Abstract
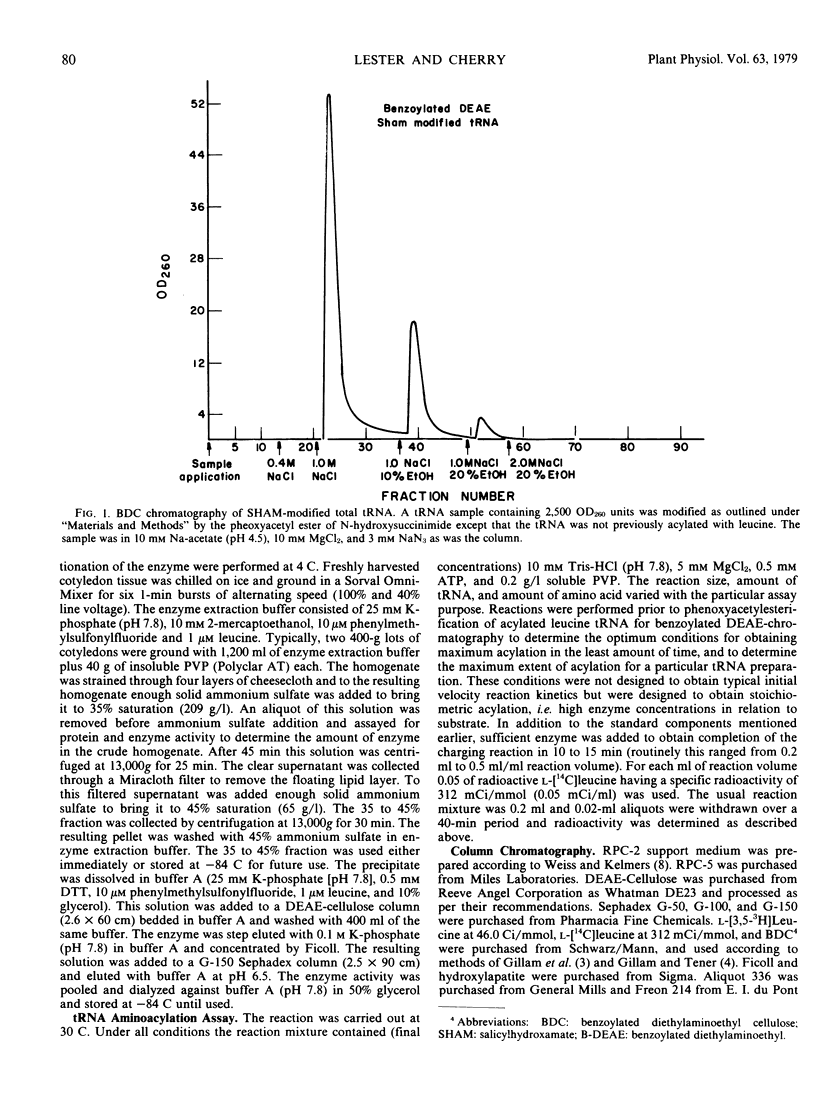
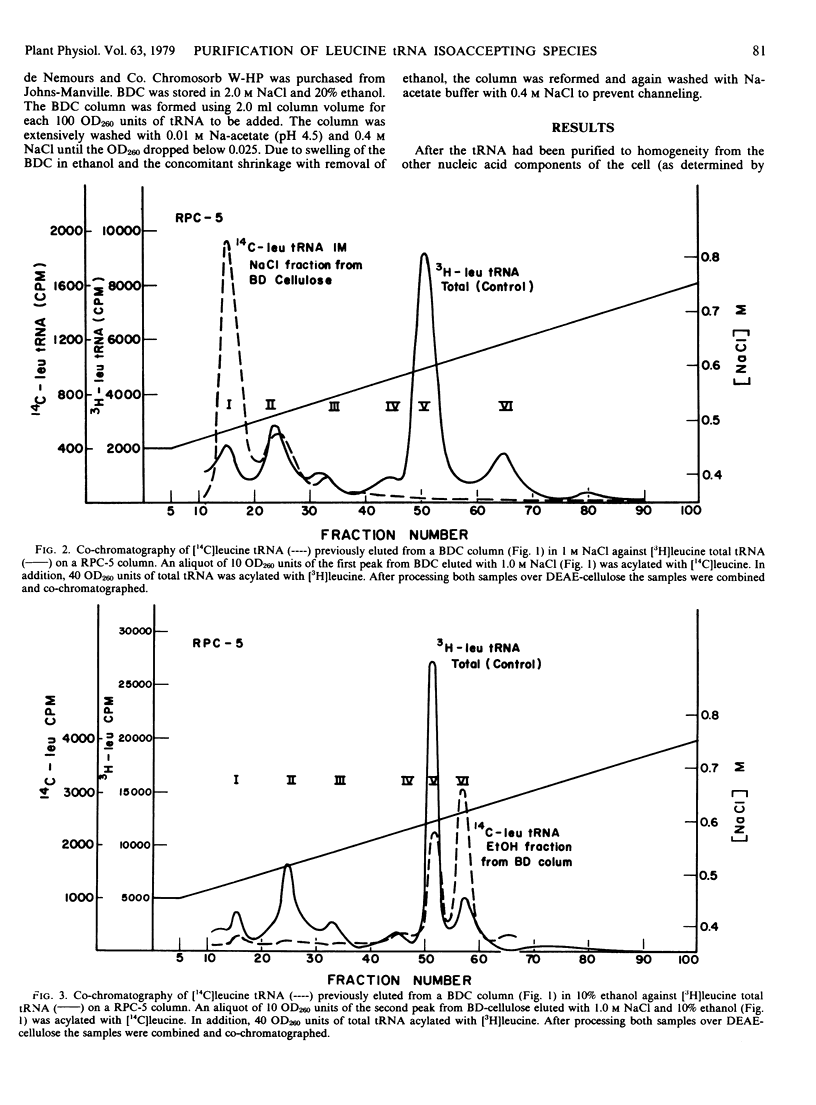
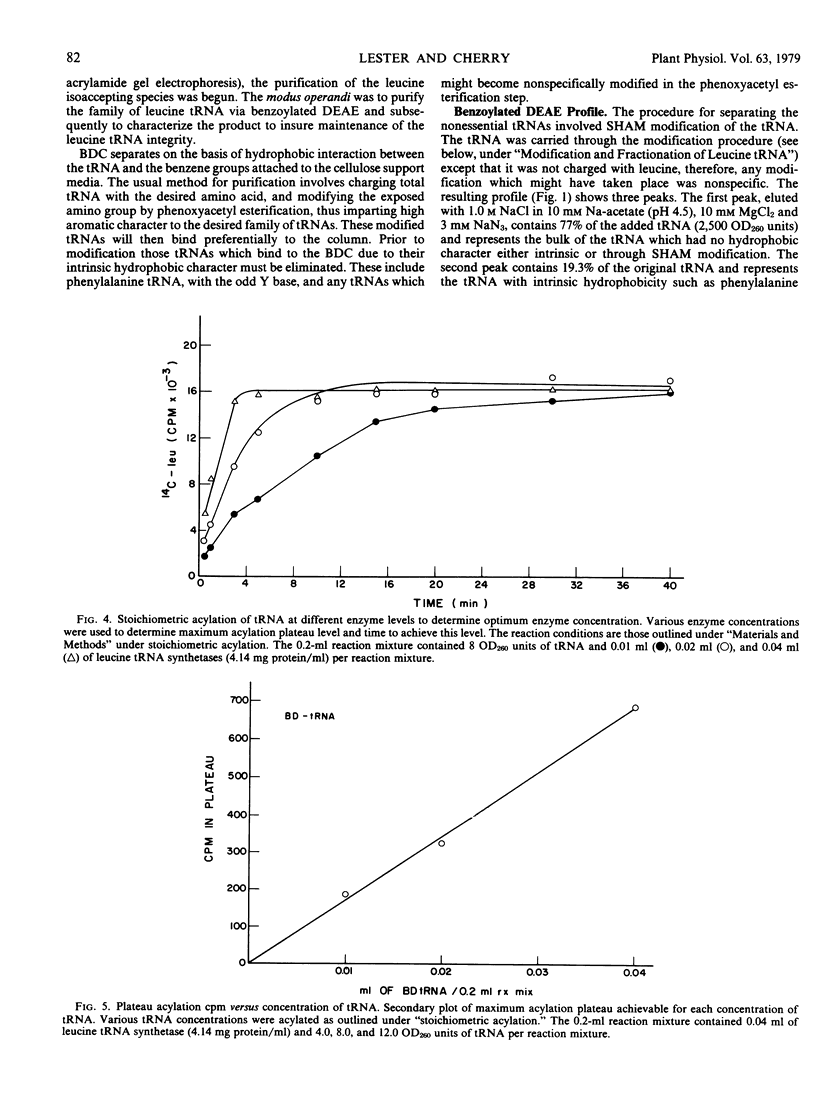
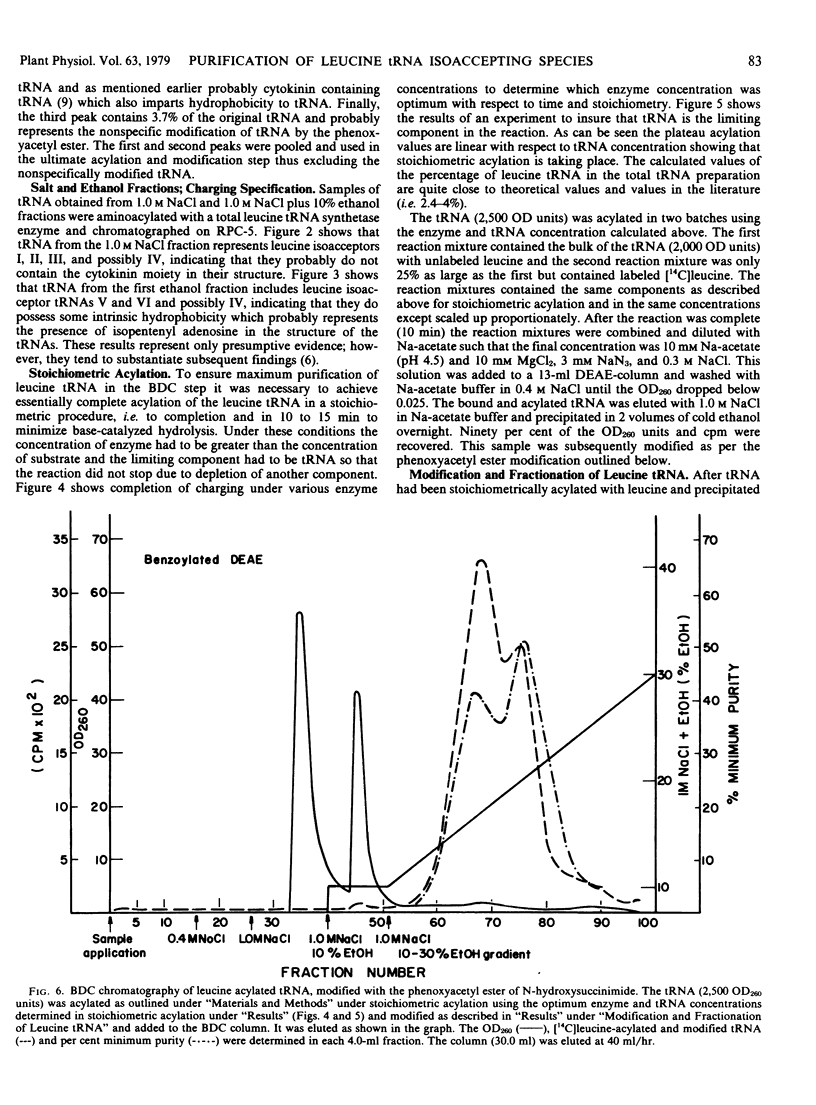
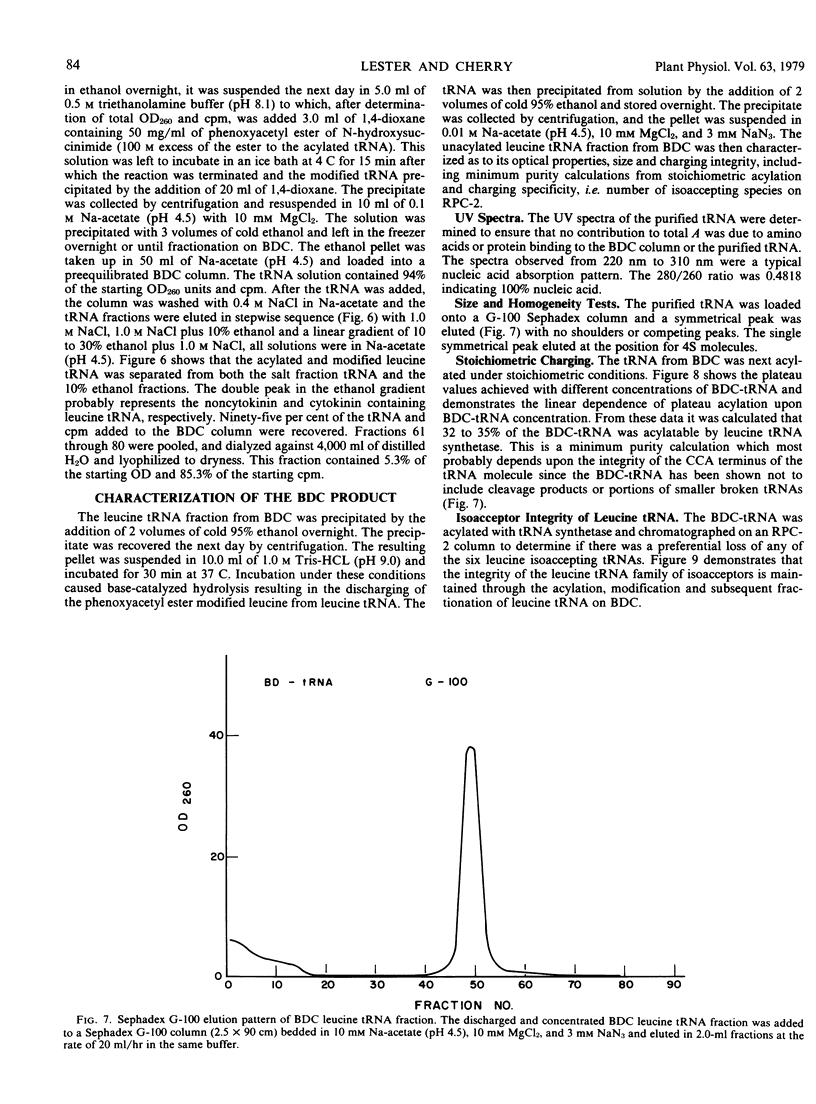
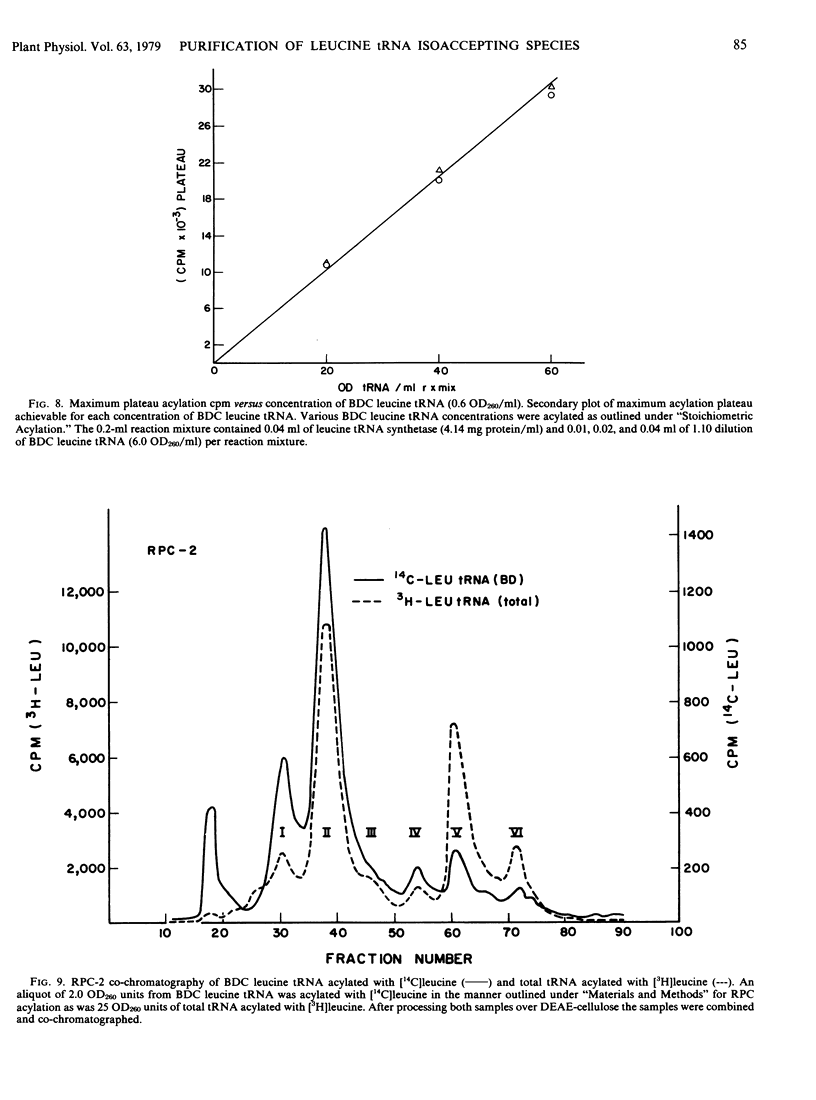
Transfer RNA from soybean (Glycine max) cotyledons was purified to homogeneity followed by the purification of the family of leucine tRNA via benzoylated diethylaminoethyl cellulose (BDC) chromatography. Nonacylated total purified tRNA was salicylhydroxamate (SHAM) modified by the phenoxyacetyl method and fractionated into three peaks on a BDC column. The first peak containing bulk tRNA with no hydrophobic character amounted to 78% of the added tRNA. The second peak