Abstract
Purpose
The goals of this study are to analyze the clinical pregnancy rate as a function of the pre-intracytoplasmic sperm injection (ICSI) oocyte spindle angle and determine factors which can be associated with different spindle angles, if clinically relevant.
Methods
Fifty-eight patients, who underwent their first ICSI cycle from January to December 2013, were included. Eight hundred thirty oocytes were collected, and 648 were metaphase II (MII) on retrieval day. Spindles were characterized in terms of visibility and position in relation to the first polar body (PB). Oocytes were separated into four groups based on angle: (group 1, n = 297) 0°–29°; (group 2 n = 212) 30°–89°; (group 3, n = 72) ≥90°; and those with no visible spindle (group 4, n = 67).
Results
The rate of blastocyst development was associated with the spindle angle (p = 0.002). The rate of good quality blastocysts were as follows: group 1 (42%), group 2 (30%), group 3 (35%), and group 4 (19%) (p = 0.02). Pregnancy and live birth rates were also affected (p = 0.007 and p = 0.046, respectively). Antral follicle count (AFC) (p = 0.001), total FSH stimulating dose (p = 0.0001), and peak serum estradiol level (p = 0.0001) were associated with spindle angle grouping. Miscarriage rates trended different (p = 0.07). On the other hand, day 3 follicle-stimulating hormone (FSH) levels and female and male age were not associated with spindle angle grouping.
Conclusions
Embryos resulting from oocytes with pre-ICSI spindle angles between 0° and 29° were associated with better blastocyst, pregnancy, live birth, and miscarriage rates when compared to oocytes that had no visible spindle. Low ovarian reserve and excessive stimulation were also associated with lack of spindle and therefore lower pregnancy outcomes.
Keywords: Spindle, Polarized light microscopy (PolScope™), Blastocyst, Clinical pregnancy, Live birth
Introduction
The developmental potential of the oocyte is among the most important factors for the success of any assisted reproductive treatment. Therefore, numerous attempts have been made to identify prognostic factors based on morphological characteristics of the oocyte which may allow for the prediction of oocyte quality, fertilization rates, and embryo development [1–4]. The predictive value of the criteria used in these studies is still controversial. The introduction of a novel polarized microscopy system coupled with image processing software has allowed the visualization of the meiotic spindle. This approach, using spindle imagining as a new marker of oocyte quality, has been proposed.
During the early transition phase from metaphase I to metaphase II, the spindle is a highly dynamic structure. Polarization light microscopy performed on oocytes in the process of leaving metaphase II, allows non-invasive visualization of the spindle due to its natural birefringent properties [5]. Rienzi et al. previously reported that oocytes with a deviation of the spindle location from the position of the PB of >90° demonstrated decreased fertilization rates [6]. Several studies have also reported on the importance of the presence of a spindle in human oocytes. In two studies, oocytes with a visible spindle showed significantly higher fertilization rates [7, 8]. However, another study did not find any significant difference in fertilization rates, when a spindle was present [9]. On the other hand, it was noted that the embryonic developmental on day 3 was superior, as was the embryo quality when a spindle was present as compared to when it lacked. A further two studies reported significantly higher blastocyst formation rate when the spindle could be visualized in the oocyte as compared to when it could not [7, 10].
The majority of authors concur that the presence of the spindle in mature oocytes can predict the fertilization rates, cleavage rates, and future embryo quality. However, the relationship between the presence of the spindle and the pregnancy and implantation potential was assessed in few studies. The results of these studies have been contradictory. One of the studies reported a correlation between spindle presence and a higher pregnancy and implantation rate [11]. However, the two other studies did not find an association [12, 13].
The goals of this study were to analyze the clinical pregnancy rate as a function of the pre-intracytoplasmic sperm injection (ICSI) oocyte spindle angle and determine factors which can be associated with different spindle angles, if clinically relevant.
Material and methods
This was a retrospective observational study evaluating the data from women who were submitted to ART in the university-based assisted reproductive center. A total of 58 patients who had at least one mature oocyte at retrieval time between January and December 2013 at MUHC Reproductive Center were included. Oocyte recipients, surrogacy, vitrified-warmed oocytes, preimplantation genetic diagnosis (PGD) cycles, and patients using donor sperm were excluded.
