Abstract
Rationale: Patients with sarcoidosis may have an increased risk of infection similar to other immune-mediated disorders. However, the data are still limited.
Objectives: To investigate the risk of hospitalized infection among patients with sarcoidosis, using a population-based cohort.
Methods: Using the Rochester Epidemiology Project record-linkage system, a cohort of incident cases of sarcoidosis in Olmsted County, Minnesota from 1976 to 2013 was identified. Diagnosis was confirmed by individual medical record review. For each patient with sarcoidosis, a sex- and age-matched comparator without sarcoidosis was randomly selected from the same population. Medical records of cases and comparators were individually reviewed for hospitalized infection that occurred after the index date. The cumulative incidence of hospitalized infection overall and by type of infection, adjusted for the competing risk of death, was estimated. Cox models were used to compare the rate of first hospitalized infection between cases and comparators and to evaluate the association between use of immunosuppressive agents and hospitalized infection among cases.
Results: Three hundred and forty-five cases and 345 comparators were identified. Patients with sarcoidosis had a higher risk of a hospitalized infection with a hazard ratio (HR) of 2.00 (95% confidence interval [CI], 1.41–2.84), adjusted for age, sex, and calendar year of index date. Use of oral glucocorticoids was a significant predictor of hospitalized infection with an HR of 3.03 (95% CI, 1.33–6.90) for oral glucocorticoids not exceeding 10 mg/day and an HR of 4.48 (95% CI, 1.54–13.03) for oral glucocorticoids greater than 10 mg/day.
Conclusions: Patients with sarcoidosis are at increased risk of hospitalized infection. Glucocorticoid therapy is strongly associated with this increased risk.
Keywords: sarcoidosis, epidemiology, infection, hospitalization
Infection is one of the most common complications of immune-mediated disorders. An increased risk of both pyogenic and opportunistic infection has been observed in several immune-mediated disorders such as rheumatoid arthritis, system lupus erythematosus, idiopathic inflammatory myositis, and vasculitis (1–4). Use of immunosuppressive agents and immune dysregulation are believed to play a pivotal role in the pathogenesis of the increased risk (5).
Sarcoidosis is an immune-mediated disorder of unclear etiology characterized by the presence of noncaseating granulomas in the affected organs. The clinical course of sarcoidosis ranges from indolent incidental findings on chest radiography to an acute self-limited process and to chronic progressive disease associated with organ failure (6, 7). Commonly used medications for the management of sarcoidosis include glucocorticoids and other immunosuppressive agents.
Similar to patients with other immune-mediated diseases, patients with sarcoidosis may have a higher risk of infection. However, data on the association between infection and sarcoidosis are limited. In the current study, a previously identified cohort of Olmsted County, Minnesota residents with incident sarcoidosis from 1976 to 2013 was used to compare the incidence of hospitalized infection with sex- and age-matched comparators (8).
Methods
Participants and Study Design
The population of Olmsted County, Minnesota provides a unique opportunity to address the epidemiology of sarcoidosis owing to resources of the Rochester Epidemiology Project (REP). The REP database provides comprehensive access to both inpatient and outpatient medical records of Olmsted County residents for over six decades from all local health care providers including the Mayo Clinic, the Olmsted Medical Center, local nursing homes, and a few private practitioners. The potential of this database for epidemiologic study was previously reported (9).
Using this medical record–linkage system, a cohort of 345 incident cases of sarcoidosis in Olmsted County, Minnesota from 1976 to 2013 was identified (8). All patients with diagnosis codes related to sarcoidosis and noncaseating granuloma were first identified from the database.
The medical records of these potential cases were then individually reviewed. Diagnosis of sarcoidosis was confirmed on the basis of the presence of histopathologic evidence of noncaseating granuloma without evidence of acid-fast bacilli or fungi, radiographic features of intrathoracic sarcoidosis, compatible clinical presentation, and exclusion of other known granulomatous inflammation. The only exception to the requirement of histopathology was stage 1 pulmonary sarcoidosis, which required only radiographic evidence of symmetric bilateral hilar adenopathy in the absence of other identifiable causes. Biopsy-proven isolated granulomatous disease of a specific organ without pulmonary sarcoidosis was also included if there was no better alternative diagnosis. However, isolated cutaneous granulomatous inflammation was not included as it could be mimicked by several conditions such as foreign body reaction (10).
