Abstract
Introduction:
Increase in extended-spectrum β-lactamases (ESBL) producing microbes in recent years has led to limitations of treatment options. This study aimed to assess the prevalence of ESBL producing E. coli and Klebsiella spp. at a tertiary hospital in Nepal.
Methods:
A total of 2209 non-repetitive mid-stream urine (MSU) samples were collected during the study period (March to September 2014). Identification of the isolates was done by Gram's staining followed by biochemical tests. Antibiotic susceptibility testing was done by modified Kirby-Bauer disc diffusion method and interpretation was done following Clinical and Laboratory Standard Institute (CLSI) guidelines, 2013. ESBL screening among E. coli and Klebsiella spp. isolates were done using ceftriaxone, cefotaxime, ceftazidime and cefpodoxime. The confirmation was done by phenotypic disc diffusion test (combined disc method) using ceftazidime (30µg) and ceftazidime plus clavulanic acid (30/10µg), and cefotaxime (30µg) and cefotaxime plus clavulanic acid (30/10µg) disc as per CLSI guidelines.
Results:
A total of 451 samples showed significant bacteriuria with 365 (80.9%) E. coli, 17 (3.8%) Klebsiella pneumoniae and 3 (0.7%) Klebsiella oxytoca. Of 451 isolates, 236 (52.3%) were found MDR strains. By combined disk test, 33 (91.7%) E. coli and 3 (8.3%) Klebsiella spp. were found ESBL producers.
Conclusion:
Higher prevalence of ESBL producing E. coli and Klebsiella spp. was observed warranting prompt need of surveillance for effective management of such MDR strains.
Keywords: E. coli, Extended-spectrum β-lactamase, ESBL, Klebsiella, Nepal, Urinary tract infection, UTI
BACKGROUND
Urinary tract infection (UTI) is a spectrum of disease caused by microbial invasion of the genitourinary tract that extends from the renal cortex of the kidney to the urethral meatus [1]. UTI is an extremely common condition that occurs in both males and females of all ages. The prevalence and incidence of infection are higher in women than in men, which is likely the result of several clinical factors including anatomical differences, hormonal effects and behavior patterns [2]. The reported positive rate of UTI among Nepalese patients attending general hospitals ranged from 23.1% to 37.4% [3].
Bacteria are the major causative organisms and are responsible for more than 95% of UTI cases. Escherichia coli is the most prevalent causative organisms of UTI and is solely responsible for more than 80% of the infections [4].
Multiple drug resistance has significantly increased in recent years. There is a growing concern for multidrug-resistant Gram-negative bacteria which produce extended-spectrum β-lactamases (ESBLs) [5]. ESBLs are Class A β-lactamases that hydrolyse penicillin, oxyimino-cephalosporins, and monobactams but not cephamycins or carbapenems [6]. They are inhibited in vitro by clavulanate. ESBLs are often plasmid-mediated enzymes and have various genotypes. The most common are the SHV, TEM, and CTX-M types. Other clinically important types include VEB, PER, BEL-1, BES-1, SFO-1, TLA, and IBC [5]. ESBLs are primarily produced by the Enterobacteriaceae family of Gram-negative organisms, in particular Klebsiella pneumoniae, K. oxytoca and E. coli [7-9].They are also produced by other Gram-negative organisms, such as Acinetobacter baumannii, Proteus spp, Pseudomonas aeruginosa and Salmonella spp. [8, 10].
Since the first isolation of ESBL-producing organism in 1983 from Germany, it has been increasingly reported worldwide to date [11, 12].The prevalence of ESBL-positive isolates depends on a range of factors including species, geographic locality, hospital/ward, group of patients and type of infection, and large variations have been reported in different studies [13].
There are limited studies showing a high rate of ESBL in Nepal, where Enterobacteriaceae were found 28% to 67% [14]. The acquisition and expression of ESBLs enzymes among Enterobactericeae have posed a serious public health problem. Many parts of Nepal still lack the facilities for urine culture and antimicrobial susceptibility testing; this clearly leads to missing ESBL isolates. Delayed reporting of ESBL producing Gram-negative bacilli is associated with prolonged hospital stay, increased morbidity, increased motility and high health care costs [6]. Information on prevailing levels of antimicrobial resistance among common pathogens is useful in making an appropriate choice of empiric therapy [15]. This study would help clinicians to be aware of the potential of treatment failures associated with serious infections caused by these bacteria and guide for appropriate empirical antimicrobial therapy.