Ovarian stimulation was performed by combination of GnRH agonist or antagonist and follicle-stimualting hormone (FSH)/hMG as previously published [14]. HCG (human chorionic gonadotropin) was administrated when the leading follicle diameter was bigger than 17 mm. Transvaginal oocyte recovery was performed 35 h after HCG administration. Immediately following egg retrieval, oocytes were briefly washed in HEPES buffer (lifeglobal, Guilford, CT) then grouped in the same dish containing fertilization media (lifeglobal, Guilford, CT) and allowed to equilibrate for at least 1 h prior to denuding. All dishes were kept in incubators at 37 °C with 6% CO2, 5% O2, and 89% N2.
One hour after egg retrieval, the cumulus corona complex was striped from the egg using enzyme and repetitive pipetting in approximately 100 μl of 80 IU/mL hyaluronidase (lifeglobal, Guilford, CT) followed by HEPES buffer. Insemination was done by ICSI in all cases 1 h later. The mature eggs were selected for spindle observation using the PolScope spindle view system (LC-PolScope™) with computerized image analysis system (spindle view, Cri) to avoid damaging the spindle during the ICSI procedure.
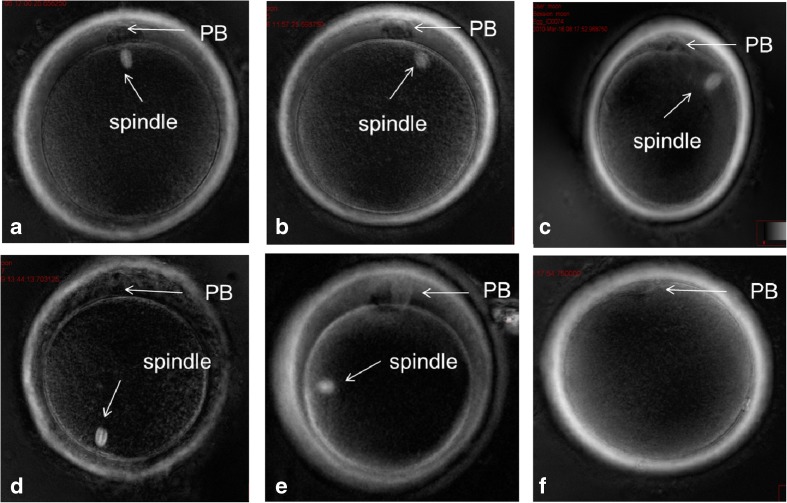
Each oocyte was put into an individual droplet of 10 μl gamete buffer (Cook, Australia) and covered with mineral oil (Cook, Australia) in a glass bottom culture dish (Fluorodish, world precision instruments, USA). The drops were maintained at 37 °C using heated stage set to 38.5 °C. The oocytes were rotated with an injection pipette until the spindle position was visible. The oocytes were separated into groups according to the spindle position and their relationship to the first PB (Fig. 1 Spindle angle position in relation to the first PB divided into four assigned groups). The assigned groups, depending on the angle position of the spindle, were as follows: group 1 0°–29°; group 2 30°–89°; group 3 ≥90°, and those with no visible spindle (group 4) [9, 10, 15]. After insemination, eggs were individually placed into 10 μl drops of culture media (lifeglobal, Guilford, CT).
Fig. 1.
a, b Oocytes in group 1 having spindle 0°–29° in relation to the polar body. c Oocyte in group 2 having spindle 30°–89° in relation to the polar body. d, e Oocytes in group 3 having spindle ≥90° in relation to the polar body. f Oocyte in group 4 with no visible spindle
Fertilization assessment was performed 16–18 h after insemination and a single-embryo transfer was performed, on day 5. On day 3, the embryos were moved to a new dish containing 20 μl drops of culture media (lifeglobal, Guilford, CT) and individually cultured until day 5 for blastocyst transfer. On day 5, blastocyst formation was assessed and graded based on the Gardner and Schoolcraft criteria which evaluated the level of expansion (graded 1 to 6) as well as inner cell mass (ICM) and trophectoderm cells (graded A to C). The embryos chosen for transfer were moved to a center 40 μl drop. Blastocyst utilization rate (BUR) was calculated by adding the number of good quality blastocysts for embryo transfer with those for cryopreservation on day 5 divided by the number of normally fertilized embryos. Blastocyst development potential and the clinical pregnancy rate were analyzed depending on the origin of a single-embryo transfer (SET) at the blastocyst stage.
