PB2 | D | No | 3PN/PB2 | B | Yes (3CB) | Not determined |
| 11 | 2PN/PB2 | D | No | 2PN/PB2 | C | No | |
| 12 | 2PN/PB2 | C | – | 2PN/PB2 | D | No | |
| 13 | 2PN/PB2 | D | No | 2PN/PB2 | D | No | |
| 14 | 2PN/PB2 | D | No | 2PN/PB2 | A | Yes (3BB) | 46,XX |
| 15 | 2PN/PB2 | D | No | Degenerated | – | ||
| 16 | 2PN/PB2 | C | No | 0PN/PB2 | C | No | |
| 17 | 2PN/PB2 | C | No | – | |||
| 18 | 0PN/no PB2 | – | – | 1PN/PB2 | Not cleaved | – | |
aThe developmental potential of each of the patient’s oocyte and its PB1-derived reconstructed oocyte was compared in the same row
bFertilization outcome was represented as pronucleus (PN) number/existing the second polar body (PB2)
cThe degrees of fragmentation in day 3 embryos were divided into four grades (A <10%; B 10–30%; C 30–50%; D >50%). Two embryos (nos. 4 and 12) were transferred on day 3 but no pregnancy was established
dThree PB1s (nos. 2, 8, and 17) have not survived after warming and had no fertilization outcome
eThe Gardner score of day 5 blastocysts was included in parentheses
NGS analysis of the PBT-derived blastocysts
Of the four blastocysts derived from fresh PB1T, three expressed results after NGS. Of these, two showed normal diploidy, and the third blastocyst had the karyotype 47,XX,+22. The three blastocysts obtained after cryopreserved PB1T yielded one diploid and two aneuploid karyotypes (48,XX,+1,+13 and 46,XX/45,XX,–17). In contrast, the case study blastocysts resulted in three diploid and one aneuploid karyotype (44 XY,-2,-15).
Discussion
The results of the present study revealed that fresh and cryopreserved PB1T, but not PB2T, could successfully generate normal fertilized zygotes with a high efficiency for developing into blastocysts. PB1T can restore the blastocyst formation capability of oocytes in a case of patient who experience repeated embryo fragmentation.
Recently, sequencing analyses of the genomes of PB1s and PB2s and the maternal pronuclei of human oocytes revealed the genome integrity of all the three products of meiosis [28]. In another study, Wang et al. [6] used array comparative genomic hybridization and reported that the PB1 and its counterpart, the spindle-chromosome complex in the human oocyte, have similar chromosome copy number without genomic alterations. Likewise, PB2 has a haploid genome as the maternal pronucleus. These research studies provide the rationales for the participation of PB1 and PB2 in normal embryonic development. PB1T has been shown to be capable of producing normal offspring [5] and generating PB1-derived parthenogenetic embryo stem cell lines in mice [29]. Our research further illustrates that blastocysts can develop from human reconstructed oocytes after PB1T; moreover, a high blastocyst formation rate was attained from cryopreserved PB1T. The use of frozen PB1s has been reported in a porcine PBT model, and it was found to result in successful fertilization and embryonic development [30]. Cryopreserved PB1T can be useful in the clinical setting because of the scarcity of donor oocytes; therefore, cryopreservation of the PB1s is desirable for future PB1T.
Wang et al. [6] showed that after substituting PB2 for the maternal pronucleus, reconstructed zygotes in mice can develop to the blastocyst stage and give rise to normal offspring. However, no blastocyst formation was achieved in this study when PB2 was used to replace the pronucleus in human zygotes. In mouse PB2T experiments, the reconstructed zygote has been found to contain a large male pronucleus and a small PB2-derived female pronucleus, similar to that observed in the present study. At the first mitosis, the PB2-derived pronucleus remained small, and chromosome staining revealed premature condensation of the PB2 chromosomes. A high developmental rate to the blastocyst stage has been reported when young recipient zygotes (short time interval after PB2 extrusion) were used for PB2T, and the developmental potential of reconstructed zygotes was found to decrease with the age of the recipient zygotes [4, 31]. In another study, it was suggested that after PB2 extrusion, the PB2 cell cycle progressed more slowly than that of the zygote, perhaps because lower mRNA levels are involved in the cell cycle progression of PB2 [32]. If the PB2 is transferred into aged recipient zygotes, the PB2-derived pronucleus seems unable to catch up with the zygote cell cycle and may not complete DNA replication at the first mitosis, resulting in the observed poor embryonic development. In this study, we replaced the female pronucleus with PB2T as soon as the pronuclei were visible. The time interval between PB2 extrusion and PB2T is short. A small pronucleus, most likely derived from the PB2, is still inevitable in the reconstructed zygotes, and the developmental potential of PB2T-derived embryos remained poor in this research. Further studies are required to address this question.
