Abstract
Background
It is unknown if a survival gap remains between HIV-infected and HIV-uninfected individuals with access to care.
Methods
We conducted a cohort study within Kaiser Permanente California during 1996–2011, using abridged life tables to estimate the expected years of life remaining (“life expectancy”) at age 20.
Results
Among 24,768 HIV-infected and 257,600 HIV-uninfected individuals, there were 2229 and 4970 deaths, with mortality rates of 1827 and 326 per 100,000 person-years, respectively. In 1996–1997, life expectancies at age 20 for HIV-infected and HIV-uninfected individuals were 19.1 and 63.4 years, respectively, corresponding with a gap of 44.3 years (95% confidence interval: 38.4 to 50.2). Life expectancy at age 20 for HIV-infected individuals increased to 47.1 years in 2008 and 53.1 years by 2011, narrowing the gap to 11.8 years (8.9–14.8 years) in 2011. In 2008–2011, life expectancies at age 20 for HIV-infected individuals ranged from a low of 45.8 years for blacks and 46.0 years for those with a history of injection drug use to a high of 52.2 years for Hispanics. HIV-infected individuals who initiated antiretroviral therapy with CD4 ≥500 cells per microliter had a life expectancy at age 20 of 54.5 years in 2008–2011, narrowing the gap relative to HIV-uninfected individuals to 7.9 years (5.1–10.6 years). For these HIV-infected individuals, the gap narrowed further in subgroups with no history of hepatitis B or C infection, smoking, drug/alcohol abuse, or any of these risk factors.
Conclusions
Even with early treatment and access to care, an 8-year gap in life expectancy remains for HIV-infected compared with HIV-uninfected individuals.
Keywords: human immunodeficiency virus (HIV), acquired immunodeficiency syndrome (AIDS), life expectancy, survival, access to health care, highly active antiretroviral therapy
INTRODUCTION
Combination antiretroviral therapy (ART) has dramatically increased the lifespan of HIV-infected individuals1,2; however, it is unknown whether a gap in life expectancy remains between HIV-infected and HIV-uninfected individuals. Although several studies have suggested that HIV-infected individuals have not yet reached a normal life expectancy,3–8 these studies relied on the general population as a comparison group. Thus, observed differences in life expectancy may be explained by differences by HIV status in sociodemographic factors, such as gender, race/ethnicity, and access to care,9,10 or by the high prevalence among HIV patients of risk factors such as substance use,11,12 viral hepatitis,13 and smoking14 that lead to comorbidities15,16 and affect survival.
The identification of an internal HIV-uninfected control group, with patient-level data on demographics and risk factors, is needed to determine how large a gap in life expectancy persists by HIV status, and how much of that gap is attributable to non-HIV–related factors. To our knowledge, only 1 study has included such a control group. Wada et al17 examined HIV-infected and HIV-uninfected men who have sex with men (MSM) in the Multicenter AIDS Cohort Study and high-risk women in the Women’s Interagency HIV Study during 1996–2008, finding that HIV patients were younger than HIV-uninfected individuals at the time of non-AIDS–related death. However, there have been no studies that have directly compared life expectancy between HIV-infected and HIV-uninfected individuals in population-based settings, or in settings with equal access to care.
Our objective was to examine life expectancy in a large cohort of HIV-infected and demographically similar HIV-uninfected individuals from within the same health care system. We evaluated life expectancies by HIV status over time, overall and within demographic subgroups. To determine the contribution of HIV-related and non-HIV–related factors to the remaining survival gap, we estimated life expectancies among HIV patients initiating ART at high CD4 counts, and in subgroups of these HIV patients and HIV-uninfected individuals without modifiable risk factors.
METHODS
Study Design, Setting, and Population
The study population was a previously described cohort18 of HIV-infected and HIV-uninfected members of Kaiser Permanente (KP) Northern and Southern California (KPNC and KPSC, respectively), large integrated health care systems providing comprehensive medical services to more than 30% of insured Californians.19 HIV status was determined using HIV registries that included all known cases of HIV/AIDS since the early 1980s for KPNC and 2000 for KPSC, with confirmation by review of medical charts or medical center case lists. Individuals not included in the HIV registries were considered HIV-uninfected. Eligible subjects included adults (aged ≥18 years) who were members of KP at any time during 1996–2011 for KPNC and 2000–2011 for KPSC. HIV-uninfected members were frequency-matched 10:1 to HIV-infected subjects by age (5-year groups), gender, medical center, and initial calendar year of follow-up, with random selection from the HIV-uninfected subgroups defined by each matching factor. The start of follow-up for each subject was the earliest date on or after January 1, 1996 (January 1, 2000, for KPSC) when eligibility criteria were met. Subjects were followed until the earliest of death or December 31, 2011.
The institutional review boards at KPNC and KPSC approved this study with a waiver of written informed consent.
Study Measurements
Deaths
Dates of death were obtained from California death certificates,20 Social Security Administration data sets,21 and the clinical and administrative databases constituting KP’s electronic health record (EHR). Deaths were completely ascertained through December 31, 2011, even if individuals left the health plan.
Demographics and Risk Factors
Other data extracted from the EHR included gender; age; race/ethnicity; laboratory test results (eg, CD4 counts); health plan enrollment periods; and inpatient or outpatient clinical diagnoses, including drug/alcohol abuse (International Classification of Disease Codes, Ninth Revision, ICD-9: 291, 292, 303–305.0, 305.2–305.5) and smoking/tobacco use (ICD-9: 305.1, V15, V65, 649, internal social history codes). Hepatitis B virus (HBV) status was determined by HBV surface antigen tests. Hepatitis C virus (HCV) status was determined by HCV-antibody tests or HCV RNA levels, or, for KPNC, by inclusion in the KPNC viral hepatitis registry, as previously described.22
In addition to HIV status, HIV registries were used to obtain HIV-transmission risk group [MSM, heterosexual sex, injection drug use (IDU)], dates of first ART use, beginning of known HIV infection, and race/ethnicity for HIV-infected individuals.