Pregnancy was assessed 11 days after embryo transfer by analyzing β-HCG levels in the blood. Levels over 10 mIU/mL were considered positive and patients were subsequently evaluated for the presence of fetal heart motion at 6 to 8 weeks of pregnancy by ultrasound. The biochemical pregnancy rate was calculated by the β-HCG-positive pregnancies per embryo transfer. The clinical pregnancy rate was calculated by fetal heart positive pregnancies per embryo transfer. The live birth rate was calculated by birthing events and ongoing pregnancies over 24 weeks per embryo transfer.
Statistical analysis: Continuous data was determined for normalcy using the Kolmogorov-Smirnov test. All continuous data was normally distributed. ANOVA and post hoc testing were used to compare the baseline data. Chi-square tests were used to compare the baseline categorical data. Logistic regression analysis was used to control for confounding effects and compute the differences in clinical outcomes. Confounding effects controlled for were as follows: female age, male age, antral follicle count (AFC), basal serum FSH levels, total dose of stimulating gonadotropin, and stimulated maximum estradiol level. Logistic regression was also performed to determine predictors of spindle abnormalities. p values ≤0.05 were considered statistically significant. The study was approved by the human subjects committee.
Results
A total of 830 eggs were collected from the 58 subjects and the characteristics of these oocytes are summarized in Table 1. From the total number of eggs collected, 648 were metaphase II on the retrieval day of which 581 (89%) had visible spindles. These were separated into groups depending on the angle position: (group 1, n = 297) 0°–29°; (group 2, n = 212) 30°–89°; (group3, n = 72) ≥90° and those with no visible spindle (group 4, n = 67).
Table 1.
Characteristics of mature oocytes based on spindle angle grouping