MATERIALS AND METHODS
Study Setting, Design and Population
This prospective cross-sectional study was done at Alka Hospital, Lalitpur (ISO 9001: 2008 standard) to assess the prevalence of ESBL production among the Gram-negative rods isolated from urine samples. All non-repetitive mid-stream urine (MSU) samples obtained during the study period (March to September 2014) were included in the study.
Processing of Samples
All MSU samples were cultured on routine culture media by semi-quantitative method as described in World Health Organization (WHO) manual [16]. In short, 1μL of urine was inoculated on MacConkey and blood agar plate (HiMedia Laboratories Pvt. Ltd., India) by streaking using calibrated loop, and incubated aerobically for 18-48hrs at 37ᵒC. Growth of 100 colonies or more, i.e. 105 colony forming units (CFU)/mL urine, was considered as culture positive. Isolation and identification of isolates were done following their morphology in Gram's staining, cultural characteristics and biochemical properties, as per the Manual of Clinical Microbiology [17].
Antimicrobial Susceptibility Testing
Antibiotic susceptibility testing of all isolates was performed by Kirby-Bauer’s disc diffusion method and interpretation of the results was done as described in CLSI 2013 [18]. Antibiotic discs (HiMedia Laboratories Pvt. Ltd., India) used were amoxicillin (10μg), cefixime (10μg), cefotaxime (30μg), cefpodoxime (30μg), ceftazidime (30μg), ceftriaxone (30μg), ciprofloxacin (5μg), cotrimoxazole (1.25/23.75μg), gentamycin (10μg), imipenem (10μg), nitrofurantoin (300μg), norfloxacin (10μg), ofloxacin (5μg), piperacillin (100μg), and vancomycin (30μg). Control strains of P. aeruginosa ATCC 27853 and E. coli ATCC 25922 were used in parallel as a part of quality control. Organisms resistant to two or more classes of antimicrobial agents were considered to be multidrug resistance (MDR).
Screening of ESBL-Producing Strains
According to CLSI guidelines, strains showing zone of inhibition of ≤25mm for ceftriaxone and/or ≤22mm for ceftazidime and/or ≤17mm for cefpodoxime and/or ≤27mm for cefotaxime were considered for conformational test for ESBL.
Confirmation of ESBL-Producing Strains
ESBL production among potential ESBL-producing isolates was confirmed phenotypically using combined disc method. Comparison of the zone of inhibition was made for the ceftazidime (30µg) and cefotaxime (30µg) discs alone vs. that of the ceftazidime and cefotaxime discs containing clavulanic acid (10µg), when placed 25mm apart (center to center). Isolates showing an increase in zone diameter of ≥5 mm around either of the clavulanate combined discs compared to that of the disc alone was considered ESBL producer. K. pneumoniae ATCC 700603 was used as control strains.
Ethical Consideration
Ethical approval was not required to carry out this work as the bacterial isolates were collected as part of routine patient care investigation in the hospital.
RESULT
This study was conducted among patients suspected of UTI visiting Alka Hospital. A total of 2209 non-repetitive MSU samples were collected from patients for urine culture. Only 451 (20.4%) samples showed significant growth.
Distribution of Samples among Patients
The ratio of number of inpatients to outpatients was 1:2.96. Similarly, number of male patients to female patients ratio was 1:2.2; females were higher in all cases. (Table 1).
Table 1.
Distribution of samples among inpatients and outpatients.
| Urine Culture | Inpatients | Outpatients | ||
|---|---|---|---|---|
| Male (%) | Female (%) | Male (%) | Female (%) | |
| Significant growth | 21(15.2) | 69(16.4) | 86(15.6) | 275(25.0) |
| Non-significant growth | 117(84.8) | 351(83.6) | 466(84.4) | 824(75.0) |
| Total | 138(100) | 420(100) | 552(100) | 1099(100) |
Distribution of Bacterial Isolates
Of the 451 isolates, the most predominant isolate was E. coli 365 (80.9%). Among 451 isolates, 236 (52.3%) were MDR; E. coli with the most MDR share i.e. 188 (79.7%). Out of total, 168 (85.2%) isolates passed screening test for ESBL but only 36 (21.4%) of them were ESBL producers. E. coli was the highest ESBL producers i.e. 33 (91.7%) and followed by K. pneumoniae i.e. 3 (8.3%) (Table 2).