Statistical Analysis
Variables for analysis included age; calendar year and era of study follow-up; gender; race/ethnicity (white, black, Hispanic); and HBV or HCV infection, drug/alcohol abuse, or smoking, all of which were defined as ever/never from 2 years before baseline through the end of follow-up. Among HIV-infected individuals, we also evaluated HIV-transmission risk group and, for individuals who initiated ART during study follow-up, the most recent CD4 count in the 6 months before ART initiation. Data were not available on sexual orientation or IDU history for HIV-uninfected individuals.
To compare survival by HIV status over time, we first computed age-adjusted, all-cause mortality rates for HIV-infected and HIV-uninfected subjects by 2-year era during 1996–2005, and by year during 2006–2011, as sample size increased in later years, particularly in older age groups. We used direct age adjustment to account for the advancing age over time, with 10-year age groups (age 20 to ≥70 years) and a standard population of HIV-uninfected subjects in 2011. Given overdispersion of the mortality data, we used negative-binomial rather than Poisson regression to assess trends.23
We then used abridged life tables to estimate the expected years of life remaining (“life expectancy”) at age 20 for HIV-infected and HIV-uninfected individuals. Life expectancy at any given age (eg, 20 years) is interpreted as the average number of years of life remaining for those surviving to that age.24 Life-table construction was based on standard methods, as previously described.4,24,25 Briefly, life tables were constructed from age-specific mortality rates for HIV-infected and HIV-uninfected subjects using 5-year age intervals starting from age 20, with a final open interval for subjects aged ≥85 years.26 When estimating life expectancy in subsets of subjects with no person-years observed at age ≥85, the final age interval of the life table was truncated to ≥80 years, and if still no person-years, to ≥75 years. If there were no deaths observed in the final age interval, eg, for HIV-infected subjects ≥85 years, we estimated the mortality rate with the assumption that the rate ratio comparing HIV-infected with HIV-uninfected subjects in the ≥85 age group was the same as the average of the rate ratios comparing HIV-infected to HIV-uninfected subjects in the 75–79 and 80–84 age groups.4 The life tables described the experience that hypothetical cohorts of HIV-infected and HIV-uninfected subjects would have had if they were subject to the observed age-specific mortality rates from age 20 until death. Using variance and SE formulas provided by Chiang,25 we generated 95% confidence intervals (CI) for life expectancy estimates (estimate ± 1.96 × SE) and differences in life expectancy by HIV status [difference ± 1.96 × √(sum of variances)]. We used z tests to obtain P values for comparisons of estimates by HIV status and other characteristics.27
Similar to the approach for mortality rates, we estimated life expectancies at age 20 for HIV-infected and HIV-uninfected subjects for each 2-year era during 1996–2005, and each year during 2006–2011, as sample size increased. We then estimated the life expectancies at age 20 by HIV status overall and stratified by gender and race/ ethnicity, and by HIV-transmission risk group for HIV-infected subjects, in 2 broad calendar eras, 1996–2007 and 2008–2011, which provided sufficient sample size for estimation of life expectancies in less common subgroups. For 2008–2011, we estimated life expectancies at age 20 for previously ART-naive HIV patients initiating ART during follow-up with CD4 ≥500 cells per microliter and HIV-uninfected subjects, and for subgroups of these HIV patients and HIV-uninfected subjects without a history of HBV or HCV infection, drug/alcohol abuse, smoking, or any of these risk factors.
Analyses were conducted in SAS 9.1 (Cary, NC) and Microsoft Excel 2010. Statistical tests were 2-sided, and statistical significance was defined as P < 0.05.
RESULTS
Study Population
Among the 24,768 HIV-infected and 257,600 HIV-uninfected subjects, there were 122,032 and 1,522,547 person-years of follow-up, with a mean of 4.9 and 5.9 person-years per subject, respectively (Table 1). Subjects were similar by HIV status with respect to the matching factors of age and sex, with an approximate mean age of 40 and 90% men, although small differences in age and other characteristics were statistically significant because of the large sample size. Among subjects with known race/ ethnicity, a higher proportion of HIV-infected compared with HIV-uninfected subjects were white (56.2% vs. 44.1%) or black (17.8% vs. 10.4%), whereas a lower proportion were Hispanic (21.0% vs. 27.3%) or Asian/Pacific Islander (4.3% vs. 16.1%; P < 0.001 overall). Compared with HIV-uninfected subjects, HIV patients more frequently had a history of smoking (45.2% vs. 31.1%; P < 0.001), drug/alcohol abuse (20.6% vs. 8.6%; P < 0.001), and HBV or HCV infection (11.5% vs. 1.7%; P < 0.001). Among HIV-infected subjects, the most common HIV-transmission risk group was MSM (74.7%), followed by heterosexual sex (16.3%) and IDU (7.0%). HIV patients had been known to be HIV-infected for an average of 3.8 years at baseline, with a mean CD4 count of 410 cells per microliter. Almost half (45.6%) had antiretroviral experience before study entry. Of the 9802 individuals who initiated ART during follow-up, 9785 had a CD4 count at the time of ART initiation, of whom 1753 (17.9%) had a count of ≥500 cells per microliter.
TABLE 1.