We selected a couple who previously experienced several cycles of severe embryo fragmentation to test the effect of PBT. Blastocysts with little fragmentation were obtained when donor oocytes were inseminated by the husband’s sperm, indicating that the embryo fragmentation was of maternal origin. Successful treatments of ooplasmic transfer for patients with previous embryo fragmentation have suggested that cytoplasmic deficiency is the cause of fragmentation [33]. We speculated that the nucleus of the oocyte or PB1 can support normal embryonic development if donor ooplasm is provided, and that cryopreserved PB1T is preferred for this preclinical application. In the treatment cycle, nearly all the patient’s own fertilized oocytes presented erratic cleavage patterns with distorted cytoplasmic movements, resulting in severe fragmentation. Distorted cytoplasmic movements at cleavage have been reported in two studies [34, 35], and both revealed the poor prognoses of embryos exhibiting this phenomenon. After PB1T with cryopreserved PB1s, four of the seven 2PN zygotes were free of erratic cleavage patterns, and all zygotes developed into blastocysts with minimal fragmentation. These results suggest that the donor ooplasm can rescue the poor developmental potential of the patient’s oocytes. However, as the pathogenesis of embryo fragmentation remains unclear, the underlying mechanism by which the donor ooplasm rescues the oocyte’s developmental potential is currently unknown [12].
In this study, one out of three (33.3%) blastocysts derived from fresh PB1T and three out of seven (42.9%) blastocysts derived from cryopreserved PB1T experienced aneuploidy. The aneuploidy rate in human blastocysts is approximately 50.0% [36, 37]. Errors in chromosome segregation during oocyte meiosis and mitosis of the early embryo have been reported to cause embryo aneuploidy [38, 39]. As the counterpart of oocytes in meiosis, an aneuploid PB1 can be the origin of the aneuploid PB1T-derived blastocysts. It is well known that the incidence of oocyte aneuploidy increases with advanced age of the woman [40]. Considering the young age of oocyte donors in this study, the probability of aneuploid PB1 may be low. In addition, aneuploidy could be caused by manipulation-induced strain on chromosomes during PB1T, such as PB1 biopsy, cryopreservation, fusion with ooplasm, and the formation of a functional spindle. Further research is required to determine the extent to which these manipulations contribute to the incidence of aneuploidy. In the future, comprehensive chromosome screening will be needed to avoid aneuploidy following PBT.
Various nuclear transfer techniques, including spindle-chromosome transfer (ST), pronuclear transfer (PNT), germinal vesicle transfer (GVT), and PBT, have been applied for ART in recent years. One of the attractive applications of nuclear transfer is to prevent transmission of mtDNA disease, as PBs contain few mitochondria and PBT may reduce the carryover of mtDNA compared to the ST and PNT techniques [6]. Apart from its applications in reducing the transmission of mtDNA disease, PNT was used to achieve successful pregnancy in a woman with a history of recurrent embryonic developmental arrest [41], and GVT was applied to overcome chromosome abnormalities in oocytes from patients with diminished ovarian reserve [42]. Our research on PBT provides another potential alternative for treating patients with ooplasmic deficiency. A technical convenience of PBT is that the nuclear DNA of the PB is contained within a portion of the cell membrane, facilitating the removal of the karyoplast, thereby reducing chances of damaging the karyoplast [7]. Further studies are needed to improve the efficiency and investigate the safety of PBT technology. For example, although the SeV extract was used for PB fusion in this study, clinical application of the viral extract may be problematic. Moreover, the ethical considerations must be carefully assessed before its clinical application, and the transfer of PBT-derived embryos should only be approached with complete transparency and informed consents in an Institutional Review Board-approved research protocol.