Baseline demographics, clinical characteristics, and laboratory investigations of cases were abstracted. Data on baseline pulmonary function test (PFT), including TLC, FVC, FEV1, and diffusing capacity of the lung for carbon monoxide (DlCO) were also collected. Baseline PFT was defined as the closest to diagnosis date in the period from 2 years prior to sarcoidosis diagnosis to 1 year after diagnosis. Cases with a diagnosis of sarcoidosis before residency in Olmsted County (i.e., prevalent cases) were excluded.
For each patient with sarcoidosis, a comparator without sarcoidosis at the time the patient received a diagnosis of sarcoidosis was randomly selected from the same population. Matching criteria included similar age (within 3 yr) and same sex. The index date of the comparator was the same date as the diagnosis date of the corresponding case. Medical records of cases and comparators were individually reviewed for community-acquired infection that required hospitalization occurring after the index date/date of diagnosis. Hospital-acquired infection was not included.
Bacteremia or septicemia was defined as isolation of a pathogenic microorganism from one or more blood cultures, with fever equal to or more than 38°C. Septic arthritis was defined as positive microbiologic culture from synovial fluid in the presence of suggestive clinical features. Urinary tract infection included pyelonephritis and urosepsis, defined as isolation of at least 100,000 colony-forming units/ml of urine in the presence of suggestive clinical features. Pneumonia was defined as the presence of new infiltrates, consolidation, or effusion seen by chest radiography and suggestive clinical features. Osteomyelitis was defined as clinical suspicion by physician, confirmed by definite radiologic findings or positive bone culture.
Lower respiratory tract infections, skin and soft tissue infections (cellulitis, abscesses, wound infections, herpes zoster, and diabetic foot infections), gastrointestinal infection (gastroenteritis and colitis), and intraabdominal infections (acute cholecystitis, ascending cholangitis, suppurative appendicitis, peritonitis, and intraabdominal abscess) were included on the basis of a physician’s diagnosis and relevant clinical findings. The category “other infections” included hospitalization for otitis media, sinusitis, eye infections, genital tract infections, tuberculosis, meningitis, brain abscess, and acute hepatitis.
Data on potential risk factors for hospitalized infection included smoking status, body mass index, diabetes mellitus, hypertension, dyslipidemia, and use of glucocorticoids as well as other immunosuppressive agents. The data were abstracted from individual medical record review. Follow-up was continued until death, migration, or January 1, 2016. The study was approved by the Mayo Clinic and Olmsted Medical Center institutional review boards. The need for informed consent was waived.
Statistical Analyses
Descriptive statistics (percentages, mean, etc.) were used to summarize the characteristics of both cohorts. Comparisons between the cohorts were performed using χ2 and rank sum tests. The cumulative incidence of ever-medication use and of the first hospitalized infection overall and by type of infection, adjusted for the competing risk of death was estimated (11). These methods are similar to the Kaplan–Meier method with censoring of patients who are still alive at last follow-up. However, patients who die before experiencing a hospitalized infection are appropriately accounted for to avoid overestimation of the rate of occurrence of hospitalized infection, which can occur if such subjects are simply censored at death. For the estimation of the overall cumulative incidence of a hospitalized infection, the date of occurrence was the earliest date of occurrence of any of the individual types of infection.
Cox proportional hazards models were used to compare the rate of development of a hospitalized infection between patients with sarcoidosis and nonsarcoidosis comparators. Analysis was also conducted according to stage of pulmonary sarcoidosis at baseline. Analyses were also performed to compare the risk between patients with sarcoidosis who were exposed to immunosuppressive agents (i.e., disease-modifying antirheumatic agents [DMARDs], biologic agents, and/or systemic glucocorticoids), patients with sarcoidosis who were not exposed to immunosuppressive agents, and nonsarcoidosis comparators. In addition, Cox proportional hazards models were used to evaluate the association of the previously described risk factors of interest, baseline clinical characteristics, and use of immunosuppressive agents on the development of hospitalized infection among patients with sarcoidosis.