Demographic and Clinical Characteristics of HIV-Infected and HIV-Uninfected Subjects, Kaiser Permanente California, 1996–2011
| HIV-Infected | HIV-Uninfected | P | |
|---|---|---|---|
| N | 24,768 | 257,600 | |
| Person-years, total (mean/subject) | 122,032 (4.9) | 1,522,547 (5.9) | |
| Age at baseline, mean (SD) | 40.7 (10.1) | 40.2 (10.3) | <0.001 |
| Year of birth, mean (SD) | 1961 (11.1) | 1961 (11.4) | 0.20 |
| Gender, % | 0.76 | ||
| Men | 90.7 | 90.6 | |
| Women | 9.3 | 9.4 | |
| Race/ethnicity, % among known | <0.001 | ||
| White | 56.2 | 44.1 | |
| Hispanic | 21.0 | 27.3 | |
| Black | 17.8 | 10.4 | |
| Asian/Pacific Islander | 4.3 | 16.1 | |
| Other | 0.8 | 2.2 | |
| Ever history of tobacco smoking, % | 45.2 | 31.1 | <0.001 |
| Ever history of drug/alcohol abuse, % | 20.6 | 8.6 | <0.001 |
| Ever history of hepatitis B or C infection, % | 11.5 | 1.7 | <0.001 |
| HIV-transmission risk group, % among known | |||
| MSM | 74.7 | — | |
| Heterosexual sex | 16.3 | — | |
| IDU | 7.0 | — | |
| Other | 2.0 | — | |
| Years known to be HIV infected, mean (SD) | 3.8 (5.5) | — | |
| CD4 cells/μL at baseline, mean (SD) | 410 (290) | — | |
| Prior antiretroviral use, % | 45.6 | — | |
| Initiated ART during follow-up, % | 39.6 | — | |
| CD4 cells/μL at ART initiation, % | |||
| <200 | 35.0 | — | |
| 200–349 | 26.7 | — | |
| 350–499 | 20.5 | — | |
| ≥500 | 17.9 | — |
HIV-infected and HIV-uninfected subjects were matched on age (5-year groups), gender, medical center, and initial calendar year of follow-up. P values were obtained from χ2 tests for categorical variables and t tests for continuous variables. Some data were missing on race/ethnicity (3.3% of HIV-infected and 21.0% of HIV-uninfected subjects), HIV-transmission risk group (20.5%), and CD4 count at ART initiation (0.2%).
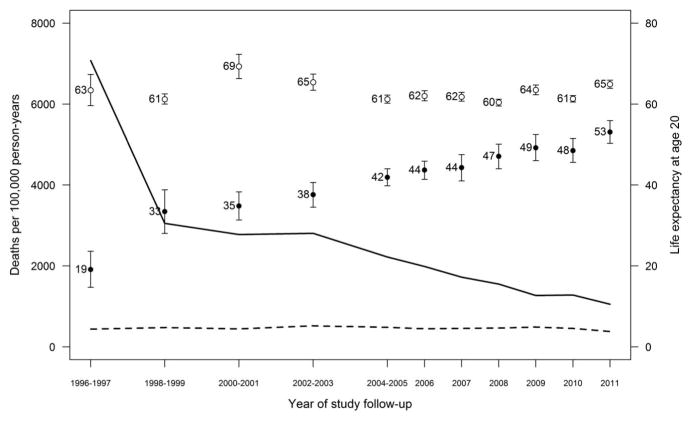
Among HIV-infected and HIV-uninfected subjects, there were 2229 and 4970 deaths during the study period, corresponding with crude mortality rates of 1827 and 326 per 100,000 person-years, respectively. Age-adjusted mortality rates for HIV-uninfected individuals were 439 per 100,000 person-years in 1996–1997 and 381 per 100,000 person-years in 2011, with no trend over time (P = 0.43), whereas rates decreased for HIV-infected individuals from 7077 per 100,000 person-years in 1996–1997 to 1054 per 100,000 person-years in 2011 (P < 0.001; Fig. 1).
FIGURE 1.
Age-adjusted mortality rates and life expectancy at age 20 for HIV-infected and HIV-uninfected individuals, Kaiser Permanente California, 1996–2011. Rates are represented by solid lines for HIV-infected and dotted lines for HIV-uninfected individuals (P < 0.001 and P = 0.43 for changes over time, respectively). Life expectancies at age 20 are represented by solid circles for HIV-infected and open circles for HIV-uninfected individuals.