Acknowledgements
This study is supported by grants from the Major State Basic Research Development Program of China (No. 2012CB944901), the National Science Foundation of China (Nos. 81222007 and 81471510), and the Program for New Century Excellent Talents in University.
Compliance with ethical standards
This study was approved by the Ethics Committee of the Reproductive and Genetic Hospital of CITIC-Xiangya (reference LL-SC-SG-2015-004).
Competing interests
The authors declare that they have no competing interests.
Footnotes
Capsule
Blastocysts with normal chromosomes can develop from human reconstructed oocytes after the first polar body transfer. Patient who suffers from repeated embryo fragmentation may benefit from this therapy.
Shuo-Ping Zhang and Chang-Fu Lu contributed equally to this work.
References
- 1.Li R, Albertini DF. The road to maturation: somatic cell interaction and self-organization of the mammalian oocyte. Nat Rev Mol Cell Biol. 2013;14:141–52. doi: 10.1038/nrm3531. [DOI] [PubMed] [Google Scholar]
- 2.Zamboni L. Ultrastructure of mammalian oocytes and ova. Biol Reprod Suppl. 1970;2:44–63. doi: 10.1095/biolreprod2.Supplement_2.44. [DOI] [PubMed] [Google Scholar]
- 3.Verlinsky Y, Ginsberg N, Lifchez A, Valle J, Moise J, Strom CM. Analysis of the first polar body: preconception genetic diagnosis. Hum Reprod. 1990;5:826–9. doi: 10.1093/oxfordjournals.humrep.a137192. [DOI] [PubMed] [Google Scholar]
- 4.Wakayama T, Hayashi Y, Ogura A. Participation of the female pronucleus derived from the second polar body in full embryonic development of mice. J Reprod Fertil. 1997;110:263–6. doi: 10.1530/jrf.0.1100263. [DOI] [PubMed] [Google Scholar]
- 5.Wakayama T, Yanagimachi R. The first polar body can be used for the production of normal offspring in mice. Biol Reprod. 1998;59:100–4. doi: 10.1095/biolreprod59.1.100. [DOI] [PubMed] [Google Scholar]
- 6.Wang T, Sha H, Ji D, Zhang HL, Chen D, Cao Y, et al. Polar body genome transfer for preventing the transmission of inherited mitochondrial diseases. Cell. 2014;157:1591–604. doi: 10.1016/j.cell.2014.04.042. [DOI] [PubMed] [Google Scholar]
- 7.Wei Y, Zhang T, Wang YP, Schatten H, Sun QY. Polar bodies in assisted reproductive technology: current progress and future perspectives. Biol Reprod. 2015;92:19. doi: 10.1095/biolreprod.114.125575. [DOI] [PubMed] [Google Scholar]
- 8.Cohen J, Scott R, Alikani M, Schimmel T, Munné S, Levron J, et al. Ooplasmic transfer in mature human oocytes. Mol Hum Reprod. 1998;4:269–80. doi: 10.1093/molehr/4.3.269. [DOI] [PubMed] [Google Scholar]
- 9.Cohen J, Scott R, Schimmel T, Levron J, Willadsen S. Birth of infant after transfer of anucleate donor oocyte cytoplasm into recipient eggs. Lancet. 1997;350:186–7. doi: 10.1016/S0140-6736(05)62353-7. [DOI] [PubMed] [Google Scholar]
- 10.Dale B, Wilding M, Botta G, Rasile M, Marino M, Di Matteo L, et al. Pregnancy after cytoplasmic transfer in a couple suffering from idiopathic infertility: case report. Hum Reprod. 2001;16:1469–72. doi: 10.1093/humrep/16.7.1469. [DOI] [PubMed] [Google Scholar]
- 11.Prados FJ, Debrock S, Lemmen JG, Agerholm I. The cleavage stage embryo. Hum Reprod. 2012;27(Suppl 1):i50–71. doi: 10.1093/humrep/des224. [DOI] [PubMed] [Google Scholar]
- 12.Fujimoto VY, Browne RW, Bloom MS, Sakkas D, Alikani M. Pathogenesis, developmental consequences, and clinical correlations of human embryo fragmentation. Fertil Steril. 2011;95:1197–204. doi: 10.1016/j.fertnstert.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 13.Meseguer M, Martínez-Conejero JA, O'Connor JE, Pellicer A, Remohí J, Garrido N. The significance of sperm DNA oxidation in embryo development and reproductive outcome in an oocyte donation program: a new model to study a male infertility prognostic factor. Fertil Steril. 2008;89:1191–9. [DOI] [PubMed]