Table 2.
Distribution of isolates.
| Organisms | Total isolates | No. of MDR strains | ESBL screening positive | ESBL confirmed |
|---|---|---|---|---|
| E. coli | 365(80.9%) | 188(79.7%) | 160 | 33 (91.7%) |
| K. oxytoca | 3 (0.7%) | 1(0.4%) | 1 | 0 (0%) |
| K. pneumoniae | 17(3.8%) | 8(3.4%) | 7 | 3 (8.3%) |
| Acinetobacter spp | 1 (0.2%) | 0 (0%) | NT | NT |
| Citrobacter spp | 4 (0.9%) | 1 (0.4%) | NT | NT |
| Enterobacter spp | 3 (0.7%) | 1 (0.4%) | NT | NT |
| Enterococcus spp | 3 (0.7%) | 2 (0.8%) | NT | NT |
| Morganella spp | 3 (0.7%) | 1 (0.4%) | NT | NT |
| Proteus mirabilis | 12 (2.7%) | 7 (3%) | NT | NT |
| Proteus vulgaris | 7 (1.6%) | 6 (2.5%) | NT | NT |
| Providencia spp | 22 (4.9%) | 12 (5.1%) | NT | NT |
| Pseudomonas spp | 7 (1.6%) | 7 (3%) | NT | NT |
| Staphylococcus spp | 4 (0.9%) | 2 (0.8%) | NT | NT |
| Total | 451 (100%) | 236 (100%) | 168 | 36 (100%) |
NT=not tested
Antibiotics Resistivity Pattern of the Isolates
E. coli were highly resistant to amoxicillin 269 (73.69%) and least resistant to imipenem 7(1.9%). Likewise, most Klebsiella spp. were highly resistant to amoxicillin 18 (90%) and least resistant to gentamycin 0 (0%) and imipenem 0 (0%) (Fig. 1).
Fig. (1).
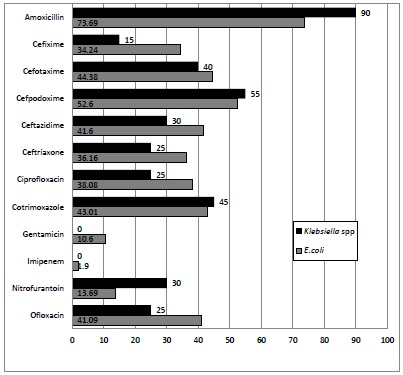
Antibiotics resistivity profile of isolates.
Distribution of ESBL Producing Isolates
The prevalence of ESBL producing E. coli were higher in female 27 (81.8%) compared to male 6 (18.2%). Similarly, the prevalence of ESBL producing Klebsiella spp. was 2 (66.7%) in female and 1(33.3%) in male (Table 3).
Table 3.
Sex wise distribution of ESBL producing isolates.
| Sex | E. coli | Klebsiella spp. | ||||||
|---|---|---|---|---|---|---|---|---|
| E. coli isolates(%) | MDR(%) | Screening positive(%) | ESBL Producers(%) | Klebsiella spp. isolates(%) | MDR(%) | Screening positive(%) | ESBL Producers(%) | |
| Male | 77 (21.1) | 43 (22.9) | 37 (23.1) | 6 (18.2) | 4 (20) | 3 (33.3) | 3 (37.5) | 1 (33.3) |
| Female | 288 (78.9) | 145 (77.1) | 123 (76.9) | 27 (81.8) | 16 (80) | 6 (66.7) | 5 (62.5) | 2 (66.7) |
| Total | 365 (100) | 188 (100) | 160 (100) | 33 (100) | 20 (100) | 9 (100) | 8 (100) | 3 (100) |
The prevalence of ESBL production in E. coli was higher in 21-40 years age group (Table 4).
Table 4.