Life expectancies at age 20 for HIV-uninfected individuals were 63.4 years (95% CI: 59.6–67.3) in 1996–1997 and 64.9 years (95% CI: 63.9 to 65.9) in 2011, while increasing for HIV-infected individuals from 19.1 years (95% CI: 14.7 to 23.6) in 1996–1997 to 53.1 years (95% CI: 50.3 to 55.9) in 2011. Significant gains in survival for HIV-infected individuals were observed across all gender, race/ethnicity, and HIV-transmission risk groups from 1996–2007 to 2008–2011 (P < 0.001 for all increases; Fig. 2). Life expectancy at age 20 increased from 36.8 years (95% CI: 32.9 to 40.8) to 50.5 years (95% CI: 47.8 to 53.2) in HIV-infected women (13.7-year increase), and from 37.6 years (95% CI: 36.2 to 38.9) to 49.2 years (95% CI: 47.6 to 50.8) in HIV-infected men (11.6-year increase), with no difference in life expectancies reached by HIV-infected women and men in 2008–2011 (P = 0.20). There were similar gains over time in life expectancy at age 20 for HIV-infected individuals by race/ethnicity, increasing from 34.7 years (95% CI: 31.5 to 37.9) to 45.8 years (95% CI: 43.1 to 48.5) among blacks (11.1-year increase), from 37.1 years (95% CI: 35.1 to 39.2) to 50.4 years (95% CI: 48.0 to 52.9) among whites (13.3-year increase), and from 39.1 years (95% CI: 36.5 to 41.7) to 52.2 years (95% CI: 49.1 to 55.2) among Hispanics (13.1-year increase), with whites and Hispanics reaching significantly higher life expectancies than blacks (P = 0.007 and P = 0.001, respectively), and no difference comparing whites and Hispanics (P = 0.19). Life expectancy at age 20 increased from 40.1 years (95% CI: 38.4 to 41.8) to 51.1 years (95% CI: 49.2 to 52.9) among MSM (11.0-year increase), from 37.5 years (95% CI: 33.9 to 41.2) to 51.3 years (95% CI: 48.2 to 54.5) among heterosexuals (13.8-year increase), and from 35.9 years (95% CI: 33.2 to 38.6) to 46.0 years (95% CI: 42.8 to 49.2) for individuals with a history of IDU (10.1-year increase). MSM and heterosexuals reached significantly higher life expectancies compared with individuals with a history of IDU in 2008–2011 (P = 0.004 and P = 0.011, respectively), with no difference comparing MSM and heterosexuals (P = 0.44). Change in life expectancy at age 20 for HIV-uninfected individuals was not statistically significant overall, but decreased from 63.0 years (95% CI: 59.9 to 66.1) to 59.2 years (95% CI: 57.9 to 60.5) for blacks (P = 0.014) and increased from 64.8 years (95% CI: 62.8 to 66.8) to 68.4 years (95% CI: 66.7 to 70.2) for Hispanics (P = 0.004).
FIGURE 2.
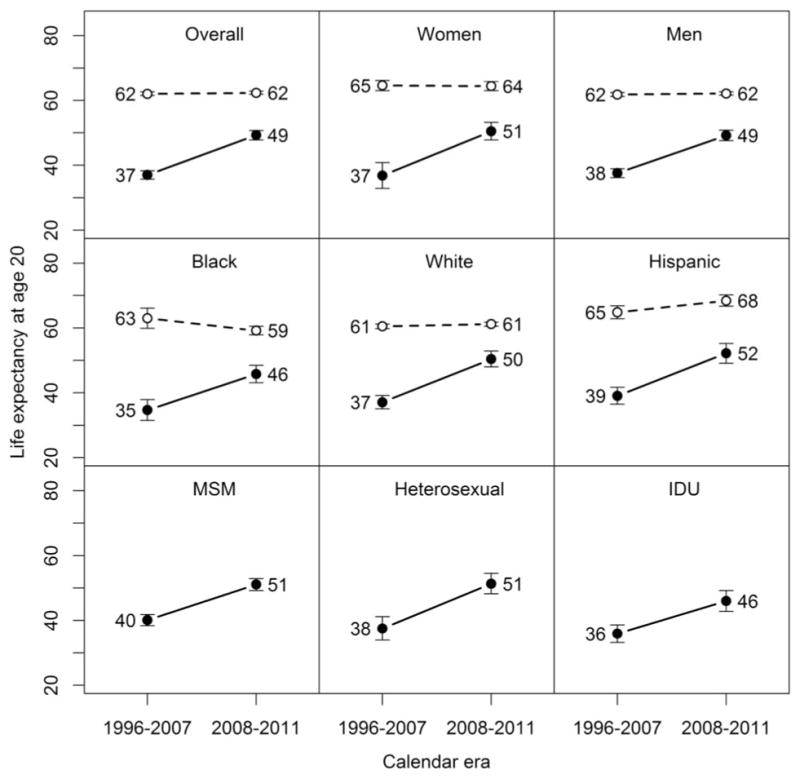
Life expectancy at age 20 for HIV-infected and HIV-uninfected individuals by demographic characteristics and HIV-transmission risk group, Kaiser Permanente California, 1996–2011. Solid lines represent changes for HIV-infected individuals, with dotted lines for HIV-uninfected individuals. All changes were statistically significant at P < 0.001 for HIV-infected individuals, but only for blacks (P = 0.014) and Hispanics (P = 0.004) for HIV-uninfected individuals.
In 2008–2011, life expectancies at age 20 among HIV-infected and HIV-uninfected individuals were 49.3 years (95% CI: 47.8 to 50.7) and 62.3 years (95% CI: 61.9 to 62.8), respectively, corresponding with a gap of 13.1 years (95% CI: 11.5 to 14.6; Table 2). HIV-infected individuals who initiated ART with CD4 ≥500 had a life expectancy at age 20 of 54.5 years (95% CI: 51.7 to 57.2), reducing the gap, relative to HIV-uninfected individuals, to 7.9 years (95% CI: 5.1 to 10.6). The gap was further narrowed in subgroups of these HIV patients and HIV-uninfected individuals with no history of HBV or HCV infection (7.2 years, 95% CI: 4.4 to 10.0), no history of drug/alcohol abuse (6.6 years, 95% CI: 3.9 to 9.3 years), no history of smoking (5.4 years, 95% CI: 2.2 to 8.7), or any of these risk factors (5.7 years, 95% CI: 2.4 to 9.0).
TABLE 2.
