Abstract
Purpose
The objective of this study is to determine if IVF outcome disparities exist among MENA women in the USA in comparison to a control group of Caucasian women.
Methods
A retrospective cohort study comparing MENA (N = 190) and Caucasian (N = 200) women undergoing their first IVF cycle between 5/2006 and 5/2014 was carried out at an academically affiliated fertility practice. All MENA cycles during that time period undergoing IVF/ICSI using autologous embryos and blastocyst transfers were compared to a control group of Caucasian women.
Results
MENA women were significantly younger (32.9 vs 34.5, P < 0.005) and had a lower BMI (25.2 vs 27.1, P < 0.001). Male factor infertility was higher among partners of MENA women (62 vs 50%, P < 0.05). MENA women experienced decreased live birth rates per blastocyst transfer compared to Caucasian women after controlling for age and BMI (OR 0.55, 95% CI 0.35–0.85 P = 0.007). The odds of a miscarriage were also significantly higher among MENA women (OR 2.55, 95% CI 1.04–6.27 P = 0.036).
Conclusion
Middle Eastern/North African women have worse IVF outcomes with decreased live birth rates per blastocyst transfer and increased miscarriage rates compared to Caucasian women.
Keywords: Ethnicity, Race, Arab American, IVF, Health disparity
Introduction
Over the course of the last three decades, live births following in vitro fertilization (IVF) went from the anecdotal to accounting for more than 1.5% of all live births in the USA [1]. However, access and outcomes in infertility treatment have not benefited everybody equally. “In the United States, economic, racial, ethnic, geographic, and other disparities exist in access to fertility treatment and in treatment outcomes” writes the ASRM Ethics Committee [2]. Moreover, the National Institutes of Health has clearly defined addressing health disparities as one of its priorities [3].
Ethnic minority groups living in the USA have decreased access to infertility treatment while also experiencing inferior outcomes following IVF [4]. A majority of previous studies have demonstrated decreased live birth and clinical pregnancy rates among women from African American/Black, Asian, and Hispanic groups while some others offer opposing viewpoints [5]. A recent review of studies utilizing the Society of Assisted Reproduction Technology (SART) reporting database concluded that all ethnic minorities studied experienced inferior outcomes though the results are limited by the relatively low rate of ethnic/racial self-identification [6]. While the largest ethnic minorities such as African Americans, Hispanics, and Asians are captured within SART reporting, smaller though significantly large ethnic minorities cannot be studied due to a lack of granularity within ethnic categories.
Middle Eastern/North African (MENA) individuals have historically been aggregated within the Caucasian ethnic group despite significant sociocultural, historical, environmental, behavioral, and genetic differences [7, 8]. Furthermore, the MENA region has among the highest rates of primary infertility in the world [9]. Most recently, Feichtinger et al. conducted the first study to specifically evaluate whether an ethnic disparity in infertility related characteristics exists between MENA women and a European Caucasian cohort [10]. While they did not find a difference in pregnancy outcome, their findings suggest that MENA women had lower oocyte yields despite presentation at a younger age.
In many regards, the grouping of MENA individuals under the umbrella of a Caucasian group is historical and bears especially little validity when used for medical or epidemiological studies. Individuals from a MENA ancestry make up at least 1.7 million people in the USA, representing a group which has grown approximately 75% since 1990 [11]. MENA women have thus far been suboptimally studied with respect to IVF ethnic disparities. The primary objective of this study is to compare live birth outcomes from IVF/ICSI in MENA women residing in the USA vs a Caucasian cohort.