Age wise distribution of ESBL Producing isolates.
| Age group (years) | E. coli | Klebsiella spp. | ||||||
|---|---|---|---|---|---|---|---|---|
| E. coli isolates(%) | MDR(%) | Screening positive(%) | ESBL producer(%) | Klebsiella spp. isolates(%) | MDR(%) | Screening positive(%) | ESBL producer(%) | |
| <=10 | 31 (8.5) | 16 (8.5) | 12 (7.5) | 1 (3) | 4 (20) | 2 (22.2) | 2 (25) | 0 (0) |
| 11-20 | 16 (4.38) | 8 (4.3) | 5 (3.13) | 1 (3) | 2 (10) | 1 (11.1) | 2 (25) | 1 (33.3) |
| 21-30 | 95 (26) | 44 (23.4) | 31 (19.4) | 9 (27.3) | 2 (10) | 1 (11.1) | 1 (12.5) | 0 (0) |
| 31-40 | 59 (16.2) | 25 (13.3) | 25 (15.6) | 8 (24.2) | 2 (10) | 1 (11.1) | 1 (12.5) | 1 (33.3) |
| 41-50 | 44 (12.1) | 27 (14.4) | 23 (14.4) | 3 (9.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 51-60 | 37 (10.1) | 15 (8) | 19 (11.9) | 6 (18.2) | 3 (15) | 1 (11.1) | 0 (0) | 0 (0) |
| 61-70 | 45 (12.3) | 30 (16) | 24 (15) | 3 (9.1) | 5 (25) | 2 (22.2) | 2 (25) | 1 (33.3) |
| 70+ | 38 (10.4) | 23 (12.2) | 20 (12.5) | 1 (3) | 2 (10) | 1 (11.1) | 0 (0) | 0 (0) |
| Total | 365 (100) | 188 (100) | 160 (100) | 33 (100) | 20 (100) | 9 (100) | 8 (100) | 3 (100) |
DISCUSSION
The emergence and rapid spread of multidrug resistant isolates are of great concern worldwide; among them, ESBL producing Enterobacteriaceae has been major concern. During the past decades, ESBLs producing Gram-negative bacilli especially E. coli and K. pneumoniae have emerged as serious pathogens both in hospital and community acquired infections worldwide. The study was conducted among the patient suspected of UTI visiting Alka Hospital, Lalitpur, Nepal. A total of 2209 non-repetitive MSU samples were included during the study period (March to September 2014). Of these samples, only 451 (20.4%) showed significant growth. Higher growth rates were reported in similar studies of 26.6% [19], 30.8% [20], 41.7% [21], but lower growth rates were reported in other similar studies of 9% [22], 17.4% [23], 19.7% [24].
In this study, the ratio of number of inpatients to outpatients was 1:2.96. And among positive cases, the ratio was 1:4.01. This signifies more prevalence of UTI in community. In this study, the number of male patients to female patients ratio was 1:2.2. And among positive cases, the ratio was 1:3.21. Females were higher in both cases which was involuntary recruitment bias. Females are more frequently affected by(particularly cystitis) due to colonization of urethra with colonic Gram-negative bacteria because of its proximity to anus and short length of urethra [25]. The patient’s sex is risk factor of UTI [26].
E. coli (80.9%) was the most common pathogens isolated. E. coli, including other enterobacteria, are likely to have caused infection after colonization of the periurethral area by gastrointestinal tract flora [27]. This accords with other studies [21, 22, 24, 28-34]. However, discords with following studies [35-38].
Antimicrobial resistance is now accepted as a major problem in public health and patient care. It is more troublesome to developing countries. In this study, E. coli were highly resistant to amoxicillin 269 (73.7%) and least resistant to imipenem 7 (1.9%). This is similar to reports of different studies [21, 23, 24, 29-31, 33, 35-41]. Likewise, most Klebsiella spp. were highly resistant to amoxicillin 18 (90%) and least resistant to gentamycin 0 (0%) and imipenem 0 (0%). Similar result was shown in different studies [29, 33, 35, 36, 38, 41, 42]. Imipenem was the most effective drug, however, it should not be administered as empirical drug unless infection is life threatening, as carbapenems are considered the drug of last resort. In case, gentamycin or nitrofurantoin could be the empiric choice. In this study, 236 (52.3%) isolates were MDR. The result accords with other studies showing 55.9% [43], 64% [23], and 64.9% [24]of MDR isolates.