Life Expectancy at Age 20 for HIV-Infected and HIV-Uninfected Individuals, Kaiser Permanente California, 2008–2011
| Life Expectancy at Age 20 (95% CI) | |||
|---|---|---|---|
|
| |||
| HIV Infected | HIV Uninfected | Difference* | |
| Overall | 49.3 (47.8 to 50.7) | 62.3 (61.9 to 62.8) | 13.1 (11.5 to 14.6) |
| HIV infected and initiated ART with CD4 ≥500 cells/μL | HIV uninfected | Difference | |
| Overall | 54.5 (51.7 to 57.2) | 62.3 (61.9 to 62.8) | 7.9 (5.1 to 10.6) |
| No hepatitis B or C | 55.4 (52.6 to 58.2) | 62.6 (62.1 to 63.1) | 7.2 (4.4 to 10.0) |
| No drug/alcohol abuse | 57.2 (54.6 to 59.9) | 63.8 (63.3 to 64.3) | 6.6 (3.9 to 9.3) |
| No smoking | 58.9 (55.8 to 62.1) | 64.3 (63.6 to 65.0) | 5.4 (2.2 to 8.7) |
| No hepatitis B or C, drug/alcohol abuse, or smoking | 59.2 (56.0 to 62.4) | 65.0 (64.2 to 65.7) | 5.7 (2.4 to 9.0) |
Life expectancy estimates for HIV-infected individuals initiating ART with CD4 ≥500 cells per microliter were among previously ART-naive patients.
All differences were statistically significant at P < 0.001, with P values derived from z tests.
DISCUSSION
In this large cohort of HIV-infected and HIV-uninfected individuals with equal access to health care, we found a steep increase in life expectancy at age 20 for HIV patients since the introduction of ART, reaching 53 years by 2011. However, even with ART initiation at CD4 counts above 500 cells per microliter, a nearly 8-year gap in survival persisted between HIV-infected and HIV-uninfected individuals in recent years, with the lowest life expectancies reached by HIV-infected blacks and individuals with a history of IDU, and the highest for Hispanics. The gap relative to HIV-uninfected individuals was narrowed in subgroups without HBV or HCV infection, drug/alcohol abuse, and smoking. These findings confirm that ART has had a substantial impact on the survival of HIV patients, and suggest that early ART initiation and risk-reduction strategies, such as smoking cessation, may further reduce the remaining gap in survival relative to HIV-uninfected individuals.
The continued disparity in life expectancy for HIV-infected individuals is consistent with several previous studies that compared HIV patients with the general population.3–8 In a study that included KPNC as 1 of 18 cohorts, the North American AIDS Cohort Collaboration on Research and Design found a life expectancy at age 20 of 51.4 years for treated HIV patients in 2006–2007, corresponding with a 6-to 10-year gap compared with the general population.3 This estimate approached the life expectancy at age 20 of 54.5 years we observed in 2008–2011 for HIV patients who initiated ART with CD4 ≥500 cells per microliter, with an 8-year gap relative to HIV-uninfected individuals. In one of the only previous studies to include an internal HIV-uninfected comparison group, Wada et al found a younger age at death for HIV-infected compared with HIV-uninfected individuals during 1996–2008.17 However, relatively few HIV-uninfected individuals were included in this study, with only 133 deaths observed among 2668 individuals in the ART era, and the mortality outcome included only non-AIDS–related deaths, precluding a direct comparison with our outcome of all-cause mortality.
Similar to previous studies,3,4,6,28 HIV-infected subjects with a history of IDU had the smallest survival gain, reaching a life expectancy at age 20 of 46 years in 2008–2011. The North American AIDS Cohort Collaboration on Research and Design found a substantially lower life expectancy at age 20 for HIV patients with a history of IDU, reaching only 29 years in 2006–20073; this lower estimate may be attributable to differences across study populations in access to care,29 ART uptake or adherence,30 or comorbidities,31 or to improvements in survival in the more recent years examined in our study.
We found that Hispanic and white HIV-infected individuals reached higher life expectancies than blacks, a pattern that mirrors that observed for HIV-uninfected individuals in our study and the general population in the United States.24 Among HIV-uninfected subjects, we also observed a small decrease over time in life expectancy for blacks, and an increase for Hispanics. Numerous studies have found higher mortality rates for HIV-infected blacks compared with other HIV-infected racial/ethnic groups,32–35 which may be attributable to reduced ART initiation36–38 or adherence39 as we have previously observed,40–42 or to a higher risk of comorbidities such as hypertension and cancer.43 These racial/ethnic disparities are a primary focus of the National HIV/AIDS Strategy.44
Our findings demonstrate that ART initiation at high CD4 counts is associated with a reduced gap in life expectancy for HIV patients, consistent with previous studies identifying timely ART initiation as a strong predictor of survival.3,4,6,45 We found that the survival gap compared with HIV-uninfected subjects was reduced from 13.1 to 7.9 years in 2008–2011 for HIV-infected individuals who initiated ART with CD4 counts above 500 cells per microliter. These findings are germane given the recent Strategic Timing of Antiretroviral Treatment trial, which found lower rates of serious AIDS-related and non-AIDS–related events among individuals starting ART immediately with CD4 counts above 500 cells per microliter compared with deferral until CD4 counts fell below 350 cells per microliter.46 The mortality rate in the immediate arm of the Strategic Timing of Antiretroviral Treatment trial was nearly half that of the deferred arm, although not statistically significant given few deaths. Our study lends additional evidence that implementation of recent guidelines supporting earlier ART initiation47,48 will continue to reduce the survival disparity for HIV patients.
While HIV-infected individuals have a higher prevalence than HIV-uninfected individuals of lifestyle risk factors, such as smoking14 and substance use,11,12 most studies of life expectancy among HIV patients have compared with the general population, with limited ability to stratify by these individual-level factors. We found that the gap in life expectancy was narrowed in subgroups without a history of HBV or HCV infection, drug/alcohol abuse, or smoking. Notably, Helleberg et al49 found that HIV-infected men on ART lose more life-years through smoking than HIV infection itself. As lifespan lengthens and AIDS-related deaths decline among HIV patients,17,50,51 the impact on survival of smoking and other risk factors is likely to increase.