containing 19% of the added tRNA and represents the tRNA with intrinsic hydrophobic properties. The third peak containing 3% of the tRNA represents the SHAM modified tRNA and nonspecifically modified tRNA. Transfer RNA peaks I and II were pooled and subsequently stoichiometrically acylated in two batches, one containing [14C]leucine while the other contained unlabeled leucine. The acylated tRNA was loaded on and step-eluted from a BDC column. The purified acylated-tRNA was phenoxyacetyl modified and following ethanol precipitation was fractionated on a BDC column. A double peak eluted from the column in the ethanol gradient contained 5.3% of the starting optical density and 85.3% of the starting counts per minute. Characterization of this leucine tRNA showed typical ultraviolet spectra properties and appeared to be homogeneous on a G-100 Sephadex column. The minimum purity of the tRNA was 32 to 35%. Finally, the acylated tRNA was chromatographed on an RPC-2 column giving six leucine isoaccepting tRNAs. The data indicate that leucine tRNA was highly purified without losing the integrity of the family of isoacceptors.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. B., Cherry J. H. Differences in leucyl-transfer rna's and synthetase in soybean seedlings. Proc Natl Acad Sci U S A. 1969 Jan;62(1):202–209. doi: 10.1073/pnas.62.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillam I., Millward S., Blew D., von Tigerstrom M., Wimmer