Materials and methods
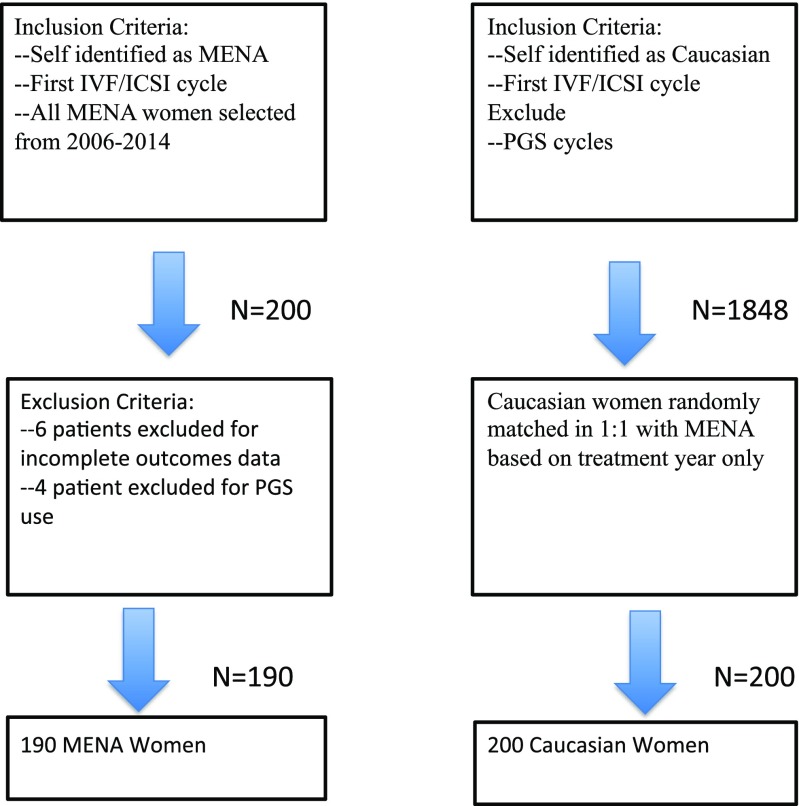
We conducted a retrospective cohort study in which all MENA women undergoing their first IVF cycle were enrolled from the period of 5/2006 to 5/2014. These women were matched on the basis of treatment year in a 1:1 ratio with Caucasian women also undergoing their first IVF cycle. All Caucasian women from a treatment year were first identified. They were assigned a number for that treatment year and then placed through a random number generator. Caucasian women were then selected for using this method until an equivalent number of women in the MENA group was achieved. Matching based on treatment year was conducted since the distribution of MENA women and Caucasian women on a year-by-year basis over the 8-year period was not equal. Matching was conducted to mitigate practice changes over this 8-year period.
All patients were enrolled from a single academically affiliated private fertility clinic located in Michigan, USA. The inclusion criteria for all patients enrolled include an IVF cycle with intracytoplasmic sperm injection (ICSI) and a fresh blastocyst transfer. Women included in the MENA group were permanently living in the USA and had self-identified as “Middle Eastern,” “Arab” or claiming an ancestry to one of the countries of origin in the Middle East/North Africa region as categorized by the United Nations. Self-reporting was completed by patients during the intake process on a form with a free text section marked “Ethnicity.” Couples utilizing donor gametes, surrogacy, or undergoing preimplantation genetic screening were excluded from the analysis. Ten MENA patients with incomplete outcome measures were excluded from the study.
Treatment protocol
All patients underwent a basic infertility work up including a hysterosalpingogram, day 3 serum FSH and LH levels, serum prolactin and TSH levels, transvaginal 2D ultrasound scan, and mid-luteal progesterone levels. Semen analysis was performed on all male partners. All patients underwent controlled ovarian stimulation with either an antagonist or mid-luteal agonist protocol. Patients received oral contraceptive pills 21–35 days in the preceding cycle. Patients who were diagnosed with polycystic ovarian syndrome (PCOS) were stimulated with recombinant FSH (150–225 IU, Gonal-F, EMD Serono or Follistim, Merck) starting on day 2 or day 3 of the cycle while other patients were stimulated with a mixed protocol (rFSH 150IU and HMG 75-150IU, Menopur, Ferring). An antagonist protocol was generally utilized in women who were older than 35, had poor ovarian reserve or in patients with PCOS. Baseline serum estradiol (E2), progesterone (P4), FSH, LH, and ultrasound were determined prior to starting rFSH or mixed protocol and at the time of each subsequent visit. The patients were seen on the sixth day of treatment and the timing and frequency of subsequent visits were determined depending on the patients’ responses. After 5 days, the dose of rFSH/combination of rFSH+HMG was adjusted according to ovarian response as determined by serial scans and measurements of serum E2 levels. When three follicles were ≥17 mm, 5000–10,000 IU human chorionic gonadotropin (HCG) was administered 36 h before oocyte retrieval. The dose of HCG was reduced to 5000 IU if the risk of severe OHSS was elevated. Intracytoplasmic sperm injection (ICSI) was performed on all mature oocytes 3 to 4 h after retrieval. Embryos were graded on both day 2 based on blastomere nuclear scoring and morphologic appearance of day 3 cleavage embryos. Blastocysts were graded according to Gardner et al. criteria [12]. All embryo transfers were conducted by the senior author (MA) with the use of transabdominal ultrasound-guided ET on day 5. Usually two top quality blastocysts were transferred but if a patient has few good embryos by day 3, a day 3 transfer was carried out. Good quality blastocysts not transferred were subsequently frozen. Luteal phase support was started on the second day after retrieval with progesterone vaginal tablets T.I.D (Endometrin 100 mg Vaginal Insert, Ferring Pharmaceuticals, Inc., Parsippany, NJ, 07054 USA) or