| Group 1 (0°–29°) | Group 2 (30°–89°) | Group 3 (≥90°) | Group 4 (no spindle) | p value | |
|---|---|---|---|---|---|
| Female age (mean ± SD) | 35.2 (±4.3) | 35.1 (±4.1) | 35.5 (±4.3) | 35.7 (±4.0) | 0.77 |
| Male age (mean ± SD) | 38.7 (±9.1) | 39.0 (±7.8) | 38.4 (±7.4) | 38.2 (±4.6) | 0.91 |
| Basal FSH IU/L (mean ± SD) | 7.5 (±3) | 7.2 (±2.2) | 6.9 (±2.0) | 8.0 (±2.4) | 0.82 |
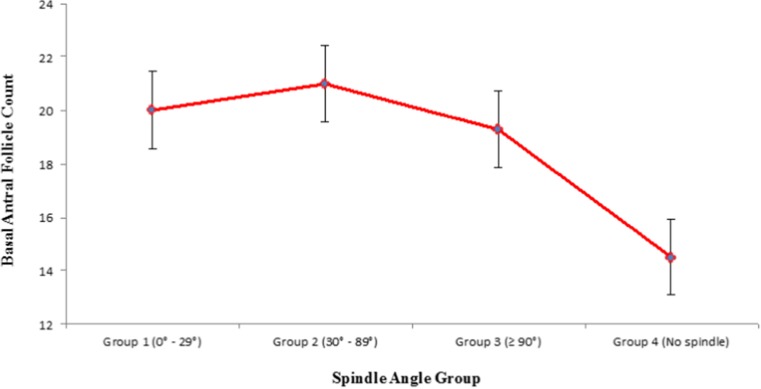
| AFC (mean ± SD) | 20.0 (±11.7) | 21.0 (±11.7) | 19.3 (±9.7) | 14.5 (±6.3) | 0.001 |
| Peak estradiol Pmol/L (mean ± SD) | 7124.8 (±4047.4) | 8332.2 (±3724.3) | 7817.2 (±1987.1) | 8914.1 (±4858.8) | 0.0001 |
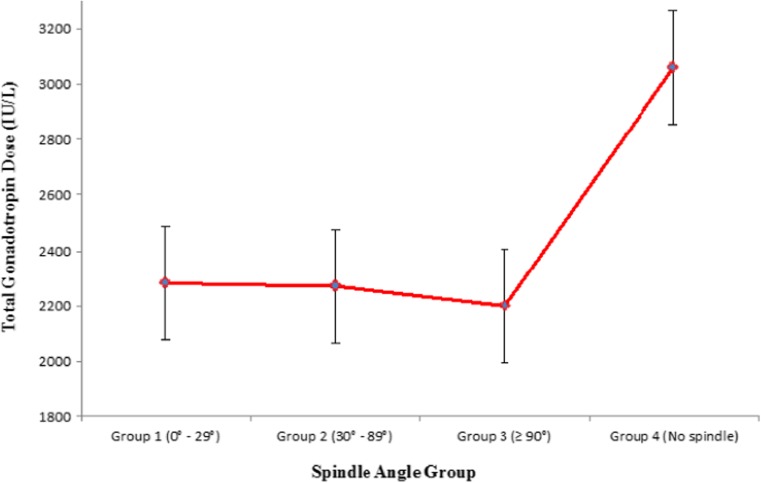
| Gonadotropin dose IU (mean ± SD) | 2280.0 (±1259.6) | 2269.3 (±1257.8) | 2198.2 (±1099.3) | 3060.7 (±1364) | 0.0001 |
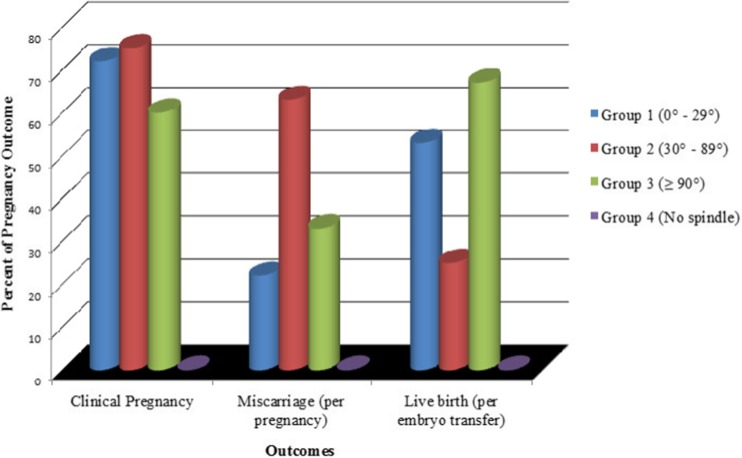
Similar to other studies, there was no significant difference in fertilization (p = 0.21) and cleavage rates among the groups (p = 0.75) [8, 9, 15]. It should be noted that the blastocyst formation rate in group 1 (0°–29°) was significantly higher than the other groups (p < 0.001) when controlling for confounding effects. The rate of blastocyst development was associated with the spindle angle (p = 0.002). The rates of good quality blastocysts were as follows: group 1 42%, group 2 30%, group 3 35%, and group 4 19% (p = 0.02) (Fig. 2 Percent of embryo development based on spindle angle groups). Pregnancy (p = 0.007) and live birth (p = 0.046) rates were also affected by the spindle angle grouping (Fig. 3 Percent of pregnancy outcome based on spindle angle groups). It is obvious from these results that outcomes were lower when the spindle was not detected with the PolScope. Therefore, a logistic regression analysis was undertaken to determine which factors predicted the lack of spindle angle being detected.
Fig. 2.
Percent of embryo development based on spindle angle groups
Fig. 3.
