Abstract
Men and women manifest different symptoms of depression and under current diagnostic criteria, depression is twice as prevalent in woman. However, little is known of the mechanisms contributing to these important sex differences. Sub-chronic variable stress (SCVS), a rodent model of depression, induces depression-like behaviors in female mice only, modelling clinical evidence of higher susceptibility to mood disorders in women. Accumulating evidence indicates that altered neuroplasticity of excitatory synapses in the nucleus accumbens (NAc) is a key pathophysiological feature of susceptibility to social stress in males. Here we investigated the effects of SCVS on pre- and post-synaptic protein levels and morphology of glutamatergic synapses of medium spiny neurons in the NAc of female and male mice. Animals underwent six-day exposure to alternating stressors including shock, tail suspension and restraint. Medium spiny neurons from the NAc were filled with a Lucifer yellow dye and spine density and type were examined using NeuronStudio. In a separate group of animals, immunofluorescence staining was performed for vesicular glutamate transporter 1 (VGLUT1) and vesicular glutamate transporter 2 (VGLUT2), in order to label cortical and subcortical glutamatergic terminals. Immunostaining for PSD95 was employed to evaluate post-synaptic density. Females demonstrated circuit specific pre-synaptic alterations in VGLUT1 and VGLUT2 containing synapses that may contribute to stress susceptibility in the absence of post-synaptic alterations in PSD 95 puncta, spine density or type. These data indicate that susceptibility to stress in females is associated with changes in the frequency of distinct glutamatergic inputs to the NAc.
Keywords: stress, sex differences, depression, nucleus accumbens, medium spiny neurons, plasticity
Introduction
Stress can precipitate or exacerbate depression in “at risk” individuals (Foland-Ross et al., 2014). A variety of rodent stress models have been developed in order to study the neurobiology of stress-induced depression (Menard et al., 2016). However, less attention has been paid to sex differences in stress and depression susceptibility, despite the fact that major depression is twice as common in females as in males (Kessler et al., 1994, Marcus et al., 2005) Important behavioral and neurobiological sex differences exist in most rodent stress models (Dalla et al., 2005, Dalla et al., 2008a, Trainor et al., 2011), which provides valuable insight into depression pathophysiology in women.
The subchronic variable stress (SCVS) paradigm involves alternating exposure to different stressors, including foot shock, tail suspension and restraint stress across a six-day period. As a result, female mice display a stress susceptible phenotype in a behavioral battery tailored to model core symptoms of depression (Hodes et al., 2015). In particular, female mice show increased passive coping in the forced swim test, decreased hedonic reactivity in the sucrose preference test, decreased self-grooming in the splash test and higher latency to eat in novelty suppressed feeding, along with no alterations of anxiety-like exploratory behaviors. Importantly, none of these behavioral deficits are observed in male mice following SCVS (LaPlant et al., 2009, Hodes et al., 2015). As such, SCVS allows us to examine which biological changes are correlated with enhanced female stress susceptibility. For example, exposure to SCVS also increases serum corticosterone levels in female mice only and reveals sex-specific alterations of the transcriptome profile in the nucleus accumbens (NAc), a key region of brain reward circuitry (Hodes et al., 2015, Pfau et al., 2016).
Recent evidence implicates the dysregulation of NAc glutamatergic transmission as a key pathophysiological feature of stress and depression susceptibility (Russo and Nestler, 2013, Thompson et al., 2015). Direct infusion of glutamate into the NAc dose-dependently decreased swimming time in the forced swim test, whereas intra NAc or systemic injection of NMDA receptor antagonists resulted in antidepressant effects (Rada et al., 2003, Autry et al., 2011). Notably, in males, stress susceptibility is associated with functional and structural neuroplasticity at excitatory synapses in the NAc (Christoffel et al., 2011b). Chronic mild stress and chronic social stress altered AMPAR profile, decreasing GluA2 and increasing GluA1 protein expression in the NAc, thus increasing excitatory synaptic strength (Toth et al., 2008, Vialou et al., 2010). Moreover, chronic mild stress and chronic social stress also act post-synaptically to alter dendritic architecture via changes in spine density and dendritic length in the NAc of males (Christoffel et al., 2011a, Bessa et al., 2013). In particular, chronic social defeat stress resulted in an increase of stubby spines, which negatively correlated with social interaction (Christoffel et al., 2011a), whereas chronic mild stress increased dendritic branching and spine number (Bessa et al., 2013). Furthermore, epigenetic regulation of the synaptic remodeling gene, RAC1, which lead to increased spine density in the NAc of male mice, increased social avoidance and sucrose preference deficits in susceptible mice (Golden et al., 2013). Sex specific effects of stress on plasticity have been reported for other brain regions, for example acute stress increased spine density in male rats on pyramidal neurons in area CA1 of the hippocampus whereas the same stressor decreased spine density in females (Shors et al., 2001). To date, it is unknown whether stress alters synaptic plasticity mechanisms in the NAc of females.
In addition to the postsynaptic plasticity described above, changes in density of vesicular glutamate transporters (VGLUTs), which mark glutamatergic presynaptic axon terminals (Fremeau et al., 2001), can impact glutamatergic signaling in NAc (Stuber et al., 2010). Among the three VGLUT isoforms, VGLUT1 and VGLUT2 control glutamate vesicle loading and pre-synaptic release of glutamate. Importantly, they are largely segregated in the brain: VGLUT1 mRNA is primarily found in neurons of the cerebral cortex, hippocampus, basolateral amygdala and cerebellar cortex (Bellocchio et al., 1998, Fremeau et al., 2001, Fremeau et al., 2004). In contrast, VGLUT2 mRNA is mainly expressed in neurons of the thalamus, brainstem and deep cerebellar nuclei (Fremeau et al., 2001, Varoqui et al., 2002, Fremeau et al., 2004). In the NAc, which receives input from both VGLUT1-expressing and VGLUT2-expressing neurons, the projections mainly segregate based on input (Hartig et al., 2003). Indeed, because of their different pattern of expression, VGLUT isoforms can serve as pre-synaptic markers to evaluate the neuroplasticity of distinct glutamate inputs to the NAc. Interestingly, the rearrangement of synaptic strength, neuronal processes and axon terminals induced by chronic stress in the NAc of male mice correlated with the susceptible behavioral phenotype, while opposite changes or no differences were observed in resilient animals with respect to unstressed controls (Vialou et al., 2010, Christoffel et al., 2011b, Christoffel et al., 2015).