vaginal cream once a day (Crinone 8% Vaginal Gel, Watson Pharmaceuticals, Morristown, NJ, 07962, USA), Progesterone in oil 100 mg I.M every other day (progesterone in oil 50 mg/mL Vial, Watson Laboratories, Inc., Corona, CA) and vaginal Estradiol B.I.D (estrace 2 mg, Warner Chilcott LLC, Rockaway, NJ). If pregnancy was achieved, the same treatment continued until 6-week gestation. At that time estrace and progesterone in oil were discontinued, and vaginal progesterone was continued until 12-week gestation. Pregnancy was confirmed by measurement of βHCG 12 days after blastocyst transfer. Biochemical pregnancy was defined as a transient rise in βHCG or a positive pregnancy test in the absence of ultrasonographic evidence of pregnancy. Miscarriage was defined as a clinical pregnancy that ended in pregnancy loss prior to 20 weeks gestation while a live birth was defined as any delivery after viability.
Data collection/statistical analysis
A centralized database was created compiling all embryology data queried from an existing electronic record along with a chart review collecting demographic and pregnancy outcome metrics. The primary outcome was live birth rate per racial/ethnic group. Secondary outcomes were metrics of embryology and ovarian response and miscarriage rate. Based on the distribution of the data, continuous data was analyzed using the Student’s t test while chi-squared analysis was carried out for categorical variables (P < 0.05 was considered to be statistically significant). A logistic regression assessed the individual association between ethnic group and live birth in our dataset. A multivariate logistic regression was conducted to ascertain the individual contribution of ethnicity to live birth rates in this dataset. Confounders which were statistically significant or known to be clinically significant were included in the models (age and BMI). However, caution was taken to not include all statistically significant parameters as this may lead to over adjustment and dissolution of a clinically relevant outcome. All data were analyzed using STATA (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX). An IRB approved for retrospective data collection was approved.
Results
A group of 400 women divided in a 1:1 manner between MENA and Caucasian women undergoing IVF/ICSI were enrolled in this retrospective cohort evaluating differences in live birth outcomes. Due to incomplete outcomes data, 10 MENA women were excluded from the analysis for a total of 390 women (MENA N = 190, 48.7% Caucasian N = 200, 51.3%). The etiologies contributing to infertility between MENA and Caucasian women were found to be relatively similar with a few notable exceptions. The prevalence of primary and secondary infertility was not different between ethnic groups. The diagnosis of polycystic ovarian syndrome approached a clinically significant difference with a higher prevalence among Caucasian women (32 vs 23% P = 0.05). Endometriosis was also higher among Caucasian women (27 vs 17% P = 0.02). There was a higher prevalence of male factor infertility among the partners of MENA women in comparison to Caucasian women (62 vs 50% P = 0.02).
Table 1 details the demographic, embryologic and infertility parameters in these two groups. MENA women tend to be younger (32.9 vs 34.5 P = 0.002) and have a lower body mass index (BMI = kg/m2) in comparison to the Caucasian cohort (25.2 vs 27.1 P < 0.001). The duration of infertility was not dissimilar between groups. Ovarian reserve measured by day 3 FSH was significantly different between the groups but this was likely attributable to the difference in age between the two groups. Total gonadotropin dose used for controlled ovarian stimulation and mature oocyte yields were not different between the groups. Table 2 describes the embryology parameters in the two groups. Fertilization rates after ICSI were noted to be significantly decreased among MENA women (73.8 vs 83.7% P < 0.005). The total number of blastocysts was not significantly different between the groups and neither were the number of fresh blastocysts transferred in MENA vs Caucasian women (2.38 vs 2.35 P = 0.7).