Identifying ESBL producing organisms is a major challenge in clinical settings and, due to the selective pressure caused by heavy use of expanded-spectrum cephalosporins, lapses in effective infection control measures and affinity of these enzymes for different substrates, outbreaks are increasing [44]. The prevalence of ESBL producing Enterobacteriaceae varies greatly among country and among the hospitals within the country. Less than 1% to greater than 70% ESBLs producers are reported worldwide. Of 385 isolates of E. coli and Klebsiella spp. 168 isolates passed ESBL screening test but only 36 (9.4%) were confirmed ESBL producers phenotypically. The prevalence of ESBL production was higher in 21 - 40 years age group. The prevalence was higher in this age group as most isolates, accounting 41%, were isolated from this group. Besides, self medication practice which is high in this age group, could have further accounted for higher prevalence [45, 46]. A higher prevalence of ESBL production was observed in E. coli followed by K. pneumoniae. The findings are in agreement with the study [11], however, contrary to the findings of other study [47] of 365 E. coli isolates, 33 (9.0%) were ESBL producers. This accords with the other studies [33, 35, 41, 48], however, discords with following studies [22, 24, 29-32, 36-38]. Likewise, of 20 Klebsiella spp. isolates, 3 (15%) were ESBL producers. This accords with other studies [22, 29, 35, 48], however, discords with following studies [32, 33, 36, 41].
ESBL-producing strains are creating significant therapeutic problems since these pathogens are resistant to a wide range of β-lactams, including third generation cephalosporins as well as have potential for plasmid mediated quinolone and carbapenem resistance. Antibacterial choice is often complicated by MDR leading to over-prescription of antibiotics. As indicated by the present finding together with previous findings, it appears to be mandatory to include ESBL detection in routine laboratory practice so as to limit the rapid spread of ESBL-producing organisms.
CONCLUSION
In this study, E. coli was found to be the most predominant MDR isolate. The prevalence of ESBL producing E. coli and Klebsiella spp. was higher. The majority of ESBL producing E. coli and Klebsiella spp. were resistant to the in-use antibiotics used for treatment of UTI. Imipenem was the most effective antibiotic and could be the drug of choice for treatment of infections caused by ESBL strains. This clinical threat of increased ESBL prevalence is creating significant therapeutic problems prompting an immediate need to formulate strategic policy initiatives to reduce their prevalence.
ACKNOWLEDGEMENTS
We would like to thank all the patients for their generous participation.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Khadka K., Khadka J., Lekhak B., Shrestha P., BR T. Incidence of urinary tract infection among the patients visiting Western Regional Hospital, Pokhara, Nepal. JHAS. 2012;2(1):35–37. [Google Scholar]
- 2.Griebling T.L. Urologic diseases in America. US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2009. Urinary tract infection in women. pp. 589–617. [Google Scholar]
- 3.Rai G.K., Upreti H.C., Rai S.K., Shah K.P., Shrestha R.M. Causative agents of urinary tract infections in children and their antibiotic sensitivity pattern: a hospital based study. Nepal Med. Coll. J. 2008;10(2):86–90. [PubMed] [Google Scholar]
- 4.Nachimuthu R., Chettipalayam S., Velramar B., Kurumandur R., Velu R. Urinary tract infection and antimicrobial susceptibility pattern of extended spectrum beta lactamase producing clinical isolates. Adv. Biol. Res. (Faisalabad) 2008;2(5-6):78–82. [Google Scholar]
- 5.Dhillon RHP, Clark J. ESBLs: A clear and present danger? 2012. [DOI] [PMC free article] [PubMed]