In the current study we characterized pre and post-synaptic plasticity of excitatory synapses in the NAc in order to elucidate potential mechanisms of sex specific stress susceptibility. In particular, we focused on the NAc shell subregion, which is considered a part of the extended amygdala and is primarily involved in the control of motivation and reward. Indeed, in the NAc shell a high degree of convergence of monosynaptic glutamatergic inputs was reported onto individual medium spiny neurons (Mulder et al., 1998; O’Donnell and Grace, 1993). Furthermore, stress and stress hormones exert shell-specific effects (Kalivas and Duffy, 1995; Barrot et al., 1999; Campioni et al., 2009).
We evaluated the immunofluorescence for VGLUT1 and VGLUT2, as pre-synaptic markers of distinct glutamatergic inputs to the NAc. The VGLUT immunofluorescence represents glutamate transporting vesicles predominately located in glutamatergic terminals (Fujiyama et al., 2001). However, the expression of VGLUT2 has been reported in a subset of dopaminergic terminals from the VTA (Stuber et al., 2010). Thus, the levels of co-localization between VGLUT2 and the catecholamine biosynthetic enzyme tyrosine hydroxylase (TH) was also assessed to further characterize the neurochemical nature of NAc VGLUT2-positive puncta(Mendez et al., 2008). To examine post-synaptic plasticity, we filled medium spiny neurons (MSNs) with a Lucifer yellow fluorescent dye and used the semi-automated program Neuron Studio to quantify spine density and examine spine morphology (Radley et al., 2008, Dumitriu et al., 2011). We further validated our post-synaptic data by examining the frequency of post-synaptic density 95 (PSD95) puncta, a protein enriched in the post-synaptic density.
Experimental Procedures
Animals
C57BL/6J female and male mice (Jackson Laboratory) were used at 8 weeks of age. Mice were group housed and maintained on a 12 h light/dark cycle with ad libitum access to food and water. Procedures were performed in accordance with the Institutional Animal Care and Use Committee guidelines of the Icahn School of Medicine at Mount. Sinai.
Subchronic variable stress
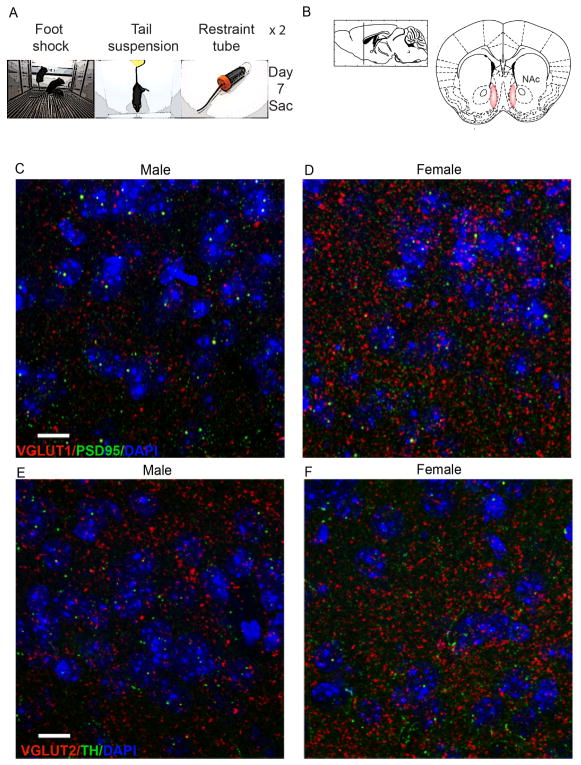
SCVS was performed as described previously (LaPlant et al., 2009; Hodes et al., 2015). Female and male mice (n = 5 – 6 per group) underwent 1-hour of variable stress each day for six days, consisting of foot shock, tail suspension and restraint. To prevent habituation, stressors were administered in the following order (Fig 1a): 100 random mild foot shocks at 0.45 mA for 1 h (Med Associates, St. Albans, Vermont) (10 mice at the same time in the chamber); tail suspension stress for 1 h; restraint stress - mice were placed inside a 50 ml falcon tube for 1 h within the home cage. The three stressors were then repeated for the next three days in the same order. After each stress, animals were returned to their home cage.
Fig. 1. VGLUT expression and colocalization with synaptic labels.
(a) Schematic of time line of study. (b) NAc coordinates. (c–d)VGLUT1 (red) and PSD95 (green) puncta in NAc of male and female control mice. (e–f) VGLUT2 puncta (red) and TH puncta and fibers (green) in NAc of male and female control mice. 100×, scale bar 10 μm.
Perfusion and tissue processing
24h after the last stressor, mice were given a lethal dose of 15% chloral hydrate and transcardially perfused with cold phosphate-buffered saline (PBS; pH 7.4) followed by fixation with cold 4% paraformaldehyde (PFA) in PBS (immunofluorescence staining tissue only). Animals used for spine analysis underwent perfusion with cold 1% PFA in PBS (pH 7.4) followed by fixations with a mixture of 4% PFA and 0.125% glutaraldehyde in PBS (pH 7.4) as previously described (Christoffel et al., 2011a). Brains were dissected and postfixed overnight in the same fixative used for perfusion. For immunofluorescence, fixed brains were cryoprotected by immersion in 15% sucrose in PBS for 24 hours, followed by immersion in 30% sucrose in PBS until sank. Brains were flash-frozen and coronal sections were prepared on a cryostat (Leica CM1850) at 35μm thickness. Serial slices were collected through the rostral-caudal dimension of the nucleus accumbens (every 6th slice) and stored at 4ºC in 0.01% sodium azide in PBS until immunofluorescence processing. For spine analysis slices were cut and processed as previously described (Radley et al., 2006, Goldwater et al., 2009, Christoffel et al., 2011a). Coronal sections were cut on a vibratome at 250 um and MSNs in the NAc shell were loaded with intracellular injection of 5% Lucifer yellow dye (Invitrogen) under direct current (1–6nA) for 10 minutes. Sections were slide mounted in Vectashield (Vector Laboratories) and cover slipped before imaging.
