Abstract
After dozens of clinical trials, there are still no Food and Drug Administration–approved drugs that improve mortality in acute respiratory distress syndrome (ARDS). These poor results may be caused in part by three unique pharmacological challenges presented by ARDS: (1) Patients with ARDS are fragile because of concomitant multiple organ dysfunction, so they do not tolerate off-target side effects of drugs; (2) inhaled drug delivery is impeded by the column of proteinaceous fluid covering the injured alveoli; and (3) ARDS is heterogeneous in its underlying pathophysiology, so targeting one pathway is unlikely to improve most patients. To address these three pharmacological problems, I present the development of pulmonary endothelium–targeted liposomes (PELs). PELs are approximately 100-nm drug carriers coated with antibodies that bind to the pulmonary capillary endothelium. In model organisms, intravenously injected PELs strongly concentrate drugs in alveoli, even in animal models displaying severe, spatially heterogeneous pathology similar to severe ARDS. By concentrating drugs in inflamed alveoli, PELs solve pharmacological challenge (1) above. By being obligate intravenous medications, they solve challenge (2). Finally, because PELs can be loaded with at least three drugs, they can solve challenge (3) with combination drug therapy. My colleagues and I are currently testing PELs loaded with numerous candidate drugs in mouse models of ARDS, and we are testing drug distribution in live pigs and ex vivo human lungs. We aim to use such preclinical validation to move PELs into a partnership with industry, and then to patients.
Keywords: acute respiratory distress syndrome, nanotechnology, nanocarrier, liposome
The American Thoracic Society (ATS) annually bestows the ATS Building Education to Advance Research (BEAR) Cage Award to an innovative translational research project. While serving as an advanced research fellow in pulmonary and critical care medicine, I received the 2016 prize for the early development of a nanotechnology-based drug delivery platform to treat acute respiratory distress syndrome (ARDS). ARDS poses several specific pharmacological challenges that have guided the design of these nanoscale drug carriers and the selection of their cargo drugs. In this article, I describe the rationale for considering a nanotechnological solution to targeting the lungs for drug treatment of ARDS, and I summarize my work in progress toward that goal.
ARDS epitomizes both the triumphs and failures of medical intensive care. The triumphs reflect advances in supportive care. Refinements in the use of positive-pressure mechanical ventilators, combined with advances in maintaining cardiovascular and metabolic homeostasis and preventing complications, have decreased the mortality of ARDS from above 80% in the 1960s to less than 40% today (1). However, the failure of medical intensive care lies in the lack of disease-modifying medications for ARDS and many other critical illnesses.
Challenges of Developing Effective Disease-modifying Drug Therapy for ARDS
Since the 1980s, dozens of drugs have failed in clinical trials for ARDS (2). Why have so many rationally chosen drugs proven ineffective? Reasons include the heterogeneity of the underlying pathology, the heterogeneous patient population, and the lack of an ideal animal model (3). But the partial triumph of advances in supportive care certainly shows that mortality reductions are possible and that interventions proven in existing animal models can benefit humans.
I propose another perspective on why so many ARDS drug therapies have failed: ARDS faces three relatively unique pharmacological challenges. First, patients with ARDS very frequently have multiple organ dysfunction syndrome, so they are fragile, with less tolerance than other patient populations to off-target side effects of drugs. This is well illustrated by the finding of the β-Agonist Lung Injury Trial-2 study investigators that one of the safest drugs in the pharmacopoeia, albuterol, actually increased mortality in ARDS, likely because of off-target side effects (4).
Second, inhaled drug delivery, so useful for airway diseases such as asthma, is much more difficult to achieve in ARDS. The pathobiology of ARDS lies in the alveoli, which in ARDS are covered by a thick column of proteinaceous liquid that acts as a unique barrier to topical drug delivery. This barrier to drug delivery partly explains why inhaled prostanoids and nitric oxide improve oxygenation (not vasodilating flooded alveoli), and it may explain why inhaled albuterol in the Albuterol to Treat Acute Lung Injury trial had weaker effects than intravenous albuterol on pulmonary edema (5).