- 14.Xia P. Intracytoplasmic sperm injection: correlation of oocyte grade based on polar body, perivitelline space and cytoplasmic inclusions with fertilization rate and embryo quality. Hum Reprod. 1997;12:1750–5. doi: 10.1093/humrep/12.8.1750. [DOI] [PubMed] [Google Scholar]
- 15.Stensen MH, Tanbo TG, Storeng R, Åbyholm T, Fedorcsak P. Fragmentation of human cleavage-stage embryos is related to the progression through meiotic and mitotic cell cycles. Fertil Steril. 2015;103:374–81. doi: 10.1016/j.fertnstert.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 16.Jurisicova A, Varmuza S, Casper RF. Programmed cell death and human embryo fragmentation. Mol Hum Reprod. 1996;2:93–8. doi: 10.1093/molehr/2.2.93. [DOI] [PubMed] [Google Scholar]
- 17.Levy R, Benchaib M, Cordonier H, Souchier C, Guerin JF. Annexin V labelling and terminal transferase-mediated DNA end labelling (TUNEL) assay in human arrested embryos. Mol Hum Reprod. 1998;4:775–83. doi: 10.1093/molehr/4.8.775. [DOI] [PubMed] [Google Scholar]
- 18.Bencomo E, Pérez R, Arteaga MF, Acosta E, Pena O, Lopez L, et al. Apoptosis of cultured granulosa-lutein cells is reduced by insulin-like growth factor I and may correlate with embryo fragmentation and pregnancy rate. Fertil Steril. 2006;85:474–80. doi: 10.1016/j.fertnstert.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 19.Alikani M, Schimmel T, Willadsen SM. Cytoplasmic fragmentation in activated eggs occurs in the cytokinetic phase of the cell cycle, in lieu of normal cytokinesis, and in response to cytoskeletal disorder. Mol Hum Reprod. 2005;11:335–44. doi: 10.1093/molehr/gah171. [DOI] [PubMed] [Google Scholar]
- 20.Fujimoto VY, Kane JP, Ishida BY, Bloom MS, Browne RW. High-density lipoprotein metabolism and the human embryo. Hum Reprod Update. 2010;16:20–38. doi: 10.1093/humupd/dmp029. [DOI] [PubMed] [Google Scholar]
- 21.Alikani M, Cohen J, Tomkin G, Garrisi GJ, Mack C, Scott RT. Human embryo fragmentation in vitro and its implications for pregnancy and implantation. Fertil Steril. 1999;71:836–42. doi: 10.1016/S0015-0282(99)00092-8. [DOI] [PubMed] [Google Scholar]
- 22.Ebner T, Yaman C, Moser M, Sommergruber M, Pölz W, Tews G. Embryo fragmentation in vitro and its impact on treatment and pregnancy outcome. Fertil Steril. 2001;76:281–5. doi: 10.1016/S0015-0282(01)01904-5. [DOI] [PubMed] [Google Scholar]
- 23.Alikani M. The origins and consequences of fragmentation in mammalian eggs and embryos. In: Elder K, Cohen J, editors. Human preimplantation embryo selection. London: Informa Healthcare; 2007. pp. 51–78. [Google Scholar]
- 24.Xu X, Duan X, Lu C, Lin G, Lu G. Dynamic distribution of NuMA and microtubules in human fetal fibroblasts, developing oocytes and somatic cell nuclear transferred embryos. Hum Reprod. 2011;26:1052–60. doi: 10.1093/humrep/der067. [DOI] [PubMed] [Google Scholar]
- 25.Gong F, Li X, Zhang S, Ma H, Cai S, Li J, et al. A modified ultra-long pituitary downregulation protocol improved endometrial receptivity and clinical outcome for infertile patients with polycystic ovarian syndrome. Exp Ther Med. 2015;10:1865–70. doi: 10.3892/etm.2015.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang SP, Tan K, Gong F, Gu YF, Tan YQ, Lu CF, et al. Blastocysts can be rebiopsied for preimplantation genetic diagnosis and screening. Fertil Steril. 2014;102:1641–5. doi: 10.1016/j.fertnstert.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Tan YQ, Yin XY, Zhang SP, Jiang H, Tan K, Li J, et al. Clinical outcome of preimplantation genetic diagnosis and screening using next generation sequencing. Gigascience. 2014;3:30. doi: 10.1186/2047-217X-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hou Y, Fan W, Yan L, Li R, Lian Y, Huang J, et al. Genome analyses of single human oocytes. Cell. 2013;155:1492–506. doi: 10.1016/j.cell.2013.11.040. [DOI] [PubMed] [Google Scholar]