Immunofluorescence staining
Sections (six per animal) were washed in PBS for 30 min and incubated in blocking solution (3% normal donkey serum (NDS), 0.3% Triton X-100 in PBS) for 2 h at room temperature under gentle shaking. Sections were then incubated in primary antibody solution overnight at 4°C under gentle shaking (3% NDS, 0.3% Tween-20 in PBS, with either guinea pig anti-VGLUT1 (1:10,000); guinea pig anti-VGLUT2 (Millipore, #ab2251) 1:10,000; goat anti-PSD95 1:1:1,000; rabbit anti-TH (Millipore, #ab152) 1:10,000). Sections were washed in PBS for 1h, incubated in secondary antibody for 2 h under gentle shaking (donkey anti-rabbit Cy2 1:200; donkey anti-goat Cy2 1:200; donkey anti-guinea pig Cy3 1:200 (Jackson ImmunoResearch). After 1h washing in PBS, slices were briefly incubated with DAPI (1 μg/ml). All sections were mounted onto superfrost plus slides, allowed to dry overnight, dehydrated in alcohol and cleared in Citrisolv, then coverslipped using DPX mounting medium (Electron Microscopy Sciences).
Imaging and analysis for immunofluorescence
VGLUT1-, VGLUT2-, PSD95- and TH-immunofluorescence were analyzed in the NAc shell (Fig. 1b -from bregma 1.70 mm to 0.98 mm, according to Paxinos and Franklin, 2001). For analysis of VGLUT puncta levels, images (one per section, n = 6 per animal) were acquired on a confocal LSM 780 upright microscope (Zeiss) at 100× magnification (Plan-Apochromat 100×/1.46 Oil DIC M27; zoom 1.0; pixel size 0.83 μm) as 2 μm z-stacks (21 slices, 0.1 μm interval) and deconvolved using AutoQuant. Analysis of puncta was performed in ImageJ, using the functions ‘max intensity’ for stacks and ‘find maxima’ to determine puncta number and ‘colocalization threshold’ for colocalization analysis of VGLUT2 and TH puncta.
Imaging and NeuronStudio
Dendritic segments (50–150 um from cell soma) were randomly selected from Lucifer yellow filled MSNs in the NAc shell. All segments were selected by previously published criteria (Christoffel et al., 2011a). Briefly, they were completely filled, at least 50 um from soma and non-overlapping with other dendritic branches. All images were acquired on a confocal LSM 710 (Carl Zeiss). Segments were imaged using the 100× lens with a zoom of 2.5. Pixel size in the x-y plane was 0.03 and in the m/z plane was 0.01 um. Image resolution was 1024 × ~300 (the y dimension was adjusted to the particular dendritic segment to expedite imaging), pixel dwell time was 1.58 μm/s, and the line average was set to 4. An average of 3 dendrites per neuron on 3 neurons per animal (n = 26 mice, n = 4–8 mice per sex/group) totaling an average of 3,851 dendritic spines per experimental group were analyzed. For quantitative analysis of spine size, shape, and volume, NeuronStudio was used as described previously (Rodriguez et al., 2008, Christoffel et al., 2011a)). NeuronStudio classifies spines as thin, mushroom, or stubby based on the following values: (1) aspect ratio, (2) head to neck ratio, and (3) head diameter. Spines with a neck can be classified as either thin or mushroom, and those without a significant neck are classified as stubby. Spines with a neck are labeled as thin or mushroom based on head diameter. These parameters have been verified by comparison with trained human operators.
Statistical analysis
Data were analyzed by 2-way ANOVA, with sex and stress as factors. Bonferroni post test was used for post hoc analysis. All statistical analyses were performed using Graph Pad Prism 6.1 software (Graphpad). Statistical significance was set a p < 0.05. Grubbs outlier test was performed on immunofluorescence images and samples that varied 2 SDs from the mean were removed and not considered for data analysis (one image out of six for CTR M, and one image out of six in CTR F). Data are reported as mean ± S.E.M.
Results
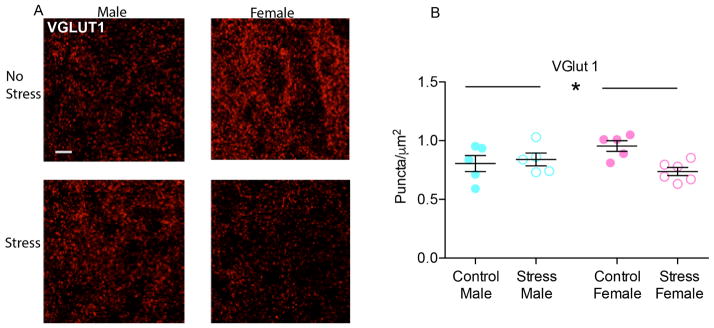
Confocal imaging revealed dense immunolabeling of VGLUT1, PSD95 (Fig. 1c–d), VGLUT2 and TH immunoreactive puncta in the NAc (Fig. 1e–f). Colocalization of TH and VGLUT2 were observed at low levels in NAc afferents (data not shown). In particular, the analysis of colocalized voxel revealed that they represented a small percentage of either TH-positive voxels (CTR M: 1.37 ± 0.95 %; CTR F: 1.63 ± 1.58 %; SCVS M: 2.19 ± 0.65 %; SCVS F: 1.05 ± 0.89 %) and VGLUT2-positive voxels (CTR M: 0.52 ± 0.58 %; CTR F: 3.14 ± 4.92 %; SCVS M: 1.09 ± 0.38 %; SCVS F: 3.94 ± 5.47 %) and 2-way ANOVA on number of co-localized voxels indicated no significant effect of stress, sex or their interaction. VGLUT1 puncta densely surrounded the NAc neurons (fig. 2a). The analysis of VGLUT1 puncta per μm2 showed a significant effect of the interaction between stress and sex (F(1, 17) = 6.133, p = 0.0241) and Bonferroni post hoc indicated a significant decrease of VGLUT1 puncta only in female mice that underwent subchronic variable stress (t = 3.086, p = 0.0402), with respect to control females; no difference was found between male and female controls or between stressed and unstressed males (fig. 2b).