A third pharmacological challenge of ARDS is the commonly cited problem of heterogeneity, with multiple cell types involved, each to differing extents in different patient subpopulations, making targeting a single pathway unlikely to work for the entire ARDS population. To solve these three unique pharmacological challenges for the diverse ARDS population, a drug should concentrate in alveoli after intravenous injection and target multiple alveolar cell types and cell processes.
Proposed Solution to the Challenges of Drug Delivery
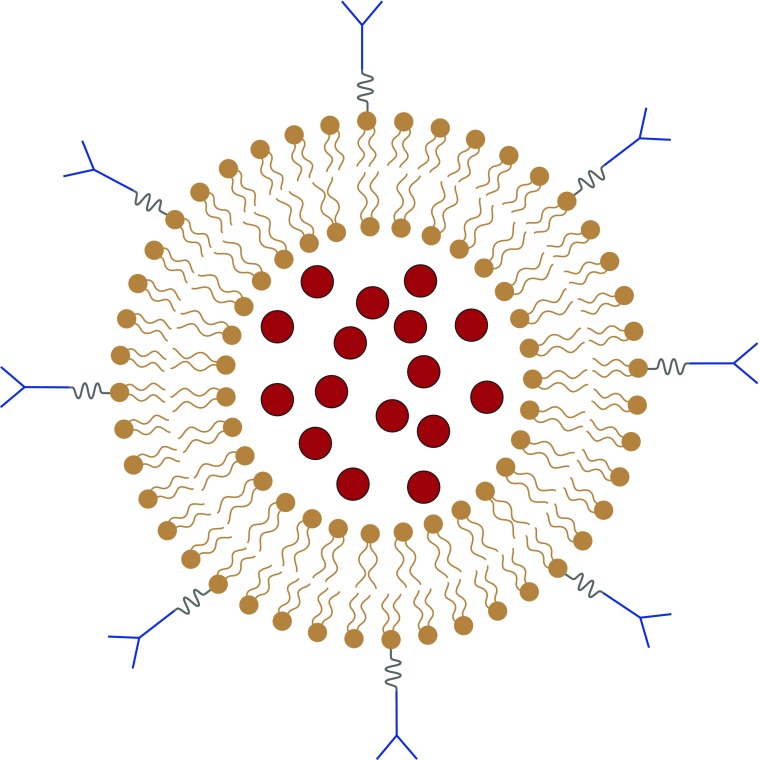
I propose such a solution in the form of pulmonary endothelium–targeted liposomes (PELs). PELs are composed of three components (Figure 1), each of which has separately been approved for clinical use: liposomes (100-nm lipid bilayers that carry drugs), cargo drugs (e.g., those that failed in ARDS trials because of off-target side effects), and antibodies that target the liposomes to the alveolar capillary endothelium.
Figure 1.
Pulmonary endothelium–targeted liposomes (PELs). PELs are composed of liposomes (phospholipid bilayers of approximately 100-nm diameter; brown), cargo drugs (red circles), and antibodies (blue Y shapes).