Exposure to immunosuppressive agents was modeled using time-dependent covariates, which changed from unexposed to exposed at the time of initiation of the first immunosuppressive agent. Current use of immunosuppressive agents was modeled using time-dependent covariates that changed from nonuse to use at the time of initiation of an immunosuppressive agent and also changed from use back to nonuse 30 days after an immunosuppressive medication was discontinued to appropriately attribute hospitalized infections occurring immediately after discontinuation to this previous use. A P value less than 0.05 was considered statistically significant for all analyses. Analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC) and R 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study Population
A total of 345 cases of incident sarcoidosis and 345 comparators without sarcoidosis were included in this study. Mean duration of follow-up for cases and comparators was 15.1 and 16.8 years, respectively. Demographics, baseline characteristics, and exposure to immunosuppressive agents of both cohorts are described in Table 1. Overall, the two cohorts shared similar characteristics except for a higher percentage of nonwhite subjects and subjects with obesity in the sarcoidosis cohort and a higher percentage of current smokers in the nonsarcoidosis comparison cohort.
Table 1.
Baseline characteristics of patients with sarcoidosis and of age- and sex-matched comparators
| Cases |
Comparators |
P Value | |
|---|---|---|---|
| (n = 345) | (n = 345) | ||
| Mean age at diagnosis/index date, yr (SD) | 45.6 (13.6) | 45.4 (13.7) | 0.87 |
| Female, % | 50 | 50 | 1.0 |
| Ethnicity, % | <0.001 | ||
| White | 90 | 95 | |
| African-American | 5 | 1 | |
| Asian | 2 | 0 | |
| Native American | 1 | 0 | |
| Other | 2 | 4 | |
| Mean length of follow-up, yr (SD) | 15.1 (10.5) | 16.8 (10.8) | |
| Smoking status at diagnosis/index date, % | <0.001 | ||
| Never | 60 | 42 | |
| Ex-smoker | 21 | 22 | |
| Current smoker | 19 | 36 | |
| Obesity, % | 41 | 21 | <0.001 |
| Hypertension, % | 22 | 22 | 1.0 |
| Dyslipidemia, % | 14 | 17 | 0.46 |
| Diabetes mellitus, % | 9 | 8 | 0.49 |
| Use of aspirin, % | 12 | 10 | 0.55 |
| Number of subjects who were ever exposed to medications, n (%)* | |||
| Methotrexate | 11 (3) | NA | |
| Hydroxychloroquine | 13 (4) | NA | |
| Other nonbiologic DMARDs | 5 (2) | NA | |
| TNF inhibitor | 5 (2) | NA | |
| Non–TNF inhibitor biologic agent | 4 (2) | NA | |
| Oral glucocorticoids | 113 (36) | NA |
Definition of abbreviations: DMARDs = disease-modifying antirheumatic drugs; NA = not available; TNF = tumor necrosis factor.
Percentage of patients who ever used medication estimated as cumulative incidence 30 years after sarcoidosis diagnosis.
Among cases, intrathoracic involvement was present in 97% of cases. In 87% of cases this consisted of intrathoracic lymphadenopathy, and 50% had evidence of pulmonary parenchymal infiltration, but only 43% had respiratory symptoms. The most common extrathoracic manifestations were skin rash (18%), arthralgia (14%), as well as ophthalmologic (7%) and hepatic (6%) involvement. Isolated extrapulmonary sarcoidosis was diagnosed in 10 patients (3%).