There are several limitations to our study. First, because risk factors were collected from the EHR, there may have been some misclassification. For example, heavy substance users without a drug/alcohol abuse diagnosis, or individuals with undiagnosed HBV or HCV infection, would have been misclassified as not having these risk factors, thus underestimating their impact on life expectancy. We also could not analyze these variables in detail. For example, a variable that captured current smoking, rather than ever smoking, would likely have had an even greater impact on life expectancy. Second, race/ethnicity data were incomplete, especially for HIV-uninfected subjects; if sicker patients had more complete data as a result of more interactions with the health care system, we may have underestimated life expectancies in racial/ethnic subgroups of HIV-uninfected subjects. Third, our study was subject to the life-table assumption that age-specific mortality rates are similar across birth cohorts. There was likely some violation of this assumption, possibly resulting in underestimated life expectancies for HIV patients, as younger birth cohorts have experienced greater benefits from recent advances in ART. However, this underestimation may have been minimized by the relative homogeneity of the cohort with respect to birth years (ie, half were born during 1954–1968). Fourth, subjects not included in the HIV registries were assumed to be HIV-uninfected; misclassification of undiagnosed HIV-infected individuals as HIV-uninfected may have resulted in underestimated life expectancies for HIV-uninfected individuals. However, we expect this to have had a negligible impact on our results given the low prevalence of HIV infection (0.12%) among KP members.52 Fifth, we did not have data on individual-level socioeconomic status, which may contribute to differences in life expectancy; however, disparities by socioeconomic status are likely to be explained by differences in access to care, which we accounted for in our analysis by comparing HIV-infected and HIV-uninfected individuals from within the same health care system. Sixth, we did not have sufficient sample size to estimate life expectancies in subgroups of optimally treated HIV patients defined by gender, race/ethnicity, or HIV-transmission risk group. Finally, most subjects were men, reflecting the HIV epidemic in California; however, we had sufficient sample size to estimate life expectancies among women with good precision.
Our study also has several strengths. First, to our knowledge, this is the first study to directly compare life expectancy by HIV status, accounting for individual-level factors and access to care. We used a large, well-characterized cohort of HIV-infected and matched HIV-uninfected subjects from the same health care system, thus allowing for stratification by race/ethnicity and risk factors, and minimizing the selection biases that can be introduced using an external comparison group. Furthermore, because KP members have comprehensive medical insurance coverage, differences in survival were unlikely to be attributable to differential access to care. Second, we completely ascertained deaths regardless of KP membership or HIV status, thus minimizing the biases that can be introduced by incomplete or differential ascertainment of deaths in life expectancy analyses.6 Third, KP’s HIV registries allowed for high-quality ascertainment of known HIV infection. Finally, the KP membership mirrors the age, sex, and race/ ethnicity distributions of the population of California,53,54 and the demographics of HIV-infected members are comparable with those of reported AIDS cases in California.55 Thus, our results are likely to be generalizable to the broader insured population.
In summary, despite a dramatic increase in life expectancy for HIV-infected individuals, an approximately eight-year gap in survival persists by HIV status in recent years, even with ART initiation at high CD4 counts. Although these results are likely generalizable to other HIV patients with access to health care, life expectancies for HIV-infected individuals without health insurance may be lower than those observed in this study. In addition to the risk factors examined here, future studies should consider other factors that may contribute to the survival disparity for HIV patients, such as cancer,15 cardiovascular disease,56 and other aging-associated conditions.57 Our results highlight the importance of timely ART initiation and risk-reduction strategies, such as smoking cessation, to increase the lifespan of HIV-infected individuals.
Acknowledgments
Supported by a research grant from Pfizer Pharmaceuticals. W.A.L. and L.X. received research grant support from Pfizer. J.L.M. received research grant support from Merck. M.A.H., C.P.Q., M.J.S., and C.R.C. received research grant support from Pfizer and Merck. W.J.T. received research grant support from Pfizer, Merck, Gilead, Bristol-Myers Squibb, ViiV Healthcare, and Vertex.
Footnotes
The remaining authors have no conflicts of interest to disclose.
Presented at the 23rd Conference on Retroviruses and Opportunistic Infections, February 22–25, 2016, Boston, MA.