Fig. 2. Effect of SCVS on VGLUT1 puncta in NAc of male and female mice.
(a) Representative pictures of VGLUT1 puncta in the experimental groups. 100×, scale bar 10 μm. Max intensity projections of a 2 μm z-stack. (b) SCVS decreased VGLUT1 puncta density only in female animals. Values represent the mean of 5–6 slices. *indicates significant interaction between stress and sex. All data represented as mean ± SEM.
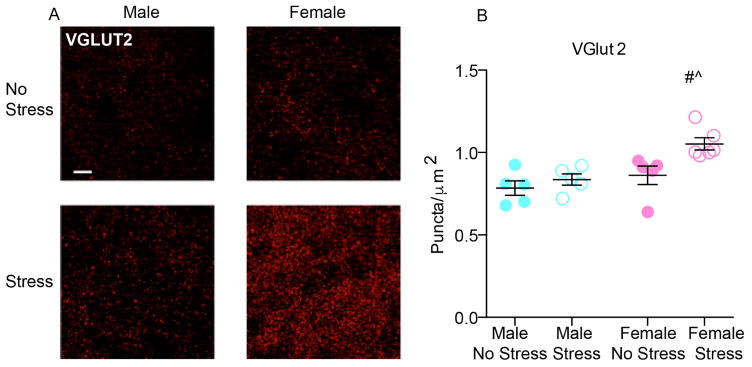
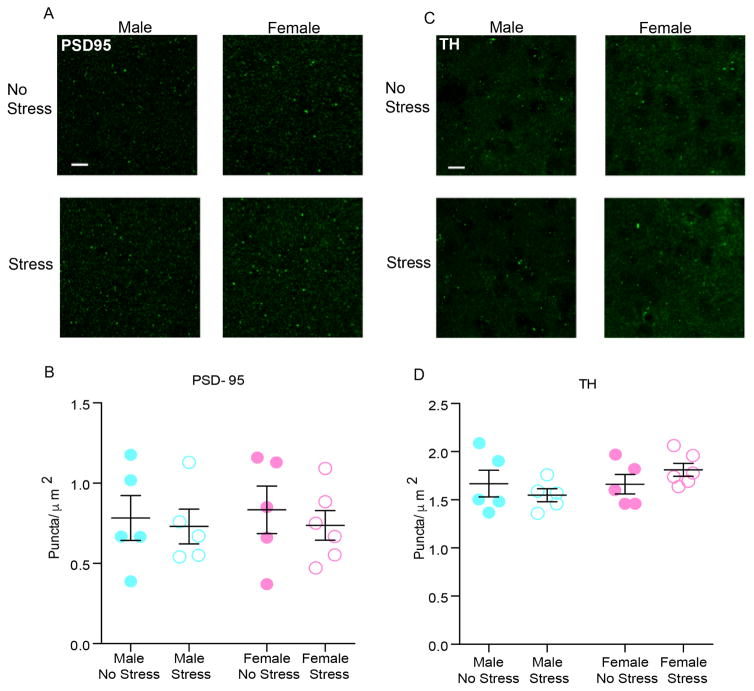
NAc VGLUT2 expressing terminals are shown in fig. 3a. Two-way ANOVA performed on the puncta density data showed a significant main effects of stress (F(1, 17) = 7.762; p = 0.0127) and sex (F(1, 17) = 11.46; p = 0.0035, fig. 3b). Stressed female mice had a 9.9% change in VGLUT2 puncta from same sex control values vs. 3% increase in male mice indicating stress effects were driven by female expression. PSD95 puncta density was not different between unstressed and stressed groups (Fig. 4a–b), suggesting that SCVS does not induce postsynaptic changes in excitatory synapses in either sex. Additionally, we found no difference in TH positive varicosities in the NAc among unstressed and stressed groups in either sex (Fig. 4c–d).
Fig. 3. Effect of SCVS on VGLUT2 puncta in Nac of male and female mice.
(a) Representative images of VGLUT2 puncta in the experimental groups. 100×, scale bar 10 μm. Max intensity projections of a 2 μm z-stack. (b) SCVS increased VGLUT2 puncta density in stressed mice, females had greater increases in puncta than males. Values represent the mean of 5–6 slices. # indicates a main effect of sex, ^ indicates a main effect of stress. All data represented as mean ± SEM.
Fig. 4. Effect of SCVS on PSD95 puncta and TH puncta in NAc of male and female mice.
(a) Representative images of PSD95 puncta in the experimental groups. 100×, scale bar 10 μm. Max intensity projections of a 2 μm z-stack (b) SCVS had no effect on PSD95 puncta density in NAc.(c) Representative pictures of TH puncta in the experimental groups. 100×, scale bar 10 μm. Max intensity projections of a 2 μm z-stack. (d) SCVS had no effect on TH puncta in NAc. All data represented and mean ± SEM.