E., Tener G. M. The separation of soluble ribonucleic acids on benzoylated diethylaminoethylcellulose. Biochemistry. 1967 Oct;6(10):3043–3056. doi: 10.1021/bi00862a011. [DOI] [PubMed] [Google Scholar]
- Kanabus J., Cherry J. H. Isolation of an organ-specific leucyl-tRNA synthetase from soybean seedling. Proc Natl Acad Sci U S A. 1971 May;68(5):873–876. doi: 10.1073/pnas.68.5.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester B. R., Morris R. O., Cherry J. H. Purification of Leucine tRNA Isoaccepting Species from Soybean Cotyledons: II. RPC-2 Purification, Ribosome Binding, and Cytokinin Content. Plant Physiol. 1979 Jan;63(1):87–92. doi: 10.1104/pp.63.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vold B. S., Clinton G. M., Spizizen J. An effect of temperature on the Bacillus subtillis transfer RNA's which respond to codons beginning with U and A correlation with cytokinin activity. Biochim Biophys Acta. 1970;209(2):396–404. doi: 10.1016/0005-2787(70)90737-9. [DOI] [PubMed] [Google Scholar]
- Weiss J. F., Kelmers A. D. A new chromatographic system for increased resolution of transfer ribonucleic acids. Biochemistry. 1967 Aug;6(8):2507–2513. doi: 10.1021/bi00860a030. [DOI] [PubMed] [Google Scholar]
- Wimmer E., Maxwell I. H., Tener G. M. A simple method for isolating highly purified yeast phenylalanine transfer ribonucleic acid. Biochemistry. 1968 Jul;7(7):2623–2628. doi: 10.1021/bi00847a026. [DOI] [PubMed] [Google Scholar]