A total of 229 cases had positive biopsy results demonstrating evidence of noncaseating granuloma (91% of 251 biopsied). The majority of patients had stage 1 disease (54%) followed by stage 2 (29%), stage 3 (15%), and stage 4 (2%). Among the total of 335 cases of pulmonary sarcoidosis, 311 patients had at least one baseline PFT in the period from 2 years before sarcoidosis diagnosis to 1 year after diagnosis. In this cohort, the means of PFT measures at baseline as a percentage of predicted values included TLC, 97.1%; FVC, 95.3%; FEV1, 91.4%; and DlCO, 91.1%. The means of these four measures among patients with non–stage 1 disease (i.e., stages 2, 3, and 4) were TLC, 93.6%; FVC, 90.9%; FEV1, 86.6%; and DlCO, 86.3%.
Less than one-half of all patients with sarcoidosis required immunosuppressive therapy (i.e., DMARDs, biologic agents, and/or systemic glucocorticoids) at some point during follow-up (151 cases, 37% by 30 yr after sarcoidosis diagnosis). The most commonly used medication was oral glucocorticoids (113 cases) followed by hydroxychloroquine (13 cases), methotrexate (11 cases), other DMARDs (5 cases), tumor necrosis factor inhibitor (5 cases), and other biologic agents (4 cases).
Risk of Infection
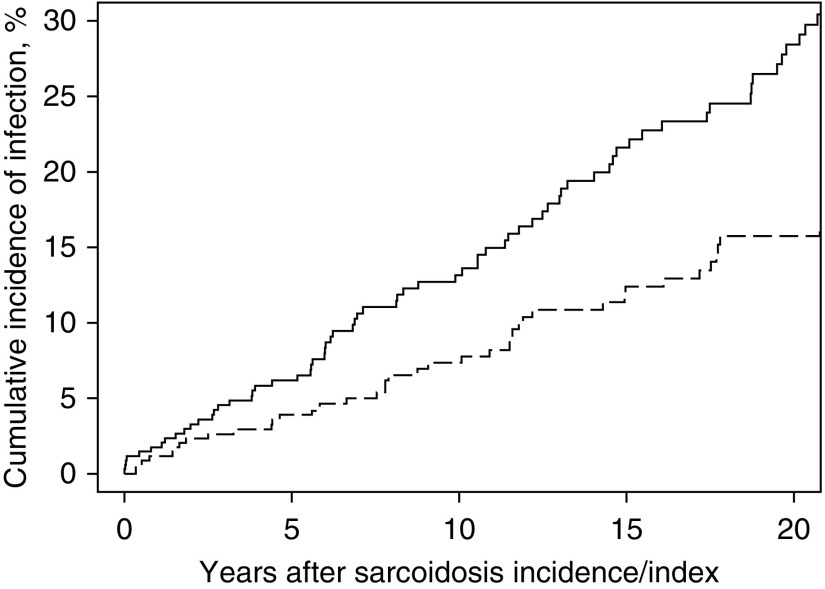
Patients with sarcoidosis had a higher risk of first hospitalized infection with a hazard ratio (HR) of 2.00 (95% confidence interval [CI], 1.41–2.84) than comparators, adjusted for age, sex, and calendar year of index date. Further adjustment for current smoking, diabetes mellitus, hypertension, dyslipidemia, and obesity yielded a similar result with an HR of 2.13 (95% CI, 1.35–3.34). Increased risk was observed for all specific types of infection, although power was insufficient to demonstrate statistical significance, except for pneumonia, soft tissue infection, and gastrointestinal infection. The number of hospitalized infections, cumulative incidence at 10 years, and HR of overall and specific types of infection are demonstrated in Table 2. Figure 1 describes the cumulative incidence of hospitalized infection after diagnosis/index date for cases and comparators.
Table 2.