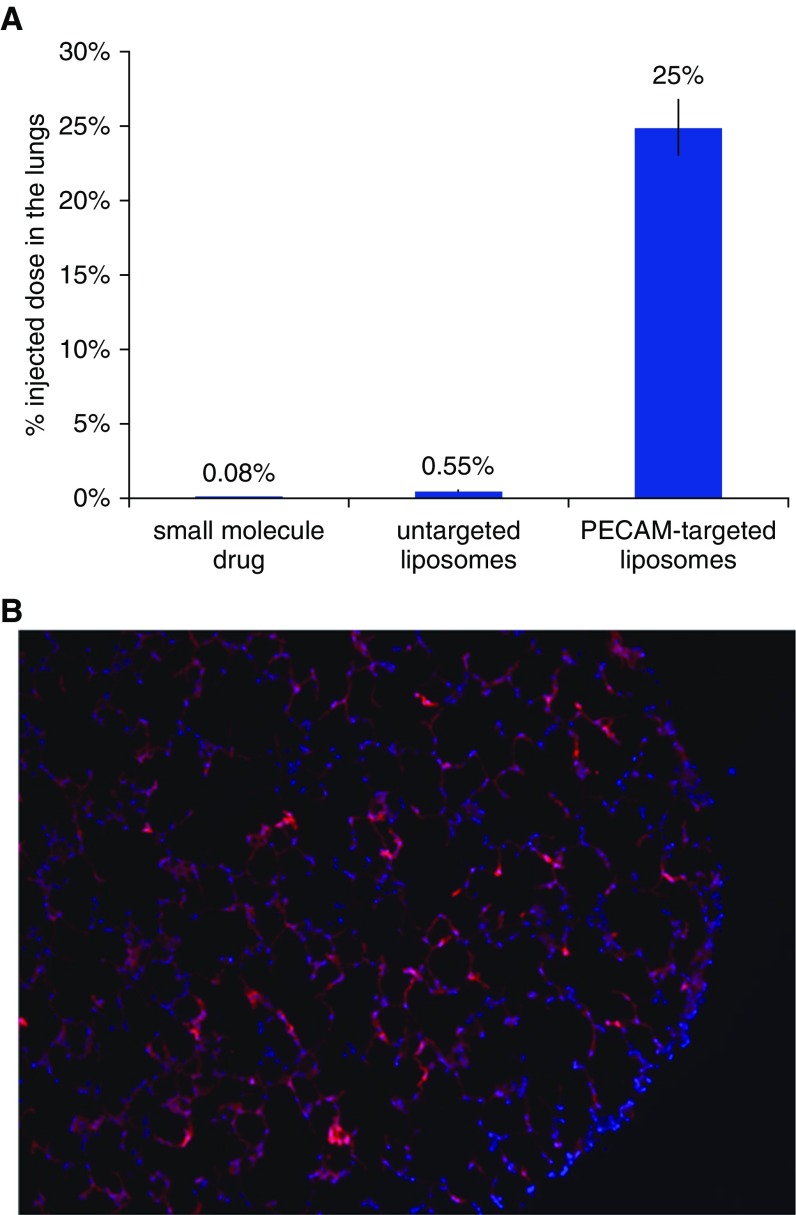
Nearly 20 years ago, my scientific mentor, Vladimir Muzykantov, discovered that coating nanoscale drug carriers with antibodies directed against endothelial epitopes (e.g., the pan- endothelial marker platelet endothelial cell adhesion molecule [PECAM]) causes the drug carriers to markedly accumulate in the lungs (6). I have recently shown that PECAM-targeted PELs accumulate in the lungs of mice at greater than 300-fold higher levels than typical small-molecule drugs (Figure 2A) (7). Further, those PELs appear to concentrate in the alveoli (Figure 2B). This lung-specific accumulation is likely due to flow dynamics, with the lungs being the first-pass organ after intravenous injection and representing a large fraction of the body’s endothelial surface area. Thus, PELs solve the above three pharmacological challenges of ARDS: They can be delivered by intravenous infusion; they strongly concentrate drugs in the alveoli; and because PELs can be loaded with up to three drugs, they can act simultaneously on multiple cells and pathways, thereby enabling “combination drug therapy.”
Figure 2.
Pulmonary endothelium–targeted liposomes concentrate cargo molecules in the lungs. (A) Fraction of radiolabeled agents in the lungs of mice 30 minutes after injection. The test small-molecule drug used was diethylenetriaminepentaacetic acid. Liposomes were targeted to platelet endothelial cell adhesion molecule (PECAM) by covalently conjugating to the liposome surface monoclonal antibodies targeting PECAM. (B) Fluorescently labeled liposomes (containing a membrane lipid conjugated to the dye rhodamine) were injected into mice, and then fresh-frozen sections of the mouse lung were made and imaged under a microscope. Red areas indicate liposomes, and blue areas (4′,6-diamidino-2-phenylindole dye) are nuclei.
PELs are part of the emerging field of nanomedicine that offers promise to greatly benefit the diseases of pulmonary and critical care medicine. Nanomedicine primarily involves the use of nanoscale (usually about 100 nm) drug carriers to improve the localization, kinetics, and sometimes pharmacodynamics of drugs.