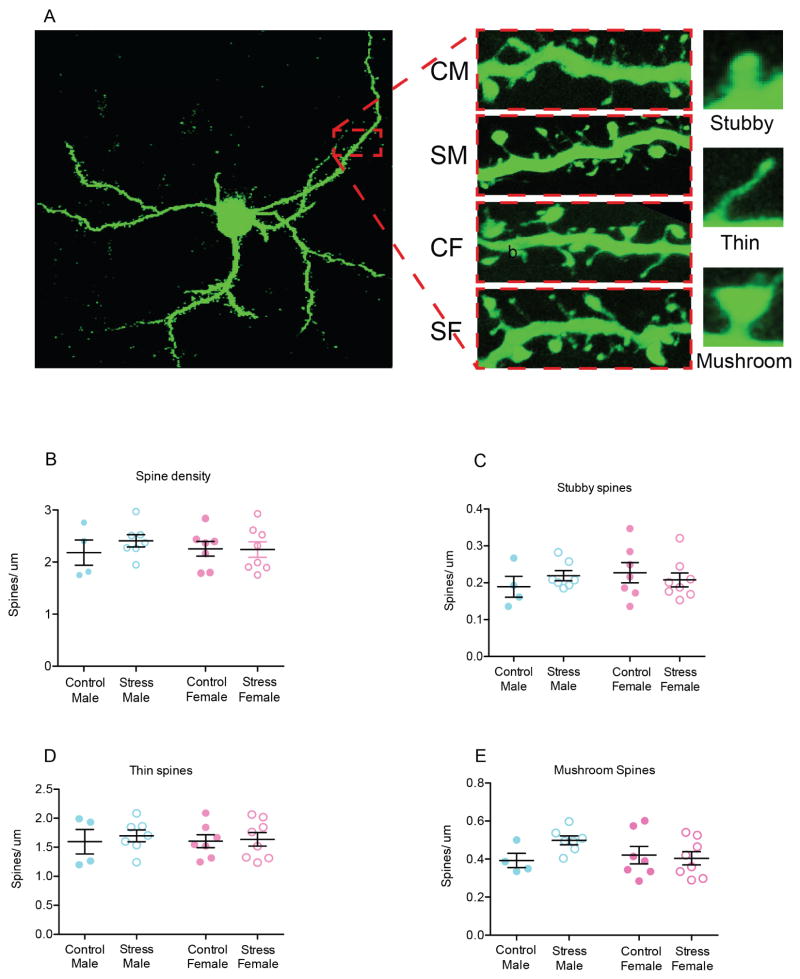
In order to further examine the effects of SCVS on postsynaptic plasticity, we examined spine density and morphology on dendritic segments from Lucifer yellow filled MSNs (Fig. 5a) and then performed semi-automated analysis of spine type using NeuronStudio (Rodriguez et al., 2008). We found no effects of sex or stress (p values < 0.05) on spine density and no interaction between these factors (p <0.05 Fig. 5b). We also found no effect of sex or stress on individual spine type (p values < 0.05, Fig. 5c–e). Together, these data suggest that SCVS specifically affects presynaptic inputs of glutamate synapses in female mice.
Fig. 5. Effect of SCVS on spine density and phenotype in NAc of male and female mice.
(a) Representative images of Lucifer yellow filled medium spiny neurons (100 x) depicting the different types of spine morphology. (b) SCVS did not alter spine density. (c). There were no effects of SCVS on stubby spine density. (d). SCVS did not alter thin spines in males or females. (e). SCVS did not alter mushroom spines in male or female. All data represented as mean ± SEM.
Discussion
This study compared the effects of SCVS on glutamatergic plasticity in the NAc of female and male mice. Our results suggest that SCVS induced synaptic alterations of NAc glutamatergic neurotransmission only in female mice that are susceptible to the SCVS paradigm. These data confirm that SCVS is a useful paradigm to study the greater susceptibility to stress and stress-induced disorders of females with respect to males (Kessler et al., 1994, Marcus et al., 2005). Previous studies demonstrated that male animals are resilient to SCVS, while females display a constellation of behavioral responses that mirror core symptoms of depression, including passive coping, decreased motivation and low preference for natural rewards (LaPlant et al., 2009; Hodes et al., 2015). Different protocols of chronic unpredictable/mild stress have been successfully employed to study stress-induced depression in male animals, but they require longer stress exposure in order to observe pro-depressant-like behavioral effects (Ménard et al., 2015). As a result, SCVS allows us to model sex differences in stress susceptibility observed in human woman, to address important mechanistic questions. Here we discovered that SCVS bi-directionally altered VGLUT1 and VGLUT2 containing pre-synaptic elements of glutamatergic synapses in the NAc of female mice. We further showed no detectable changes in post-synaptic density, spine morphology or dopaminergic input to neurons. Because VGLUT1 and VGLUT2 are enriched within separate presynaptic cell types throughout the brain, our data suggest that SCVS induces circuit specific reorganization in female mice.
VGLUT1 has a key role in glutamate release in the forebrain (Wojcik et al., 2004) and recent clinical studies reported decreased levels of VGLUT1 in the entorhinal cortex of depressed human subjects (Uezato et al., 2009). We found that levels of VGLUT1 were decreased only in stressed females. It is possible that an extended period of stress (ie. 21 or 28 days), would alter VGLUT expression in males as previous reports demonstrated that six weeks of chronic mild stress, a protocol that induces depression-like behavior in male mice, decreased VGLUT1 protein levels in the hippocampus and frontal cortex (Elizalde et al., 2010). Moreover, heterozygous VGLUT1 knockout decreased sucrose preference and increased immobility in the forced swim test (Garcia-Garcia et al., 2009, Elizalde et al., 2010). Other stress models have also been tied to alterations in VGLUT1. A study that examined male mice that were behaviorally susceptible to chronic social stress found a trend towards decreased VGLUT1-positive puncta in the NAc (Christoffel et al., 2015). In addition, optogenetic stimulation of glutamatergic input from prefrontal cortex, one of the VGLUT1-containing glutamatergic inputs to the NAc, induced resilience to chronic social stress in male mice (Covington et al., 2010, Bagot et al., 2015). The decrease of VGLUT1 positive terminals in the NAc of female mice susceptible to SCVS reported here could represent a sex-specific neurobiological alteration arising from neuroplasticity of glutamatergic inputs from the prefrontal cortex, amygdala or ventral hippocampus. Further studies are needed to evaluate the circuit-specific source of SCVS-induced decreased VGLUT1-positive inputs in female mice.