Table 1.
Patient characteristics of MENA vs Caucasian groups
| MENA | Caucasian | P | |
|---|---|---|---|
| N | 190 | 200 | |
| Age (years) | 32.9 ± 5.8 | 34.5 ± 5.0 | 0.002* |
| BMI (kg/m2) | 25.2 ± 4.1 | 27.1 ± 6.6 | 0.0006* |
| Day 3 FSH (IU/L) | 6.6 ± 2.7 | 7.3 ± 2.9 | 0.008* |
| Antral follicle count | 12.0 ± 10.0 | 13.1 ± 10.2 | 0.27 |
| Primary infertility | 43% | 44% | 0.8 |
| Secondary infertility | 57% | 56% | 0.8 |
| Male factor | 62% | 50% | 0.02* |
| PCOS | 23% | 32% | 0.05 |
| Tubal factor | 24% | 27% | 0.5 |
| Endometriosis | 17% | 27% | 0.02* |
| Uterine | 30.5% | 38.4% | 0.1 |
| Infertility duration (years) | 4.1 ± 3.4 | 3.7 ± 3.2 | 0.2 |
*P < 0.05
Table 2.
Controlled ovarian stimulation embryology parameters of MENA vs Caucasian women
| MENA N = 190 | Caucasian N = 200 | P | |
|---|---|---|---|
| Agonist protocol | 31.1% (59) | 25% (50) | 0.18 |
| Antagonist protocol | 69.9% (131) | 75% (150) | 0.18 |
| Total gonadotropin dose (IU) | 2714 ± 1104 | 2759 ± 818 | 0.65 |
| Mature oocytes (no.) | 8.6 ± 5.9 | 8.5 ± 5.2 | 0.8 |
| Fertilization rate | 73.8 ± 23.7% | 83.7 ± 14.2% | <0.005 |
| No. blastocysts/cycle | 3.2 ± 3.8 | 3.4 ± 3.8 | 0.6 |
| No. embryos transferred | 2.38 ± 0.9 | 2.35 ± 0.9 | 0.7 |
The live birth rate per transfer for MENA women was 45.2% (N = 86) and 52% (N = 104) for Caucasian women. In a multivariate logistic regression analysis adjusted for age and BMI, there was a statistically significant lower odds of a live birth in the MENA group compared to Caucasians (OR 0.55, 95% CI 0.35–0.85, P = 0.007). Furthermore, the odds of a miscarriage was significantly higher among MENA women (OR 2.55, 95% CI 1.04–6.27, P = 0.036) as can be seen in Table 3.
Table 3.
Pregnancy outcomes in MENA vs Caucasian
| MENA (N = 190) | Caucasian (N = 200) | P | |
|---|---|---|---|
| Positive βhcg | 57% (109) | 69% (138) | 0.017* |
| Miscarriage rate/transfer | 14% (15) | 6% (8) | 0.036* |
| Live birth rate/transfer | 45% (86) | 52% (104) | 0.18 |
*P < 0.05
Discussion
This study demonstrates significantly decreased odds of a live birth per blastocyst embryo transfer following IVF/ICSI in MENA women as compared to a Caucasian cohort. Furthermore, MENA ethnicity is associated with an increased chance of a miscarriage per pregnancy. These findings are concerning especially given that MENA women tend to present at an earlier age, with a lower BMI and undergo equivalent blastocyst transfers with equivalent embryologic parameters. Lower fertilization rates and increased male factor infertility may point to a causal link but this conclusion cannot be drawn from this study. While MENA women in the USA have never been previously studied for outcome disparities, this study is partially supported by the findings uncovered in a recent study comparing MENA women and a European Caucasian cohort [10]. Feichtinger et al. compared 218 MENA women to 2703 European Caucasians undergoing their first IVF cycle. They found that MENA women presented at a younger age despite having a longer period of infertility. MENA women also had higher BMIs with a higher prevalence of polycystic ovarian syndrome. In contrast to our findings, Feichtinger et al. did not demonstrate a lower clinical pregnancy rate among MENA women (22.4% Caucasian vs 22.9% MENA); however, MENA women were found to have a lower oocyte yield (7 Caucasian vs 6 MENA, P = 0.04). Among their cohort, MENA women had an increase in cycle cancelation due to poor ovarian response or fertilization failure. They propose that MENA women may have a lower ovarian reserve as evidenced by their higher day 3 FSH. Biochemical and live birth rates were comparably higher in our cohort though a direct comparison cannot be made between the studies as our study investigated live birth rates per blastocyst embryo transfer.