Nanomedicine first entered the critical care armamentarium in 1990 with the Food and Drug Administration’s approval of amphotericin B (AmBisome; Astellas Pharma, Northbrook, IL), a liposomal formulation of amphotericin for severe fungal infection (8). Since then, the field of nanomedicine has only scored hits with two oncology drugs. However, pulmonary nanomedicine may soon witness a new dawn because promising nanotechnologies, such as inhaled liposomal amikacin for bronchiectasis, are supported by strong data in late-stage clinical trials (9).
Pulmonary nanomedicine has the long-term potential to benefit nearly all lung diseases by dramatically increasing local concentrations of drugs in the lungs relative to other organs, thereby expanding the repertoire of drug formulations that can be used with attractive efficacy and safety profiles. Most nanomedicine-enhanced applications are likely to employ delivery by inhalation. However, I believe that intravenously delivered formulations will soon prove comparably attractive for certain applications, such as treatment of ARDS.
Development in Progress
For the ATS BEAR Cage Award competition, I proposed the development and validation of PELs for ARDS. I began by further optimizing the delivery system. For example, I developed a mouse model of spatially heterogeneous ARDS to investigate whether our PELs would be shunted away from flooded alveoli via hypoxic vasoconstriction (potentially a fourth pharmacological challenge of ARDS). Indeed, PECAM-targeted PELs were strongly shunted away from flooded alveoli. However, by switching to target another epitope, intercellular adhesion molecule, I was able to concentrate PELs preferentially in inflamed, instead of healthy, lung regions (7).
After making other optimizations of the targeting system, I am now systematically testing in ARDS mouse models the treatment effect of multiple drugs loaded into PELs to determine if PELs increase the therapeutic index of each drug. To further validate the potential clinical utility, I am also testing PEL targeting in live, large animals (pigs) and human lungs in an ex vivo lung perfusion model. The goal is to develop a multidrug-loaded PEL ready to move from academia into large-scale preclinical and clinical testing by partnering with industry.
Future Prospects for Targeted Drug Treatment of ARDS
ARDS is ripe for drug development. First, ARDS affects about 190,000 patients in the United States every year (10), making it among the most common diseases that can benefit from the “orphan drug laws” (which apply to diseases with <200,000 prevalent patients) (11). Second, there is significant room for improved outcomes in ARDS, with a mortality rate up to 40%, prolonged physical debilitation (approximately 90% were dependent on caregivers at 1 yr in one study [12]), and cognitive impairment at 1 year impairing roughly 50% of ARDS survivors (13). Third, ARDS is extremely costly to hospitals and insurers, averaging approximately $300,000 for the first year, not to mention the costs of rehabilitation and prolonged disability (10). Finally, there are no approved ARDS drugs to compete against. This rosy market landscape makes ARDS drug development financially prudent, especially if a new pharmacological approach is taken.
The ATS BEAR Cage Award in Perspective
The ATS BEAR Cage Award significantly aided my pursuit of a pulmonary nanomedicine formulation for ARDS. The annual competition holds promise for advancing other innovations in at least two ways. First, the BEAR Cage Award provides a platform for discussion of new translational technologies within academia. The National Institutes of Health generally focuses its funding on science (finding new knowledge) rather than on engineering (building technologies), which makes developing translational technologies difficult within academia. The BEAR Cage Award provides a rare accolade to technological development, opening numerous doors for funding and publication. Second, the BEAR Cage Award facilitates academic interaction with industry. The BEAR Cage Award Committee is composed of experts from academia and the pharmaceutical industry and serves as a diverse mentoring team to BEAR Cage Award recipients, whose projects need a bridge from academia to industry.
I am thankful to the ATS BEAR Cage Award Committee for a tremendous boost to my work and for continuing support. Together, I hope we can further the field of pulmonary nanomedicine. More important, I hope that, through collaboration between academia, industry, and government, we can turn the decades of ARDS drug failures into triumphs.