- 29.Wakayama S, Hikichi T, Suetsugu R, Sakaide Y, Bui HT, Mizutani E, et al. Efficient establishment of mouse embryonic stem cell lines from single blastomeres and polar bodies. Stem Cells. 2007;25:986–93. doi: 10.1634/stemcells.2006-0615. [DOI] [PubMed] [Google Scholar]
- 30.Wang GJ, Yu JN, Tan XD, Zhou XL, Xu XB, Fan BQ. Injection of frozen-thawed porcine first polar bodies into enucleated oocytes results in fertilization and embryonic development. Theriogenology. 2011;75:826–31. doi: 10.1016/j.theriogenology.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 31.Evsikov SV, Evsikov AV. Preimplantation development of manipulated mouse zygotes fused with the second polar bodies: a cytogenetic study. Int J Dev Biol. 1994;38:725–30. [PubMed] [Google Scholar]
- 32.VerMilyea MD, Maneck M, Yoshida N, Blochberger I, Suzuki E, Suzuki T, et al. Transcriptome asymmetry within mouse zygotes but not between early embryonic sister blastomeres. EMBO J. 2011;30:1841–51. doi: 10.1038/emboj.2011.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barritt J, Willadsen S, Brenner C, Cohen J. Cytoplasmic transfer in assisted reproduction. Hum Reprod Update. 2001;7:428–35. doi: 10.1093/humupd/7.4.428. [DOI] [PubMed] [Google Scholar]
- 34.Athayde Wirka K, Chen AA, Conaghan J, Ivani K, Gvakharia M, Behr B, et al. A typical embryo phenotypes identified by time-lapse microscopy: high prevalence and association with embryo development. Fertil Steril. 2014;101:1637–48. doi: 10.1016/j.fertnstert.2014.02.050. [DOI] [PubMed] [Google Scholar]
- 35.Yang ST, Shi JX, Gong F, Zhang SP, Lu CF, Tan K, et al. Cleavage pattern predicts developmental potential of day 3 human embryos produced by IVF. Reprod Biomed Online. 2015;30:625–34. doi: 10.1016/j.rbmo.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 36.Fragouli E, Wells D. Aneuploidy in the human blastocyst. Cytogenet Genome Res. 2011;133:149–59. doi: 10.1159/000323500. [DOI] [PubMed] [Google Scholar]
- 37.Capalbo A, Rienzi L, Cimadomo D, Maggiulli R, Elliott T, Wright G, et al. Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Hum Reprod. 2014;29:1173–81. doi: 10.1093/humrep/deu033. [DOI] [PubMed] [Google Scholar]
- 38.Howe K, FitzHarris G. Recent insights into spindle function in mammalian oocytes and early embryos. Biol Reprod. 2013;89:71. doi: 10.1095/biolreprod.113.112151. [DOI] [PubMed] [Google Scholar]
- 39.Jones KT, Lane SI. Molecular causes of aneuploidy in mammalian eggs. Development. 2013;140:3719–30. doi: 10.1242/dev.090589. [DOI] [PubMed] [Google Scholar]
- 40.Jones KT. Meiosis in oocytes: predisposition to aneuploidy and its increased incidence with age. Hum Reprod Update. 2008;14:143–58. doi: 10.1093/humupd/dmm043. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J, Zhuang G, Zeng Y, Grifo J, Acosta C, Shu Y, et al. Pregnancy derived from human zygote pronuclear transfer in a patient who had arrested embryos after IVF. Reprod Biomed Online. 2016;33:529–33. doi: 10.1016/j.rbmo.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J. Revisiting germinal vesicle transfer as a treatment for aneuploidy in infertile women with diminished ovarian reserve. J Assist Reprod Genet. 2015;32:313–7. doi: 10.1007/s10815-014-0400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]