- 6.Mehrgan H., Rahbar M. Prevalence of extended-spectrum beta-lactamase-producing Escherichia coli in a tertiary care hospital in Tehran, Iran. Int. J. Antimicrob. Agents. 2008;31(2):147–151. doi: 10.1016/j.ijantimicag.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Paterson D.L., Bonomo R.A. Extended-spectrum beta-lactamases: a clinical update. Clin. Microbiol. Rev. 2005;18(4):657–686. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vandana K.E., Honnavar P., Amp C. Beta lactamases among ESBL producing Escherichia coli and Klebsiella pneumoniae If you don’t look, you won’t find. J. Clin. Diagn. Res. 2009;3:1653–1656. [Google Scholar]
- 9.Falagas M.E., Karageorgopoulos D.E. Extended-spectrum beta-lactamase-producing organisms. J. Hosp. Infect. 2009;73(4):345–354. doi: 10.1016/j.jhin.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 10.Jacoby G.A., Munoz-Price L.S. The new β-lactamases. N. Engl. J. Med. 2005;352(4):380–391. doi: 10.1056/NEJMra041359. [DOI] [PubMed] [Google Scholar]
- 11.Aminzadeh Z., Sadat Kashi M., Shabani M. Bacteriuria by extended-spectrum Beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: isolates in a governmental hospital in South of Tehran, Iran. Iran. J. Kidney Dis. 2008;2(4):197–200. [PubMed] [Google Scholar]
- 12.Khanfar H.S., Bindayna K.M., Senok A.C., Botta G.A. Extended spectrum beta-lactamases (ESBL) in Escherichia coli and Klebsiella pneumoniae: trends in the hospital and community settings. J. Infect. Dev. Ctries. 2009;3(4):295–299. doi: 10.3855/jidc.127. [DOI] [PubMed] [Google Scholar]
- 13.Livermore D.M., Canton R., Gniadkowski M., Nordmann P., Rossolini G.M., Arlet G., Ayala J., Coque T.M., Kern-Zdanowicz I., Luzzaro F., Poirel L., Woodford N. CTX-M: changing the face of ESBLs in Europe. J. Antimicrob. Chemother. 2007;59(2):165–174. doi: 10.1093/jac/dkl483. [DOI] [PubMed] [Google Scholar]
- 14.Hammer D.A., Dongol S., Anderson T.P., Wong J.S., Werno A.M., Murdoch D.R. High prevalence of extended-spectrum beta-lactamase-producing Enterobacteriaceae in Nepal. Int. J. Antimicrob. Agents. 2007;30(5):471–472. doi: 10.1016/j.ijantimicag.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Ko K.S., Suh J.Y., Peck K.R., Lee M.Y., Oh W.S., Kwon K.T., Jung D.S., Lee N.Y., Song J.H. In vitro activity of fosfomycin against ciprofloxacin-resistant or extended-spectrum β-lactamase-producing Escherichia coli isolated from urine and blood. Diagn. Microbiol. Infect. Dis. 2007;58(1):111–115. doi: 10.1016/j.diagmicrobio.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Vandepitte J., Engbaek K., Rohner P., Piot P., Heuck C.C., World Health Organization . Basic laboratory procedures in clinical bacteriology. 2nd ed. Geneva: World Health Organization; 2003. [Google Scholar]
- 17.Versalovic J., Carroll K.C., Funke G., Jorgensen J.H., Landry M.L., Warnock D.W., editors. Manual of Clinical Microbiology. 10th ed. American Society of Microbiology; 2011. [Google Scholar]
- 18.Clinical and Laboratory Standards Institute (CLSI) Performance Standards for Antimicrobial Susceptibility Testing. Wayne, PA: Twenty-thi. Clinical and Laboratory Standards Institute; 2013. [Google Scholar]
- 19.Kattel H.P., Mishra S.K., Acharya J., Singh A.S., Rijal B.P., Pokhrel B.M. Relationship between pyuria and bacteriuria in suspected urinary tract infection. J Nepal Assoc Med Lab Sci. 2009;10:19–21. [Google Scholar]
- 20.Baral P., Neupane S., Marasini B.P., Ghimire K.R., Lekhak B., Shrestha B. High prevalence of multidrug resistance in bacterial uropathogens from Kathmandu, Nepal. BMC Res. Notes. 2012;5:38. doi: 10.1186/1756-0500-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhatt C., Shrestha B., Khadka S., Swar S., Shah B., Pun K. Etiology of urinary tract infection and drug resistance cases of uropathoges. J Kathmandu Med Coll. 2012;1(2(2)):114–0. [Google Scholar]