Supplementary Material
Acknowledgments
Acknowledgment
The author thanks Vladimir Muzykantov, who has served as his research advisor for the last 3 years, conceived of the approach described with me, and in whose laboratory I conducted all of the supporting experiments. The author also thanks his research mentors in the University of Pennsylvania Pulmonary, Allergy, and Critical Care Division, especially Steve Albelda, Mike Beers, Nilam Mangalmurti, Scott Worthen, and Jason Christie. The author is also grateful to John Hansen-Flaschen for encouraging the author to apply for the BEAR Cage Award and for critical review of the manuscript.
Footnotes
Supported by National Institutes of Health grant F32 HL129665-01 (J.S.B.).
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, et al. LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 2.Brenner JS, Greineder C, Shuvaev V, Muzykantov V. Endothelial nanomedicine for the treatment of pulmonary disease. Expert Opin Drug Deliv. 2015;12:239–261. doi: 10.1517/17425247.2015.961418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beitler JR, Goligher EC, Schmidt M, Spieth PM, Zanella A, Martin-Loeches I, Calfee CS, Cavalcanti AB ARDSne(x)t Investigators. Personalized medicine for ARDS: the 2035 research agenda. Intensive Care Med. 2016;42:756–767. doi: 10.1007/s00134-016-4331-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao Smith F, Perkins GD, Gates S, Young D, McAuley DF, Tunnicliffe W, Khan Z, Lamb SE BALTI-2 study investigators. Effect of intravenous β-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet. 2012;379:229–235. doi: 10.1016/S0140-6736(11)61623-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Randomized, placebo-controlled clinical trial of an aerosolized β2-agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011;184:561–568. doi: 10.1164/rccm.201012-2090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muzykantov VR, Christofidou-Solomidou M, Balyasnikova I, Harshaw DW, Schultz L, Fisher AB, Albelda SM. Streptavidin facilitates internalization and pulmonary targeting of an anti-endothelial cell antibody (platelet-endothelial cell adhesion molecule 1): a strategy for vascular immunotargeting of drugs. Proc Natl Acad Sci USA. 1999;96:2379–2384. doi: 10.1073/pnas.96.5.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenner JS, Bhamidipati K, Glassman P, Ramakrishnan N, Jiang D, Paris AJ, Myerson JW, Pan DC, Shuvaev VV, Villa C, et al. Mechanisms that determine nanocarrier targeting to healthy versus inflamed lung regions Nanomedicine[online ahead of print] 2017. Jan 5DOI: 10.1016/j.nano.2016.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steimbach LM, Tonin FS, Virtuoso S, Borba HHL, Sanches ACC, Wiens A, Fernandez-Llimós F, Pontarolo R. Efficacy and safety of amphotericin B lipid-based formulations—a systematic review and meta-analysis. Mycoses. 2017;60:146–154. doi: 10.1111/myc.12585. [DOI] [PubMed] [Google Scholar]
- 9.Olivier KN, Griffith DE, Eagle G, McGinnis JP, II, Micioni L, Liu K, Daley CL, Winthrop KL, Ruoss S, Addrizzo-Harris DJ, et al. Randomized trial of liposomal amikacin for inhalation in nontuberculous mycobacterial lung disease Am J Respir Crit Care Med 2016. Oct 17DOI: 10.1164/rccm.201604-0700OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 11.Wellman-Labadie O, Zhou Y. The US Orphan Drug Act: rare disease research stimulator or commercial opportunity? Health Policy. 2010;95:216–228. doi: 10.1016/j.healthpol.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Unroe M, Kahn JM, Carson SS, Govert JA, Martinu T, Sathy SJ, Clay AS, Chia J, Gray A, Tulsky JA, et al. One-year trajectories of care and resource utilization for recipients of prolonged mechanical ventilation: a cohort study. Ann Intern Med. 2010;153:167–175. doi: 10.1059/0003-4819-153-3-201008030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilcox ME, Brummel NE, Archer K, Ely EW, Jackson JC, Hopkins RO. Cognitive dysfunction in ICU patients: risk factors, predictors, and rehabilitation interventions. Crit Care Med. 2013;41(9) Suppl 1:S81–S98. doi: 10.1097/CCM.0b013e3182a16946. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.