In general, our findings substantiate the trend for longer periods of infertility and a presentation at a younger age. Despite an earlier infertility treatment, both studies demonstrate unfavorable outcomes among MENA women.
MENA women traditionally are required to self-report as “Caucasian” having made this a difficult group to study outside of the Middle East. There is a substantial base of evidence that may explain the disparate access to infertility care and diminished outcomes that we have demonstrated. MENA men experiencing infertility have been well documented to perceive societal barriers to ART within the same geographic locale as our patient population [13]. Moreover, the genetic implications of consanguinity may play a contributing role in the increased rates of male factor infertility [14]. While we did not uncover specific ovarian reserve marker differences between MENA and Caucasian groups there is a previous genome wide association study which supports this genetic underpinning to ethnic disparities [15]. This may indirectly support previous findings that suggest a higher incidence of infertility in the indigenous MENA population [9].
The first articles investigating the relationship between ethnicity and IVF outcomes demonstrated inferior results for African American, Hispanic, and Asian women and while there were subsequent challenges to this notion, the most recent evidence suggests a strong relationship between ethnic minorities and inferior IVF outcomes. Sharara et al. reported on the perceived disparity in IVF outcomes in black vs white women demonstrating a lower implantation and clinical pregnancy rate among black women [16]. Bendikson et al. found no difference in pregnancy outcomes after IVF between white patients and African American, Hispanic, or Asian women though the ethnic minority groups represented a small proportion of the total sample [17]. Dayal et al. studied IVF outcomes between African American and Caucasian women in a university-based IVF program where they did not observe a difference in pregnancy outcomes despite a lower embryo yield among African American women [18]. Subsequent larger studies would go on to find significant differences between ethnic groups in terms of IVF outcomes. Fujimoto et al. revealed decreased live birth rates of Black, Hispanic, and Asian women compared to Caucasian women in a historical cohort analysis utilizing the SART database evaluating over 139,000 cycles from 2004 to 2006 [19]. While this is a convincing study secondary to its power, the authors note that it is limited by incomplete self-reporting of race/ethnicity.
A large population based study in the UK by Jayaprakasan et al. sheds light on ethnic disparities in a system in which ART is a covered benefit [20]. Again, compared to European white women, all ethnic minorities were found to have inferior IVF outcomes. This study separately identified 14 Middle Eastern women who were found to have the worst outcomes following IVF though the sample size was too small to reach a statistically significant outcome. Wellons et al. conducted a systemic review of studies utilizing SART data and concluded that Hispanic, Asian, and African American women had the lowest live birth rates following ART [6]. The authors highlight the difficulty of interpreting these results as more than 35% of cycles in the SART database are missing data on ethnicity/race. Quinn and Fujimoto published a review paper in which they conclude that ethnic disparities in both access to ART and outcomes after ART exist and may be increasing [7]. Taken together, the weight of the literature leans heavily toward significant disparities in ART outcomes among ethnic minorities.