- 22.Behrooozi A., Rahbar M., Yousefi J.V. Frequency of extended spectrum beta-lactamase (ESBLs) producing Escherichia coli and Klebseilla pneumonia isolated from urine in an Iranian 1000-bed tertiary care hospital. Afr. J. Microbiol. Res. 2010;4(9):881–884. [Google Scholar]
- 23.Thakur S., Pokhrel N., Sharma M. Prevalence of multidrug resistant enterobacteriaceae and extended spectrum β lactamase producing Escherichia coli in urinary tract infection. Res J Pharm Biol Chem Sci. 2013;4(4):1615–1624. [Google Scholar]
- 24.Parajuli N.P., Maharjan P., Parajuli H., Joshi G., Paudel D., Sayami S., Khanal P.R. High rates of multidrug resistance among uropathogenic Escherichia coli in children and analyses of ESBL producers from Nepal. Antimicrob. Resist. Infect. Control. 2017;6(1):9. doi: 10.1186/s13756-016-0168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forbes B.A., Sahm D.F., Weissfeld A.S. Bailey & Scott’s Diagnostic Microbiology. 12th ed. USA: Mosby Elsevier; 2007. [Google Scholar]
- 26.Magliano E, Grazioli V, Deflorio L, et al. Gender and agedependent etiology of communityacquired urinary tract infections. Sci. World J. 2012;2012:349597. doi: 10.1100/2012/349597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLellan L.K., Hunstad D.A. Urinary tract infection: pathogenesis and outlook. Trends Mol. Med. 2016;22(11):946–957. doi: 10.1016/j.molmed.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Mahmood A.M., Atimi A.T., Tirmidhi A.B., Mohammed A. Antimicrobial susceptibility of some quinolone antibiotics against some urinary tract pathogens in a tertiary hospital, Yola, Adamawa State. Nig J Clin Med Res. 2009;1(2):26–34. [Google Scholar]
- 29.Bhandari R., Pant N.D., Poudel A., Sharma M. Assessment of the effectiveness of three different cephalosporin/clavulanate combinations for the phenotypic confirmation of extended-spectrum beta-lactamase producing bacteria isolated from urine samples at National Public Health Laboratory, Kathmandu, Nepal. BMC Res. Notes. 2016;9(1):390. doi: 10.1186/s13104-016-2192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chakrawarti a Dongol P, Khanal H, Subba P, Benerjee JJ. Extended spectrum beta lactamases detection and multiple antibiotic resistance indexing of E. Coli from urine samples of patients from a referral hospital of eastern Nepal. Int J Appl Sci Biotechnol. 2015;3:423. doi: 10.3126/ijasbt.v3i3.12924. [DOI] [Google Scholar]
- 31.Neupane S., Pant N.D., Khatiwada S., Chaudhary R., Banjara M.R. Correlation between biofilm formation and resistance toward different commonly used antibiotics along with extended spectrum beta lactamase production in uropathogenic Escherichia coli isolated from the patients suspected of urinary tract infections visiting Shree Birendra Hospital, Chhauni, Kathmandu, Nepal. Antimicrob. Resist. Infect. Control. 2016;5:5. doi: 10.1186/s13756-016-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma M., Pathak S., Srivastava P. Prevalence and antibiogram of Extended Spectrum β-Lactamase (ESBL) producing gram negative bacilli and further molecular characterization of ESBL producing Escherichia coli and Klebsiella spp. J. Clin. Diagn. Res. 2013;7(10):2173–2177. doi: 10.7860/JCDR/2013/6460.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Somily A.M., Habib H.A., Absar M.M., Arshad M.Z., Manneh K., Al Subaie S.S., Al Hedaithy M.A., Sayyed S.B., Shakoor Z., Murray T.S. ESBL-producing Escherichia coli and Klebsiella pneumoniae at a tertiary care hospital in Saudi Arabia. J. Infect. Dev. Ctries. 2014;8(9):1129–1136. doi: 10.3855/jidc.4292. [DOI] [PubMed] [Google Scholar]
- 34.Sibi G., Devi A.P., Fouzia K., Patil B.R. Prevalence, microbiologic profile of urinary tract infection and its treatment with trimethoprim in diabetic patients. Res J Microbiol. 2011;6(6):543–551. doi: 10.3923/jm.2011.543.551. [DOI] [Google Scholar]