The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
References
- 1.Bhaskaran K, Hamouda O, Sannes M, et al. Changes in the risk of death after HIV seroconversion compared with mortality in the general population. JAMA. 2008;300:51–59. doi: 10.1001/jama.300.1.51. [DOI] [PubMed] [Google Scholar]
- 2.Lima VD, Hogg RS, Harrigan PR, et al. Continued improvement in survival among HIV-infected individuals with newer forms of highly active antiretroviral therapy. AIDS. 2007;21:685–692. doi: 10.1097/QAD.0b013e32802ef30c. [DOI] [PubMed] [Google Scholar]
- 3.Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8:e81355. doi: 10.1371/journal.pone.0081355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lohse N, Hansen AB, Pedersen G, et al. Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med. 2007;146:87–95. doi: 10.7326/0003-4819-146-2-200701160-00003. [DOI] [PubMed] [Google Scholar]
- 6.Patterson S, Cescon A, Samji H, et al. Life expectancy of HIV-positive individuals on combination antiretroviral therapy in Canada. BMC Infect Dis. 2015;15:274. doi: 10.1186/s12879-015-0969-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrison KM, Song R, Zhang X. Life expectancy after HIV diagnosis based on national HIV surveillance data from 25 states, United States. J Acquir Immune Defic Syndr. 2010;53:124–130. doi: 10.1097/QAI.0b013e3181b563e7. [DOI] [PubMed] [Google Scholar]
- 8.May M, Gompels M, Delpech V, et al. Impact of late diagnosis and treatment on life expectancy in people with HIV-1: UK Collaborative HIV Cohort (UK CHIC) Study. BMJ. 2011;343:d6016. doi: 10.1136/bmj.d6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall HI, Frazier EL, Rhodes P, et al. Differences in human immunodeficiency virus care and treatment among subpopulations in the United States. JAMA Intern Med. 2013;173:1337–1344. doi: 10.1001/jamainternmed.2013.6841. [DOI] [PubMed] [Google Scholar]
- 10.Simard EP, Fransua M, Naishadham D, et al. The influence of sex, race/ethnicity, and educational attainment on human immunodeficiency virus death rates among adults, 1993–2007. Arch Intern Med. 2012;172:1591–1598. doi: 10.1001/archinternmed.2012.4508. [DOI] [PubMed] [Google Scholar]
- 11.Galvan FH, Bing EG, Fleishman JA, et al. The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: results from the HIV cost and services utilization study. J Stud Alcohol. 2002;63:179–186. doi: 10.15288/jsa.2002.63.179. [DOI] [PubMed] [Google Scholar]
- 12.Mimiaga MJ, Reisner SL, Grasso C, et al. Substance use among HIV-infected patients engaged in primary care in the United States: findings from the centers for AIDS research network of integrated clinical systems cohort. Am J Public Health. 2013;103:1457–1467. doi: 10.2105/AJPH.2012.301162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006;44(suppl 1):S6–S9. doi: 10.1016/j.jhep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Mdodo R, Frazier EL, Dube SR, et al. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med. 2015;162:335–344. doi: 10.7326/M14-0954. [DOI] [PubMed] [Google Scholar]
- 15.Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148:728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 16.Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 17.Wada N, Jacobson LP, Cohen M, et al. Cause-specific life expectancies after 35 years of age for human immunodeficiency syndrome-infected and human immunodeficiency syndrome-negative individuals followed simultaneously in long-term cohort studies, 1984–2008. Am J Epidemiol. 2013;177:116–125. doi: 10.1093/aje/kws321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silverberg MJ, Chao C, Leyden WA, et al. HIV infection, immunodeficiency, viral replication, and the risk of cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:2551–2559. doi: 10.1158/1055-9965.EPI-11-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon N. [Accessed August 24, 2015];Similarity of the Adult Kaiser Permanente Membership in Northern California to the Insured and General Population in Northern California: Statistics from the 2011 California Health Interview Survey. 2015 Available at: http://www.dor.kaiser.org/external/chis_non_kp_2011/
- 20.Death Data Files. California, CA: Department of Public Health; 2016. [Accessed February 29, 2016]. Available at: http://www.cdph.ca.gov/data/dataresources/requests/Pages/DeathDataFiles.aspx. [Google Scholar]
- 21.Death Master File Monthly Updates. National Technical Information Service, U.S. Department of Commerce; 2016. [Accessed February 29, 2016]. Available at: https://dmf.ntis.gov/monthly/ [Google Scholar]
- 22.Marcus JL, Leyden WA, Chao CR, et al. Differences in response to antiretroviral therapy by sex and hepatitis C infection status. AIDS Patient Care STDS. 2015;29:370–378. doi: 10.1089/apc.2015.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JH, Han G, Fulp WJ, et al. Analysis of overdispersed count data: application to the human Papillomavirus infection in men (HIM) study. Epidemiol Infect. 2012;140:1087–1094. doi: 10.1017/S095026881100166X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arias E. United States life tables, 2010. Natl Vital Stat Rep. 2014;63:1–63. [PubMed] [Google Scholar]
- 25.Chiang CL. The Life Table and its Construction. Introduction to Stochastic Processes in Biostatistics. New York, NY: John Wiley and Sons; 1983. pp. 189–214. [Google Scholar]
- 26.Eayres D, Williams ES. Evaluation of methodologies for small area life expectancy estimation. J Epidemiol Community Health. 2004;58:243–249. doi: 10.1136/jech.2003.009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith DP. Formal Demography. New York, NY: Springer Science, Business Media New York; 1992. [Google Scholar]
- 28.Lloyd-Smith E, Brodkin E, Wood E, et al. Impact of HAART and injection drug use on life expectancy of two HIV-positive cohorts in British Columbia. AIDS. 2006;20:445–450. doi: 10.1097/01.aids.0000206508.32030.92. [DOI] [PubMed] [Google Scholar]
- 29.Lourenco L, Colley G, Nosyk B, et al. High levels of heterogeneity in the HIV cascade of care across different population subgroups in British Columbia, Canada. PLoS One. 2014;9:e115277. doi: 10.1371/journal.pone.0115277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber R, Huber M, Rickenbach M, et al. Uptake of and virological response to antiretroviral therapy among HIV-infected former and current injecting drug users and persons in an opiate substitution treatment programme: the Swiss HIV cohort study. HIV Med. 2009;10:407–416. doi: 10.1111/j.1468-1293.2009.00701.x. [DOI] [PubMed] [Google Scholar]