Studying ethnic disparities is fraught with methodological challenges as we attempt to isolate sociocultural from biological determinants of health. Our retrospective cohort captures women over an 8-year period in order to include the largest number of MENA women. In order to offset heterogeneity in treatment protocols, technology and success rates over this time period, we randomly selected Caucasian women from the same treatment year as MENA women in a 1:1 manner. However, matching has the potential drawback of overmatching and thus introducing a sampling bias. This study investigated live birth rates per blastocyst transfer between two groups which may not capture the overall IVF/ICSI success of these two populations as it selects for a good prognosis cohort. Sociocultural differences may explain why MENA women present at an earlier age; however, this happens only after they have experienced infertility for a longer period of time reaffirming the findings that minority populations experience barriers which delay their care [21, 22]. All women in this study were private payers which may have contributed to a diminished effect of the sociocultural disparity of ART access in the USA. Patients were required to self-report their ethnicity which may introduce a skew in reporting. However, the clinic had specific cultural and linguistic support for MENA women making it unlikely there would be an underreporting bias. It should also be noted that while the outcome of interest in this study is a single live birth, this may not fully capture the reproductive goals of individuals from a MENA background.
This study is strengthened by a homogenous comparison between MENA and Caucasian women. Women underwent IVF/ICSI protocols in the same fertility center and all underwent embryo transfer by the same physician. Furthermore, matching women in a 1:1 basis on treatment year alone should alleviate year-to-year treatment heterogeneity. We also report on the clinically relevant outcome of live births instead of clinical pregnancy rate. Finally, this is the only study to our knowledge that directly compares MENA women with a Caucasian cohort in the USA though the findings are supported by similar evidence in a MENA vs European cohort.
Increased male factor infertility, decreased fertilization rates and increased miscarriage rates among the MENA group may underlie a causal link and warrants further investigation. However, the social determinants of health should not be overlooked as there are many explanatory variables which would affect IVF/ICSI treatment over the course of the actual treatment cycle. In clinics treating a small number of ethnically diverse patients, cultural and linguistic barriers may lead to nonadherence of a complex treatment protocol. Furthermore, patient-physician shared decision-making which is central to fertility treatment may also be compromised if there are cultural or communication barriers.
Conclusion
This is the first study to demonstrate inferior IVF/ICSI live birth outcomes in MENA women compared to Caucasian women. While the underlying etiology for these disparities is unknown, it is imperative to observe these outcomes on a larger scale. We suggest collecting the data for MENA women as a separate category as opposed to unifying it with the Caucasian group as it currently stands. As objective evidence of disparities in IVF continues to surface, it is incumbent on the field to introduce practices that aim to further understand and alleviate the infertility treatment gap.
Acknowledgements
The authors want to especially thank the patients of IVF Michigan for their participation along with Prof. Hakam, Dr. H Salem, and Dr. Wadhera for inspiration.
Appendix
Fig. 1.
Patient selection flowchart
Compliance with ethical standards
Funding
This study was not provided any external or institutional funding.
Conflict of interest
The authors declare that they have no conflicts of interests.
References
- 1.Sunderam S, Kissin DM, Crawford SB, Folger SG, Jamieson DJ, Warner L, et al. Assisted reproductive technology surveillance—United States, 2013. Morb Mortal Wkly Rep Surveill Summ Wash DC 2002. 2015;64:1–25. doi: 10.15585/mmwr.ss6411a1. [DOI] [PubMed] [Google Scholar]
- 2.Ethics Committee of the American Society for Reproductive Medicine Disparities in access to effective treatment for infertility in the United States: an ethics committee opinion. Fertil Steril. 2015;104:1104–1110. doi: 10.1016/j.fertnstert.2015.07.1139. [DOI] [PubMed] [Google Scholar]