Percent of pregnancy outcome based on spindle angle groups
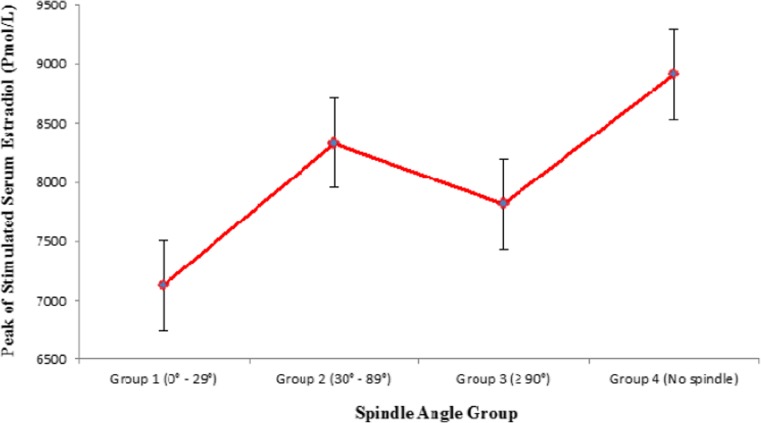
When evaluating for factors which predicted spindle angle being present or not: AFC (p = 0.005) (CI 1.02–1.09), total FSH stimulating dose (p = 0.05) (CI 1.03–1.15) and peak serum estradiol level (p = 0.001) (CI 1.10–1.19) were associated with spindle angle detection. On the other hand, female age (p = 0.86) (CI 0.93–1.10), male age (p = 0.90) (CI 0.96–1.04), day 3 FSH levels (p = 0.87) (CI 0.90–1.13) were not associated with spindle angle grouping. No other variables were used in this analysis. Plot analysis revealed that inclusion in group 4 was more common among lower AFC subjects than groups 1 to 3 (Fig. 4 Basal antral follicle count based on spindle angle groups), as was use of higher FSH doses (Fig. 5 Total gonadotropin dose based on spindle angle groups), and higher peak stimulated estradiol (Fig. 6 Peak stimulated serum estradiol based on spindle angle groups).
Fig. 4.
Basal antral follicle count based on spindle angle groups
Fig. 5.
Total gonadotropin dose based on spindle angle groups
Fig. 6.
Peak stimulated serum estradiol based on spindle angle groups
Discussion
Better pregnancy rates were observed in the cycles where the embryo had been derived from group 1 (0°–29°) spindle angle. Additionally, not having a detectable spindle was associated with worse outcomes than all other groups. So far, all studies published had evaluated the clinical relevance of using polarization microscope to select the oocytes by comparing the results of oocytes with identifiable or not unidentified meiotic spindles. The percentage of in vivo matured human oocytes with visible spindles is reported to range from 63% [11] to 91% [6]. It was previously suggested that these differences may be explained by variation in the patient population, ovarian stimulation protocols, or different culture environments. Our data suggests that lower ovarian reserve as measured by AFC predicted the lack of spindle being predicted. Interestingly, excessive ovarian stimulation also seems to favor a compromised oocyte due to the lack of spindle development. Both increased FSH dose and higher serum peak estradiol levels were associated with the lack of spindle detection with the PolScope. Although, increased FSH stimulation could be another marker of ovarian reserve. The presence of raised estradiol levels suggests a possible mechanism that explained how excessive ovarian stimulation may damage oocytes and compromise clinical outcomes.
Several investigators have reported that the meiotic spindle is a dynamic structure and that various environmental conditions can produce temporary depolymerisation without the occurrence of a real impairment of oocyte quality [16–18]. This depolymerisation may be caused by changes in culture conditions such as temperature and pH. However, under these conditions, the lack of meiotic spindle does not prevent the occurrence of adequate fertilization and development. Some papers published have demonstrated that oocytes with the presence of detectible meiotic spindles have higher fertilization rates, a higher proportion of blastocyst formation, and a better implantation and pregnancy rates. On the other hand, some studies did not observe any significant difference in fertilization, pregnancy, and implantation rates based on the presence of a spindle [9, 19]. Once a meiotic spindle is identified, its position can easily be analyzed. The position of meiotic spindle in relation with the PB was also thought to be important due to the capability to damage the meiotic spindle during ICSI causing potential degeneration of the oocyte [20]. Additionally, the misalignment between the meiotic spindle and first PB increase the risk of abnormal fertilization [6]. Other studies have correlated the position of the meiotic spindle close to the PB with better fertilization rate and cleavage rate and embryo morphology in the early stages of development [19, 21]. In this study, we found that oocytes without detectible meiotic spindles had compromised outcomes. Both blastocyst developmental potential and the pregnancy and clinical pregnancy rates were lower if the spindle was not detectible with the PolScope. Miscarriage rates were just sub-significantly affected. Further studies are needed to confirm this finding.