Numbers of patients with a hospitalized infection, cumulative incidence at 10 years, and hazard ratio for overall and specific types of infection, comparing patients with sarcoidosis with subjects without sarcoidosis
| Subtype of Infection | Number of Patients with an Event after Index Date for Case/Comparator | Cumulative Incidence at 10 yr for Cases (95% CI) | Cumulative Incidence at 10 yr for Comparators (95% CI) | HR (95% CI) Adjusted for Age, Sex, and Calendar Year of Sarcoidosis Diagnosis |
|---|---|---|---|---|
| Sepsis | 17/11 | 2.0 (0.4–3.6) | 1.5 (0.2–2.7) | 1.66 (0.78–3.56) |
| Septic arthritis | 3/1 | NA | NA | 4.43 (0.42–46.94) |
| Osteomyelitis | 3/1 | NA | NA | 3.31 (0.34–31.95) |
| Pneumonia | 37/20 | 6.8 (3.8–9.8) | 2.0 (0.4–3.5) | 1.99 (1.15–3.43) |
| Pyelonephritis | 19/12 | 3.4 (1.2–5.6) | 1.4 (0.0–2.8) | 1.62 (0.78–3.34) |
| Soft tissue infection | 18/8 | 1.3 (0.0–2.6) | 1.4 (0.0–2.8) | 2.39 (1.04–5.51) |
| Gastrointestinal infection | 27/11 | 2.9 (0.9–4.9) | 1.5 (0.0–2.9) | 2.66 (1.32–5.38) |
| Intraabdominal infection | 3/1 | NA | NA | 3.23 (0.33–31.14) |
| Other infection | 5/1 | 0.9 (0.0–1.9) | 0.3 (0.0–0.9) | 5.96 (0.69–51.52) |
| Any infection | 89/49 | 13.2 (9.1–17.0) | 7.4 (4.3–10.3) | 2.00 (1.41-2.84) |
Definition of abbreviations: CI = confidence interval; HR = hazard ratio; NA = not available.
Figure 1.
Cumulative incidence of hospitalized infection among patients with sarcoidosis (solid line) and comparators without sarcoidosis (dashed line).
Compared with subjects without sarcoidosis, the increased risk of hospitalized infection was seen in all stages with an HR of 1.70 (95% CI, 1.12–2.58; P = 0.013) in patients with stage 1 sarcoidosis, an HR of 2.00 (95% CI, 1.22–3.29; P = 0.006) among those with stage 2 disease, and an HR of 2.63 (95% CI, 1.58–4.39; P < 0.001) among those with stage 3–4 disease. Patients with sarcoidosis who did not receive immunosuppressive treatment (or prior to the start of immunosuppressive treatment) had a significantly elevated risk of hospitalized infection (HR, 1.73; 95% CI, 1.16–2.60; P = 0.008) compared with those without sarcoidosis, with even higher risk among those who received immunosuppressive treatment (HR, 2.41; 95% CI, 1.60–3.64; P < 0.001) compared with nonsarcoidosis subjects.
Predictors of Infection in Sarcoidosis
Analyses were undertaken to examine the association between the baseline characteristics, use of immunosuppressive agents, and hospitalized infection among patients with sarcoidosis, as shown in Table 3. Baseline DlCO was associated with increased risk of overall hospitalized infection, with an HR of 1.15 per decrease of 10% predicted in DlCO (95% CI, 1.01–1.32). Baseline FVC was associated with increased risk of hospitalized pneumonia, with an HR of 1.15 per decrease of 10% predicted in FVC (95% CI, 1.01–1.32). The presence of extrathoracic disease and positive biopsy result were not associated with increased risks for hospitalized infection.
Table 3.
Risk factors for hospitalized infection among patients with sarcoidosis
| Risk Factor | Any Infection |
Pneumonia |
|---|---|---|
| [Hazard Ratio* (95% CI)] | [Hazard Ratio* (95% CI)] | |
| Extrathoracic disease | 1.14 (0.74–1.75) | 1.73 (0.90–3.33) |
| DlCO† | 1.15 (1.01–1.32) | 1.10 (0.90–1.35) |
| FVC† | 1.08 (0.95–1.23) | 1.26 (1.05–1.51) |
| Noncaseating granuloma‡ | 0.92 (0.42–2.04) | 0.89 (0.26–2.99) |
| Any immunosuppressive treatment§ | 1.43 (0.94–2.19) | 1.64 (0.85–3.17) |
Definition of abbreviations: CI = confidence interval; DlCO = diffusing capacity of the lung for carbon monoxide.