We also report that SCVS significantly increased VGLUT2 levels in the NAc of female mice and to a lesser degree male mice, however, we found no effect of SCVS in either sex on the number of co-localized VGLUT2-TH puncta, which label a subset of midbrain dopaminergic terminals whose axons express VGLUT2 and may co-release dopamine and glutamate into the NAc (Tecuapetla et al., 2010; Stuber et al., 2010). Our finding parallels previous reports showing no effect of chronic stress on dopaminergic transmission in the NAc of both female and male rats (Dalla et al., 2008b). This suggests that SCVS is affecting other glutamatergic inputs emanating from outside of the VTA. One possible candidate is the intralaminar thalamus (ILT). Although statistical analysis did not highlight a significant interaction between stress and gender, the significant effect of stress on VGLUT2 puncta was driven by the large increase in females and is consistent with our previous observations. We have shown previously that, in males exposed to chronic social stress, VGLUT2 puncta was increased in the NAc of susceptible but not resilient mice (Christoffel et al., 2015). We further showed that optogenetic stimulation of VGLUT2 containing inputs to the NAC from ILT increases depression-like behaviors in males. Thus, the increase in VGLUT2 expression may contribute to circuit specific increases of ILT-NAc pre- synaptic glutamate release, potentially strengthening these connections, coinciding with a weakening the VGLUT1 inputs from other brain regions.
Despite observing significant changes within presynaptic glutamate synaptic markers, we found no effects of SCVS on PSD95, a core component of the post-synaptic density scaffold (Nair et al., 2013). PSD95-positive immunofluorescence can be thought of as a surrogate marker of post-synaptic compartments directly onto MSNs. We also found no effects of stress or sex on spine density or spine morphology on MSNs, which supports the idea that female susceptibility to SCVS is associated with changes in presynaptic innervation of the NAc. However, based on the current study we cannot rule out that circuit or even cell type specific changes in post-synaptic plasticity occlude these findings. It is possible that there are changes in specific VGLUT2 inputs associated with post-synaptic dendritic remodeling that are not evident with currently available methods. Furthermore, changes in post-synaptic density and morphology may occur on a specific NAc cell type (ie. D1 or D2 MSNs). Indeed, stress produces distinct functional synaptic plasticity on D1 versus D2 MSNs in susceptible versus resilient mice (Francis et al., 2015, Francis and Lobo, 2016, Khibnik et al., 2016). In addition, the stage of the estrous cycle was not tracked in the current study. Previous research demonstrated that spine density on pyramidal cells in the hippocampus was altered by circulating levels of estrogen and progesterone (Woolley and McEwen, 1993, 1994) and that stress effects on spine density in females could be altered by ovariectomy (Shors et al., 2001, Leuner and Shors, 2013). Therefore it is possible that hormonal effects occluded changes in spine density on MSNs, future research will be necessary to tease out potential hormonal effects. Given the limitations of the current study, our data indicate that SCVS induced a substantial rewiring of presynaptic glutamatergic inputs in susceptible female mice. Thus, we can speculate that SCVS-induced behavioral susceptibility is associated with decreased glutamate inputs from the prefrontal cortex, hippocampus and/or basolateral amygdala to the NAc, and increased glutamatergic input from the thalamus. In the NAc the integration among distinct excitatory inputs is crucial to shape goal-directed behaviors and it is increasingly recognized that distinct glutamate inputs convey different kinds of information related to the pro-depressant effects of chronic stress (Christoffel et al., 2015). Future studies will be needed to determine the functional roles of individual glutamatergic inputs to the NAc in mediating sex differences in stress susceptibility.
Conclusion
This is the first study to characterize the effects of SCVS on pre- and post-synaptic plasticity of glutamatergic synapses in the NAc of male and female mice. These data demonstrate that SCVS induces rearrangement of pre-synaptic VGLUT-1 and VGLUT-2 inputs, which likely reflect changes in the strength of distinct glutamatergic inputs to the NAc. Further studies are needed to explore the complex framework of glutamatergic dysfunctions in the NAc in females’ susceptibility to stress and depression.
Highlights.
6 days of variable stress sex specifically decreases VGLUT1 in NAc.
Variable stress increases VGLUT2 in NAc 3-fold higher in females than males.
Variable stress does not alter spine density or morphology in either sex.
Variable stress does not alter expression of PSD-95 or TH in either sex.
Stressed females undergo circuit specific activation of glutamatergic synapses.
Acknowledgments
This research was supported by R01MH090264; R21MH099562; P50MH096890; NARSAD Young Investigator Award.
Footnotes
Author contributions
A.B., D.B., H.F.A. M.P. C. M. collected data for the study. A.B., S.J.R. and G.E.H. were involved in the design and statistical analysis of the manuscript. A.B., C.M., C.C. S.J.R. and G.E.H. wrote and edited the manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot RC, Parise EM, Pena CJ, Zhang HX, Maze I, Chaudhury D, Persaud B, Cachope R, Bolanos-Guzman CA, Cheer JF, Deisseroth K, Han MH, Nestler EJ. Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat Commun. 2015;6:7062. doi: 10.1038/ncomms8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrot M, Marinelli M, Abrous DN, Rougé-Pont F, Le Moal M, Piazza PV. Functional heterogeneity in dopamine release and in the expression of Fos-like proteins within the rat striatal complex. Eur J Neurosci. 1999;11(4):1155–66. doi: 10.1046/j.1460-9568.1999.00525.x. [DOI] [PubMed] [Google Scholar]