Unlike previous studies which hypothesized that laboratory factors may transiently affect spindle depolarization, this study demonstrated clinical relevant patient parameters which seemed to play a role. The lack of spindle detection was also found to be clinically important.
Conclusion
The oocytes in which the birefringent spindle can be visualized have a higher embryonic developmental competence than the oocyte without any meiotic spindle. Embryos resulting from oocytes with pre-ICSI spindle angles between 0° and 29° were associated with better blastocyst, pregnancy, live birth rate, and miscarriage rates when compared to oocytes that did not have a visible spindle. Low ovarian reserve and excessive stimulation were also associated with lack of spindle and therefore lower pregnancy outcomes. Meiotic spindle of oocytes located between 0° and 29°, in relation to the first PB, is a positive predictor for blastocyst development.
Compliance with ethical standards
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of the study, the local IRB does not require a formal consent.
References
- 1.De Sutter P, Dozortsev D, Qian C, Dhont M. Oocyte morphology does not correlate with fertilization rate and embryo quality after intracytoplasmic sperm injection. Hum Reprod. 1996;11:595–7. doi: 10.1093/HUMREP/11.3.595. [DOI] [PubMed] [Google Scholar]
- 2.Serhal PF, Ranieri DM, Kinis A, Marchant S, Davies M, Khadum IM. Oocyte morphology predicts outcome of intracytoplasmic sperm injection. Hum Reprod. 1997;12:1267–70. doi: 10.1093/humrep/12.6.1267. [DOI] [PubMed] [Google Scholar]
- 3.Xia P. Intracytoplasmic sperm injection: correlation of oocyte grade based on polar body, perivitelline space and cytoplasmic inclusions with fertilization rate and embryo quality. Hum Reprod. 1997;12:1750–5. doi: 10.1093/humrep/12.8.1750. [DOI] [PubMed] [Google Scholar]
- 4.Balaban B, Urman B, Sertac A, Alatas C, Aksoy S, Mercan R. Oocyte morphology does not affect fertilization rate, embryo quality and implantation rate after intracytoplasmic sperm injection. Hum Reprod. 1998;13:3431–3. doi: 10.1093/humrep/13.12.3431. [DOI] [PubMed] [Google Scholar]
- 5.Silva CP, Kommineni K, Oldenbourg R, Keefe DL. The first polar body does not predict accurately the location of the metaphase II meiotic spindle in mammalian oocytes. Fertil Steril. 1999;71:719–21. doi: 10.1016/S0015-0282(98)00530-5. [DOI] [PubMed] [Google Scholar]
- 6.Rienzi L, Ubaldi F, Martinez F, Iacobelli M, Minasi MG, Ferrero S, et al. Relationship between meiotic spindle location with regard to the polar body position and oocyte developmental potential after ICSI. Hum Reprod. 2003;18:1289–93. doi: 10.1093/humrep/deg274. [DOI] [PubMed] [Google Scholar]
- 7.Wang WH, Meng L, Hackett RJ, Keefe DL. Developmental ability of human oocytes with or without birefringent spindles imaged by Polscope before insemination. Hum Reprod. 2001;16:1464–8. doi: 10.1093/humrep/16.7.1464. [DOI] [PubMed] [Google Scholar]
- 8.Cohen Y, Malcov M, Schwartz T, Mey-Raz N, Carmon A, Cohen T, et al. Spindle imaging: a new marker for optimal timing of ICSI? Hum Reprod. 2004;19:649–54. doi: 10.1093/humrep/deh113. [DOI] [PubMed] [Google Scholar]
- 9.Moon JH, Hyun CS, Lee SW, Son WY, Yoon SH, Lim JH. Visualization of the metaphase II meiotic spindle in living human oocytes using the Polscope enables the prediction of embryonic developmental competence after ICSI. Hum Reprod. 2003;18:817–20. doi: 10.1093/humrep/deg165. [DOI] [PubMed] [Google Scholar]