Adjusted for age, sex, and calendar year of sarcoidosis diagnosis.
Among patients with pulmonary sarcoidosis with pulmonary function testing within 2 yr prior to 1 yr after sarcoidosis diagnosis (283, DlCO; and 297, FVC); hazard ratio reported per 10-unit decrease in percent predicted.
Among 251 patients who were biopsied.
Disease-modifying antirheumatic agents, biologic agents, and/or systemic glucocorticoids.
Among patients with sarcoidosis, exposure to immunosuppressive agents (i.e., DMARDs, biologic agents, and/or systemic glucocorticoids) was not significantly associated with the risk of a hospitalized infection (HR, 1.43; 95% CI, 0.94–2.19). However, current use of oral glucocorticoids (with or without other immunosuppressants) was a significant predictor of hospitalized infection, with an HR of 3.03 (95% CI, 1.33–6.90) for oral glucocorticoids not exceeding 10 mg/day prednisone equivalent, and an HR of 4.48 (95% CI, 1.54–13.03) for oral glucocorticoids greater than 10 mg/day prednisone equivalent compared with patients who were not using glucocorticoids.
Discussion
Increased incidence of infection has been observed in several immune-mediated disorders, and infection remains one of the most common causes of morbidity and mortality among patients with these diseases. The current study is the first to examine the risk of serious infection requiring hospitalization, using a population-based cohort. The risk of hospitalized infection among patients with sarcoidosis was increased twofold compared with sex- and age-matched comparators. This excess risk is observed in varying degrees for all infections examined. Because of their significant implications for patient outcomes and health care use, only hospitalized infection, rather than any infection, was used as the study end point.
There are a number of possible explanations for the increased risk of hospitalized infection among patients with sarcoidosis. First, the increased risk could be a consequence of immunosuppression resulting from exposure to immunosuppressive agents used to treat sarcoidosis. Increased susceptibility to infection is a well-recognized complication of therapy with immunosuppressive agents, particularly glucocorticoids (12).
In this study, glucocorticoid use was a significant predictor, in a dose-dependent fashion, of hospitalized infection among patients with sarcoidosis. Glucocorticoids have numerous effects on the immune system. They act primarily through the cytoplasmic glucocorticoid receptor. On ligand binding, the ligand-bound glucocorticoid receptor will translocate to the nucleus to serve as a transcription factor and facilitate antiinflammatory gene expression. In addition, the cytoplasmic glucocorticoid receptor also suppresses the expression of proinflammatory genes by a tethering mechanism with cointegrators such as TRIP6 and STAMP (13, 14). This gene expression modulation has several effects on immune cells, resulting in a decrease in proliferation and migration of lymphocytes, impaired adhesion of neutrophils, reduction of inflammatory cytokine production by monocytes, and decreased antigen-presenting activity of dendritic cells (1, 13). A smaller magnitude of increased infection risk was also observed with overall exposure to nonsteroid immunosuppressive agents (i.e., DMARDs and biologic agents) albeit without statistical significance. It is possible that this study was underpowered to detect smaller magnitudes of risk or that considering patients as exposure from the time of initiation to their last follow-up, even after discontinuation of immunosuppression, could have diluted the effect.
Nonetheless, exposure to immunosuppressive agents is not the only mechanism behind the increased risk, as patients with sarcoidosis who did not receive immunosuppressive therapy also demonstrated a significantly higher risk of hospitalized infection than nonsarcoidosis comparators. It is certainly possible that the association between use of glucocorticoids and hospitalized infection is not causative as this treatment is usually reserved for those who do not have spontaneous resolution or those with more extensive disease, so use of glucocorticoids could simply be a marker of more severe disease.
The second possible explanation is related to immune dysregulation found in sarcoidosis. Although cellular immunity is up-regulated in affected tissues, the peripheral immune response appears to be suppressed in sarcoidosis. Peripheral T-cell lymphopenia, especially CD4+ T cells, resulting in a decreased CD4+/CD8+ T-cell ratio, has been demonstrated in patients with sarcoidosis, particularly those with more extensive disease (15).