- Bellocchio EE, Hu H, Pohorille A, Chan J, Pickel VM, Edwards RH. The localization of the brain-specific inorganic phosphate transporter suggests a specific presynaptic role in glutamatergic transmission. J Neurosci. 1998;18:8648–8659. doi: 10.1523/JNEUROSCI.18-21-08648.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessa JM, Morais M, Marques F, Pinto L, Palha JA, Almeida OF, Sousa N. Stress-induced anhedonia is associated with hypertrophy of medium spiny neurons of the nucleus accumbens. Transl Psychiatry. 2013;3:e266. doi: 10.1038/tp.2013.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campioni MR, Xu M, McGehee DS. Stress-induced changes in nucleus accumbens glutamate synaptic plasticity. J Neurophysiol. 2009;101(6):3192–8. doi: 10.1152/jn.91111.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, Krishnan V, Reyes CM, Han MH, Ables JL, Eisch AJ, Dietz DM, Ferguson D, Neve RL, Greengard P, Kim Y, Morrison JH, Russo SJ. IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci. 2011a;31:314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Russo SJ. Structural and synaptic plasticity in stress-related disorders. Rev Neurosci. 2011b;22:535–549. doi: 10.1515/RNS.2011.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Walsh JJ, Guise KG, Heshmati M, Friedman AK, Dey A, Smith M, Rebusi N, Pfau M, Ables JL, Aleyasin H, Khibnik LA, Hodes GE, Ben-Dor GA, Deisseroth K, Shapiro ML, Malenka RC, Ibanez-Tallon I, Han MH, Russo SJ. Excitatory transmission at thalamo-striatal synapses mediates susceptibility to social stress. Nat Neurosci. 2015;18:962–964. doi: 10.1038/nn.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, 3rd, Lobo MK, Maze I, Vialou V, Hyman JM, Zaman S, LaPlant Q, Mouzon E, Ghose S, Tamminga CA, Neve RL, Deisseroth K, Nestler EJ. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J Neurosci. 2010;30:16082–16090. doi: 10.1523/JNEUROSCI.1731-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla C, Antoniou K, Drossopoulou G, Xagoraris M, Kokras N, Sfikakis A, Papadopoulou-Daifoti Z. Chronic mild stress impact: are females more vulnerable? Neuroscience. 2005;135:703–714. doi: 10.1016/j.neuroscience.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Dalla C, Edgecomb C, Whetstone AS, Shors TJ. Females do not express learned helplessness like males do. Neuropsychopharmacology. 2008a;33:1559–1569. doi: 10.1038/sj.npp.1301533. [DOI] [PubMed] [Google Scholar]
- Dalla C, Antoniou K, Kokras N, Drossopoulou G, Papathanasiou G, Bekris S, Daskas S, Papadopoulou-Daifoti Z. Sex differences in the effects of two stress paradigms on dopaminergic neurotransmission. Physiology & Behavior. 2008b;93:595–605. doi: 10.1016/j.physbeh.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Rodriguez A, Morrison JH. High-throughput, detailed, cell-specific neuroanatomy of dendritic spines using microinjection and confocal microscopy. Nat Protoc. 2011;6:1391–1411. doi: 10.1038/nprot.2011.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elizalde N, Pastor PM, Garcia-Garcia AL, Serres F, Venzala E, Huarte J, Ramirez MJ, Del Rio J, Sharp T, Tordera RM. Regulation of markers of synaptic function in mouse models of depression: chronic mild stress and decreased expression of VGLUT1. J Neurochem. 2010;114:1302–1314. doi: 10.1111/j.1471-4159.2010.06854.x. [DOI] [PubMed] [Google Scholar]
- Foland-Ross LC, Kircanski K, Gotlib IH. Coping with having a depressed mother: the role of stress and coping in hypothalamic-pituitary-adrenal axis dysfunction in girls at familial risk for major depression. Dev Psychopathol. 2014;26:1401–1409. doi: 10.1017/S0954579414001102. [DOI] [PubMed] [Google Scholar]
- Francis TC, Chandra R, Friend DM, Finkel E, Dayrit G, Miranda J, Brooks JM, Iniguez SD, O’Donnell P, Kravitz A, Lobo MK. Nucleus accumbens medium spiny neuron subtypes mediate depression-related outcomes to social defeat stress. Biol Psychiatry. 2015;77:212–222. doi: 10.1016/j.biopsych.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis TC, Lobo MK. Emerging role for nucleus accumbens medium spiny neuron subtypes in depression. Biol Psychiatry. 2016 doi: 10.1016/j.biopsych.2016.09.007. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Kam K, Qureshi T, Johnson J, Copenhagen DR, Storm-Mathisen J, Chaudhry FA, Nicoll RA, Edwards RH. Vesicular glutamate transporters 1 and 2 target to functionally distinct synaptic release sites. Science. 2004;304:1815–1819. doi: 10.1126/science.1097468. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Fujiyama F, Furuta T, Kaneko T. Immunocytochemical localization of candidates for vesicular glutamate transporters in the rat cerebral cortex. J Comp Neurol. 2001;435:379–387. doi: 10.1002/cne.1037. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia AL, Elizalde N, Matrov D, Harro J, Wojcik SM, Venzala E, Ramirez MJ, Del Rio J, Tordera RM. Increased vulnerability to depressive-like behavior of mice with decreased expression of VGLUT1. Biol Psychiatry. 2009;66:275–282. doi: 10.1016/j.biopsych.2009.02.027. [DOI] [PubMed] [Google Scholar]
- Golden SA, Christoffel DJ, Heshmati M, Hodes GE, Magida J, Davis K, Cahill ME, Dias C, Ribeiro E, Ables JL, Kennedy PJ, Robison AJ, Gonzalez-Maeso J, Neve RL, Turecki G, Ghose S, Tamminga CA, Russo SJ. Epigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression. Nature medicine. 2013;19:337–344. doi: 10.1038/nm.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldwater DS, Pavlides C, Hunter RG, Bloss EB, Hof PR, McEwen BS, Morrison JH. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience. 2009;164:798–808. doi: 10.1016/j.neuroscience.2009.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartig W, Riedel A, Grosche J, Edwards RH, Fremeau RT, Jr, Harkany T, Brauer K, Arendt T. Complementary distribution of vesicular glutamate transporters 1 and 2 in the nucleus accumbens of rat: Relationship to calretinin-containing extrinsic innervation and calbindin-immunoreactive neurons. J Comp Neurol. 2003;465:1–10. doi: 10.1002/cne.10789. [DOI] [PubMed] [Google Scholar]
- Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ, Magida J, Brancato A, Takahashi A, Flanigan ME, Menard C, Aleyasin H, Koo JW, Lorsch ZS, Feng J, Heshmati M, Wang M, Turecki G, Neve R, Zhang B, Shen L, Nestler EJ, Russo SJ. Sex differences in nucleus accumbens transcriptome profiles associated with susceptibility versus resilience to subchronic variable stress. J Neurosci. 2015;35:16362–16376. doi: 10.1523/JNEUROSCI.1392-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Res. 1995;675:325–328. doi: 10.1016/0006-8993(95)00013-g. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Khibnik LA, Beaumont M, Doyle M, Heshmati M, Slesinger PA, Nestler EJ, Russo SJ. Stress and cocaine trigger divergent and cell type-specific regulation of synaptic transmission at single spines in nucleus accumbens. Biol Psychiatry. 2016;79:898–905. doi: 10.1016/j.biopsych.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPlant Q, Chakravarty S, Vialou V, Mukherjee S, Koo JW, Kalahasti G, Bradbury KR, Taylor SV, Maze I, Kumar A, Graham A, Birnbaum SG, Krishnan V, Truong HT, Neve RL, Nestler EJ, Russo SJ. Role of nuclear factor kappaB in ovarian hormone-mediated stress hypersensitivity in female mice. Biol Psychiatry. 2009;65:874–880. doi: 10.1016/j.biopsych.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Shors TJ. Stress, anxiety, and dendritic spines: what are the connections? Neuroscience. 2013;251:108–119. doi: 10.1016/j.neuroscience.2012.04.021. [DOI] [PubMed] [Google Scholar]
- Marcus SM, Young EA, Kerber KB, Kornstein S, Farabaugh AH, Mitchell J, Wisniewski SR, Balasubramani GK, Trivedi MH, Rush AJ. Gender differences in depression: findings from the STAR*D study. J Affect Disord. 2005;87:141–150. doi: 10.1016/j.jad.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Menard C, Hodes GE, Russo SJ. Pathogenesis of depression: Insights from human and rodent studies. Neuroscience. 2016;321:138–162. doi: 10.1016/j.neuroscience.2015.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez JA, Bourque MJ, Dal Bo G, Bourdeau ML, Danik M, Williams S, Lacaille JC, Trudeau LE. Developmental and target-dependent regulation of vesicular glutamate transporter expression by dopamine neurons. J Neurosci. 2008;28:6309–6318. doi: 10.1523/JNEUROSCI.1331-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder AB, Hodenpijl MG, Lopes da Silva FH. Electrophysiology of the hippocampal and amygdaloid projections to the nucleus accumbens of the rat: convergence, segregation, and interaction of inputs. J Neurosci. 1998;18(13):5095–5102. doi: 10.1523/JNEUROSCI.18-13-05095.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Physiological and morphological properties of accumbens core and shell neurons recorded in vitro. Synapse. 1993;13(2):135–160. doi: 10.1002/syn.890130206. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The mouse brain in stereotaxic coordinates. Academic Press; San Diego: 2001. [Google Scholar]
- Pfau ML, Purushothaman I, Feng J, Golden SA, Aleyasin H, Lorsch ZS, Cates HM, Flanigan ME, Menard C, Heshmati M, Wang Z, Ma’ayan A, Shen L, Hodes GE, Russo SJ. Integrative analysis of sex-specific microRNA networks following stress in mouse nucleus accumbens. F Front Mol Neurosci. 2016;9:144. doi: 10.3389/fnmol.2016.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada P, Moreno SA, Tucci S, Gonzalez LE, Harrison T, Chau DT, Hoebel BG, Hernandez L. Glutamate release in the nucleus accumbens is involved in behavioral depression during the PORSOLT swim test. Neuroscience. 2003;119:557–565. doi: 10.1016/s0306-4522(03)00162-3. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, Morrison JH, Wearne SL, Hof PR. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol. 2008;507:1141–1150. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A, Ehlenberger DB, Dickstein DL, Hof PR, Wearne SL. Automated three-dimensional detection and shape classification of dendritic spines from fluorescence microscopy images. PLoS One. 2008;3:e1997. doi: 10.1371/journal.pone.0001997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci. 2010;30:8229–8233. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecuapetla F, Patel JC, Xenias H, English D, Tadros I, Shah F, Berlin J, Deisseroth K, Rice ME, Tepper JM, Koos T. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. J Neurosci. 2010;30:7105–7110. doi: 10.1523/JNEUROSCI.0265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SM, Kallarackal AJ, Kvarta MD, Van Dyke AM, LeGates TA, Cai X. An excitatory synapse hypothesis of depression. Trends Neurosci. 2015;38:279–294. doi: 10.1016/j.tins.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth E, Gersner R, Wilf-Yarkoni A, Raizel H, Dar DE, Richter-Levin G, Levit O, Zangen A. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J Neurochem. 2008;107:522–532. doi: 10.1111/j.1471-4159.2008.05642.x. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Pride MC, Villalon Landeros R, Knoblauch NW, Takahashi EY, Silva AL, Crean KK. Sex differences in social interaction behavior following social defeat stress in the monogamous California mouse (Peromyscus californicus) PLoS One. 2011;6:e17405. doi: 10.1371/journal.pone.0017405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uezato A, Meador-Woodruff JH, McCullumsmith RE. Vesicular glutamate transporter mRNA expression in the medial temporal lobe in major depressive disorder, bipolar disorder, and schizophrenia. Bipolar Disord. 2009;11:711–725. doi: 10.1111/j.1399-5618.2009.00752.x. [DOI] [PubMed] [Google Scholar]
- Varoqui H, Schafer MK, Zhu H, Weihe E, Erickson JD. Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J Neurosci. 2002;22:142–155. doi: 10.1523/JNEUROSCI.22-01-00142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Robison AJ, Laplant QC, Covington HE, 3rd, Dietz DM, Ohnishi YN, Mouzon E, Rush AJ, 3rd, Watts EL, Wallace DL, Iniguez SD, Ohnishi YH, Steiner MA, Warren BL, Krishnan V, Bolanos CA, Neve RL, Ghose S, Berton O, Tamminga CA, Nestler EJ. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2010;13:745–752. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik SM, Rhee JS, Herzog E, Sigler A, Jahn R, Takamori S, Brose N, Rosenmund C. An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size. Proc Natl Acad Sci U S A. 2004;101:7158–7163. doi: 10.1073/pnas.0401764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor-dependent mechanism. J Neurosci. 1994;14:7680–7687. doi: 10.1523/JNEUROSCI.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]