- 10.Rama Raju GA, Prakash GJ, Krishna KM, Madan K. Meiotic spindle and zona pellucida characteristics as predictors of embryonic development: a preliminary study using polscope imaging. Reprod Biomed Online. 2007;14:166–74. doi: 10.1016/S1472-6483(10)60784-5. [DOI] [PubMed] [Google Scholar]
- 11.Madaschi C, de Souza Bonetti TC. de Almeida Ferreira Braga DP, Pasqualotto FF, Iaconelli A, Jr, Borges E, Jr. Spindle imaging: a marker for embryo development and implantation. Fertil Steril. 2008;90:194–8. doi: 10.1016/j.fertnstert.2007.05.071. [DOI] [PubMed] [Google Scholar]
- 12.Chamayou S, Ragolia C, Alecci C, Storaci G, Maglia E, Russo E, et al. Meiotic spindle presence and oocyte morphology do not predict clinical ICSI outcomes: a study of 967 transferred embryos. Reprod Biomed Online. 2006;13:661–7. doi: 10.1016/S1472-6483(10)60656-6. [DOI] [PubMed] [Google Scholar]
- 13.Heindryckx B, De Gheselle S, Lierman S, Gerris J, De Sutter P. Efficiency of polarized microscopy as a predictive tool for human oocyte quality. Hum Reprod. 2011;26:535–44. doi: 10.1093/humrep/deq376. [DOI] [PubMed] [Google Scholar]
- 14.Dahan MH, Agdi M, Shehata F, Son W, Tan SL. A comparison of outcomes from in vitro fertilization cycles stimulated with either recombinant luteinizing hormone (LH) or human chorionic gonadotropin acting as an LH analogue delivered as human menopausal gonadotropins, in subjects with good or poor ovarian reserve: a retrospective analysis. Eur J Obstet Gynecol Reprod Biol. 2014;172:70–3. doi: 10.1016/j.ejogrb.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 15.Picinato MC, Martins WP, Giorgenon RC, Santos CK, Ferriani RA, Navarro PA, et al. The impact of examining the meiotic spindle by polarization microscopy on assisted reproduction outcomes. Fertil Steril. 2014;101:379–84. doi: 10.1016/j.fertnstert.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Eichenlaub-Ritter U, Shen Y, Tinneberg HR. Manipulation of the oocyte: possible damage to the spindle apparatus. Reprod Biomed Online. 2002;5:117–24. doi: 10.1016/S1472-6483(10)61613-6. [DOI] [PubMed] [Google Scholar]
- 17.Hu Y, Betzendahl I, Cortvrindt R, Smitz J, Eichenlaub-Ritter U. Effects of low O2 and ageing on spindles and chromosomes in mouse oocytes from pre-antral follicle culture. Hum Reprod. 2001;16:737–48. doi: 10.1093/humrep/16.4.737. [DOI] [PubMed] [Google Scholar]
- 18.Mullen SF, Agca Y, Broermann DC, Jenkins CL, Johnson CA, Critser JK. The effect of osmotic stress on the metaphase II spindle of human oocytes, and the relevance to cryopreservation. Hum Reprod. 2004;19:1148–54. doi: 10.1093/humrep/deh201. [DOI] [PubMed] [Google Scholar]
- 19.Fang C, Tang M, Li T, Peng WL, Zhou CQ, Zhuang GL, et al. Visualization of meiotic spindle and subsequent embryonic development in in vitro and in vivo matured human oocytes. J Assist Reprod Genet. 2007;24:547–51. doi: 10.1007/s10815-007-9171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avery S, Blayney M. Effect of the position of the meiotic spindle on the outcome of intracytoplasmic sperm injection. Hum Fertil (Camb) 2003;6:19–22. doi: 10.1080/1464770312331368933. [DOI] [PubMed] [Google Scholar]
- 21.Cooke S, Tyler JP, Driscoll GL. Meiotic spindle location and identification and its effect on embryonic cleavage plane and early development. Hum Reprod. 2003;18:2397–405. doi: 10.1093/humrep/deg447. [DOI] [PubMed] [Google Scholar]