- 3.NIH policy on reporting race and ethnicity data: subjects in clinical research [Internet]. 2016. Available from: http://grants.nih.gov/grants/guide/notice-files/NOT-OD-01-053.html
- 4.Huddleston HG, Cedars MI, Sohn SH, Giudice LC, Fujimoto VY. Racial and ethnic disparities in reproductive endocrinology and infertility. Am J Obstet Gynecol. 2010;202:413–419. doi: 10.1016/j.ajog.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 5.Jain T, Hornstein MD. Disparities in access to infertility services in a state with mandated insurance coverage. Fertil Steril. 2005;84:221–223. doi: 10.1016/j.fertnstert.2005.01.118. [DOI] [PubMed] [Google Scholar]
- 6.Wellons MF, Fujimoto VY, Baker VL, Barrington DS, Broomfield D, Catherino WH, et al. Race matters: a systematic review of racial/ethnic disparity in Society for Assisted Reproductive Technology reported outcomes. Fertil Steril. 2012;98:406–409. doi: 10.1016/j.fertnstert.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aboul-Enein BH, Aboul-Enein FH. The cultural gap delivering health care services to Arab American populations in the United States. J Cult Divers. 2010;17:20–23. [PubMed] [Google Scholar]
- 8.Krogstad JM. Census Bureau explores new Middle East/North Africa ethnic category. Pew Res. Cent. [Internet]. 2016; Available from: http://www.pewresearch.org/fact-tank/2014/03/24/census-bureau-explores-new-middle-eastnorth-africa-ethnic-category/
- 9.Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;9:e1001356. doi: 10.1371/journal.pmed.1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feichtinger M, Göbl C, Weghofer A, Feichtinger W. Reproductive outcome in European and Middle Eastern/North African patients. Reprod BioMed Online. 2016;33:684–689. doi: 10.1016/j.rbmo.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Maryam Asi and Daniel Beaulieu. Arab Households in the United States: 2006–2010 [Internet]. 2013. Available from: https://www.census.gov/prod/2013pubs/acsbr10-20.pdf
- 12.Gardner DK, Surrey E, Minjarez D, Leitz A, Stevens J, Schoolcraft WB. Single blastocyst transfer: a prospective randomized trial. Fertil Steril. 2004;81:551–555. doi: 10.1016/j.fertnstert.2003.07.023. [DOI] [PubMed] [Google Scholar]
- 13.Inhorn MC, Fakih MH. Arab Americans, African Americans, and infertility: barriers to reproduction and medical care. Fertil Steril. 2006;85:844–852. doi: 10.1016/j.fertnstert.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 14.Inhorn MC, Kobeissi L, Nassar Z, Lakkis D, Fakih MH. Consanguinity and family clustering of male factor infertility in Lebanon. Fertil Steril. 2009;91:1104–1109. doi: 10.1016/j.fertnstert.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Schuh-Huerta SM, Johnson NA, Rosen MP, Sternfeld B, Cedars MI, Reijo Pera RA. Genetic markers of ovarian follicle number and menopause in women of multiple ethnicities. Hum Genet. 2012;131:1709–1724. doi: 10.1007/s00439-012-1184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharara FI, McClamrock HD. Differences in in vitro fertilization (IVF) outcome between white and black women in an inner-city, university-based IVF program. Fertil Steril. 2000;73:1170–1173. doi: 10.1016/S0015-0282(00)00524-0. [DOI] [PubMed] [Google Scholar]
- 17.Bendikson K, Cramer DW, Vitonis A, Hornstein MD. Ethnic background and in vitro fertilization outcomes. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2005;88:342–346. doi: 10.1016/j.ijgo.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 18.Dayal MB, Gindoff P, Dubey A, Spitzer TLB, Bergin A, Peak D, et al. Does ethnicity influence in vitro fertilization (IVF) birth outcomes? Fertil Steril. 2009;91:2414–2418. doi: 10.1016/j.fertnstert.2008.03.055. [DOI] [PubMed] [Google Scholar]
- 19.Fujimoto VY, Jain T, Alvero R, Nelson LM, Catherino WH, Olatinwo M, et al. Proceedings from the conference on Reproductive Problems in Women of Color. Fertil. Steril. 2010;94:7–10. [DOI] [PMC free article] [PubMed]
- 20.Jayaprakasan K, Pandian D, Hopkisson J, Campbell BK, Maalouf WE. Effect of ethnicity on live birth rates after in vitro fertilisation or intracytoplasmic sperm injection treatment. BJOG Int J Obstet Gynaecol. 2014;121:300–306. doi: 10.1111/1471-0528.12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Missmer SA, Seifer DB, Jain T. Cultural factors contributing to health care disparities among patients with infertility in Midwestern United States. Fertil Steril. 2011;95:1943–1949. doi: 10.1016/j.fertnstert.2011.02.039. [DOI] [PubMed] [Google Scholar]
- 22.Chandra A, Copen CE, Stephen EH. Infertility service use in the United States: data from the National Survey of Family Growth, 1982–2010. Natl. Health Stat. Rep. 2014;1–21. [PubMed]