- 31.May MT, Justice AC, Birnie K, et al. Injection drug Use and hepatitis C as risk factors for mortality in HIV-infected individuals: the antiretroviral therapy cohort collaboration. J Acquir Immune Defic Syndr. 2015;69:348–354. doi: 10.1097/QAI.0000000000000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubin MS, Colen CG, Link BG. Examination of inequalities in HIV/AIDS mortality in the United States from a fundamental cause perspective. Am J Public Health. 2010;100:1053–1059. doi: 10.2105/AJPH.2009.170241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levine RS, Briggs NC, Kilbourne BS, et al. Black-White mortality from HIV in the United States before and after introduction of highly active antiretroviral therapy in 1996. Am J Public Health. 2007;97:1884–1892. doi: 10.2105/AJPH.2005.081489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemly DC, Shepherd BE, Hulgan T, et al. Race and sex differences in antiretroviral therapy use and mortality among HIV-infected persons in care. J Infect Dis. 2009;199:991–998. doi: 10.1086/597124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siddiqi AE, Hu X, Hall HI. Mortality among blacks or African Americans with HIV infection–United States, 2008–2012. MMWR Morb Mortal Wkly Rep. 2015;64:81–86. [PMC free article] [PubMed] [Google Scholar]
- 36.Gebo KA, Fleishman JA, Conviser R, et al. Racial and gender disparities in receipt of highly active antiretroviral therapy persist in a multistate sample of HIV patients in 2001. J Acquir Immune Defic Syndr. 2005;38:96–103. doi: 10.1097/00126334-200501010-00017. [DOI] [PubMed] [Google Scholar]
- 37.Shapiro MF, Morton SC, McCaffrey DF, et al. Variations in the care of HIV-infected adults in the United States: results from the HIV cost and services utilization study. JAMA. 1999;281:2305–2315. doi: 10.1001/jama.281.24.2305. [DOI] [PubMed] [Google Scholar]
- 38.Moore RD, Stanton D, Gopalan R, et al. Racial differences in the use of drug therapy for HIV disease in an urban community. N Engl J Med. 1994;330:763–768. doi: 10.1056/NEJM199403173301107. [DOI] [PubMed] [Google Scholar]
- 39.Golin CE, Liu H, Hays RD, et al. A prospective study of predictors of adherence to combination antiretroviral medication. J Gen Intern Med. 2002;17:756–765. doi: 10.1046/j.1525-1497.2002.11214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horberg MA, Hurley L, Towner WJ, et al. The HIV care cascade measured over time by age, sex, and race in a large national integrated care system. AIDS Patient Care STDS. 2015;29:582–590. doi: 10.1089/apc.2015.0139. [DOI] [PubMed] [Google Scholar]
- 41.Horberg MA, Hurley L, Klein DB, et al. The HIV Care Cascade Measured Over Multiple Time Periods Varies by Race and Ethnicity. Miami, FL: NIMH/IAPAC Treatment Adherence Conference; 2015. [Google Scholar]
- 42.Silverberg MJ, Leyden W, Quesenberry CP, Jr, et al. Race/ethnicity and risk of AIDS and death among HIV-infected patients with access to care. J Gen Intern Med. 2009;24:1065–1072. doi: 10.1007/s11606-009-1049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Centers for Disease Control and Prevention. Health disparities experienced by black or African Americans–United States. MMWR Morb Mortal Wkly Rep. 2005;54:1–3. [PubMed] [Google Scholar]
- 44.National HIV/AIDS Strategy for the United States: Updated to 2020. The White House; 2015. [Accessed October 26, 2015]. Available at: https://www.aids.gov/federal-resources/national-hiv-aids-strategy/nhas-update.pdf. [Google Scholar]
- 45.May MT, Gompels M, Delpech V, et al. Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS. 2014;28:1193–1202. doi: 10.1097/QAD.0000000000000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.INSIGHT START Study Group. Lundgren JD, Babiker AG, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health and Human Services; 2014. [Accessed August 25, 2015]. Available at: https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-treatment-guidelines/0. [Google Scholar]
- 48.World Health Organization. [Accessed August 25, 2015];Consolidated Guidelines on HIV Prevention, Diagnosis, Treatment and Care for Key Populations. 2014 Available at: http://apps.who.int/iris/bitstream/10665/128048/1/9789241507431_eng.pdf?ua=1&ua=1. [PubMed]
- 49.Helleberg M, May MT, Ingle SM, et al. Smoking and life expectancy among HIV-infected individuals on antiretroviral therapy in Europe and North America. AIDS. 2015;29:221–229. doi: 10.1097/QAD.0000000000000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lima VD, Lourenco L, Yip B, et al. Trends in AIDS incidence and AIDS-related mortality in British Columbia between 1981 and 2013. Lancet HIV. 2015;2:e92–e97. doi: 10.1016/S2352-3018(15)00017-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith CJ, Ryom L, Weber R, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet. 2014;384:241–248. doi: 10.1016/S0140-6736(14)60604-8. [DOI] [PubMed] [Google Scholar]
- 52.Satre DD, Parthasarathy S, Altschuler A, et al. Demographic, insurance, and health characteristics of newly enrolled HIV patients after implementation of the Affordable Care Act in California. Am J Public Health. 2016 Apr 14;:e1–e3. doi: 10.2105/AJPH.2016.303126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gordon NP. [Accessed May 21, 2013];How Does the Adult Kaiser Permanente Membership in Northern California Compare with the Larger Community? 2006 Available at: http://www.dor.kaiser.org/external/uploadedFiles/content/research/mhs/_2011_Revised_Site/Documents_Special_Reports/comparison_kaiser_vs_nonKaiser_adults_kpnc(1).pdf.
- 54.Koebnick C, Langer-Gould AM, Gould MK, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16:37–41. doi: 10.7812/tpp/12-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.California Department of Health Services, Office of AIDS. [Accessed May 21, 2013];HIV/AIDS Surveillance in California. 2010 Available at: http://www.cdph.ca.gov/programs/aids/Documents/SSSemiAnnualRptDec2011.pdf.
- 56.Silverberg MJ, Leyden WA, Xu L, et al. Immunodeficiency and risk of myocardial infarction among HIV-positive individuals with access to care. J Acquir Immune Defic Syndr. 2014;65:160–166. doi: 10.1097/QAI.0000000000000009. [DOI] [PubMed] [Google Scholar]
- 57.Hasse B, Ledergerber B, Furrer H, et al. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis. 2011;53:1130–1139. doi: 10.1093/cid/cir626. [DOI] [PubMed] [Google Scholar]