- 35.Chander A., Shrestha C.D. Prevalence of extended spectrum beta lactamase producing Escherichia coli and Klebsiella pneumoniae urinary isolates in a tertiary care hospital in Kathmandu, Nepal. BMC Res. Notes. 2013;6:487. doi: 10.1186/1756-0500-6-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahdi Yahya Mohsen S., Hamzah H.A., Muhammad Imad Al-Deen M., Baharudin R. Antimicrobial susceptibility of klebsiella pneumoniae and Escherichia coli with extended-spectrum β-lactamase associated genes in hospital Tengku Ampuan Afzan, Kuantan, Pahang. Malays. J. Med. Sci. 2016;23(2):14–20. [PMC free article] [PubMed] [Google Scholar]
- 37.Pokhrel R.H., Thapa B., Kafle R., Shah P.K., Tribuddharat C. Co-existence of beta-lactamases in clinical isolates of Escherichia coli from Kathmandu, Nepal. BMC Res. Notes. 2014;7:694. doi: 10.1186/1756-0500-7-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yadav K.K., Adhikari N., Khadka R., Pant A.D., Shah B. Multidrug resistant Enterobacteriaceae and extended spectrum β-lactamase producing Escherichia coli: a cross-sectional study in National Kidney Center, Nepal. Antimicrob. Resist. Infect. Control. 2015;4:42. doi: 10.1186/s13756-015-0085-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lakshmi B., Lakshmi M.S. Microbiological profile of urinary tract infections and prevalence of extended spectrum beta lactamase producing. J Evol Med Dent Sci. 2014;3(32):8766–8774. [Google Scholar]
- 40.Shah R., Singh Y., Sanjana R. Study of Extended spectrum beta-lactamases (ESBLs) producing Klebsiella species in various clinical specimens: A preliminary report. J Coll Med Sci. 2010;6(3):19–23. [Google Scholar]
- 41.Akpaka P.E., Swanston W.H. Phenotypic detection and occurrence of extended-spectrum beta-lactamases in clinical isolates of Klebsiella pneumoniae and Escherichia coli at a tertiary hospital in Trinidad & Tobago. Braz. J. Infect. Dis. 2008;12(6):516–520. doi: 10.1590/S1413-86702008000600014. [DOI] [PubMed] [Google Scholar]
- 42.Mekki A.H., Hassan A.N., Elsayed D.E. Extended spectrum beta lactamases among multi drug resistant Escherichia coli and Klebsiella species causing urinary tract infections in Khartoum. J Bacteriol Res. 2010;2(3):18–21. [Google Scholar]
- 43.Poudyal S., Bhatta D.R., Shakya G., Upadhyaya B., Dumre S.P., Buda G., Kandel B.P. Extended spectrum â-lactamase producing multidrug resistant clinical bacterial isolates at National Public Health Laboratory, Nepal. Nepal Med. Coll. J. 2011;13(1):34–38. [PubMed] [Google Scholar]
- 44.Roopa T.J., Sudha S.S. Antimicrobial susceptibility of extended spectrum β -lactamase (ESBL) producing uropathogens isolated from ICU patients. Int J Biol Technol. 2010;1(3):23–31. [Google Scholar]
- 45.Gyawali S., Shankar P.R., Poudel P.P., Saha A. Knowledge, attitude and practice of self-medication among basic science undergraduate medical students in a medical school in western Nepal. J. Clin. Diagn. Res. 2015;9(12):FC17–FC22. doi: 10.7860/JCDR/2015/16553.6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shankar P.R., Partha P., Shenoy N. Self-medication and non-doctor prescription practices in Pokhara valley, Western Nepal: a questionnaire-based study. BMC Fam. Pract. 2002;3:17. doi: 10.1186/1471-2296-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ullah F., Malik S.A., Ahmed J. Antibiotic susceptibility pattern and ESBL prevalence in nosocomial Escherichia coli from urinary tract infections in Pakistan. Afr. J. Biotechnol. 2009;8(16):3921–3926. [Google Scholar]
- 48.Han S.B., Lee S.C., Lee S.Y., Jeong D.C., Kang J.H. Aminoglycoside therapy for childhood urinary tract infection due to extended-spectrum β-lactamase-producing Escherichia coli or Klebsiella pneumoniae. BMC Infect. Dis. 2015;15:414. doi: 10.1186/s12879-015-1153-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