Clinical anergy to various skin test antigens in patients with sarcoidosis, such as tuberculin and extracts of Trichophyton and Candida, has long been recognized (16). In vitro studies have also demonstrated reduced peripheral lymphocyte response to mitogen and recall antigen (17, 18). A more recent study has suggested that this anergic state is possibly modulated by diminished dendritic cell function (19). Thus, the ability of the immune system of patients with sarcoidosis to respond to infection may be compromised.
The data from this cohort demonstrate that patients with more severe sarcoidosis tend to develop hospitalized infection more often than those with less severe disease. For example, decreased PFT at baseline was predictive of hospitalized infection, and the HR of hospitalized infection was higher among those with a higher stage of pulmonary sarcoidosis. Hence, more severe disease with diminished pulmonary reserve and significant immune dysregulation contribute to the higher risk of infection.
Last, local factors may also predispose patients to specific organ infections. For example, skin lesions from sarcoidosis may predispose the patients to skin infection.
Strengths and Limitations
The major strength of this study is the study design that uses a population-based cohort to investigate the risk of hospitalized infection. The comprehensive database allows capture of nearly all the clinically recognized cases of sarcoidosis in the population. Thus, this cohort represents the true spectrum of sarcoidosis in the community without referral bias. The diagnosis of sarcoidosis and infection is also well validated as all medical records were individually reviewed. Therefore, misclassification of the disease and events of interest, a common concern for coding-based studies, is minimized. The long duration of follow-up of more than 15 years for both cases and comparators is an additional strength of the study.
Limitations of the study are inherent in the retrospective design. The clinical information was extracted from existing medical records that were not systematically obtained and recorded. The REP database contains only the hospitalization data from the hospitals in Olmsted County, Minnesota (Mayo Clinic and Olmsted Medical Center) and mentions or records of hospitalization of county residents, which may have occurred elsewhere during travel. Therefore, hospitalized infections outside the county were not included in the analysis. Nonetheless, event rates would generally not be expected to differ between cases and comparators (9).
Generalizability of the findings to other populations is another limitation as clinical manifestations and severity of sarcoidosis vary among patients with different geographic and ethnic backgrounds (7, 20). The majority of the Olmsted County population, as well as the majority of patients with sarcoidosis in this cohort, are of Northern European descent. Olmsted County also has a higher-than-average proportion of health care workers, which could affect the pattern of health care use and hospitalization. Moreover, with the relative scarcity of data from clinical trials, treatment of sarcoidosis is less standardized. Therefore, the risk of infection may only reflect the pattern of the use of immunosuppressive agents by local health care providers for this population. Examination of the relationship between infection risk due to sarcoidosis and its extent, and infection risk from immunosuppressive therapy, is affected by these patterns of therapeutic interventions and confounding by indication, and could not be adequately assessed.
Conclusions
An increased risk of hospitalized infection was observed in this cohort of patients with incident sarcoidosis. The risk was increased by approximately twofold compared with sex- and age-matched comparators. Use of glucocorticoids was a significant predictor for hospitalized infection among patients with sarcoidosis.
Supplementary Material
Footnotes
Supported by resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676, and CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions: P.U.: Conception and design, acquisition and interpretation of data, drafting of the manuscript, and statistical analysis; C.S.C.: Conception and design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, and statistical analysis; E.L.M.: Conception and design, acquisition and interpretation of data, critical revision of the manuscript for important intellectual content, statistical analysis, and supervision.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Schmidt J, Smail A, Roche B, Gay P, Salle V, Pellet H, Duhaut P. Incidence of severe infections and infection-related mortality during the course of giant cell arteritis: a multicenter, prospective, double-cohort study. Arthritis Rheumatol. 2016;68:1477–1482. doi: 10.1002/art.39596. [DOI] [PubMed] [Google Scholar]
- 2.Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2281–2293. doi: 10.1002/art.10524. [DOI] [PubMed] [Google Scholar]
- 3.Herrinton LJ, Liu L, Goldfien R, Michaels MA, Tran TN. Risk of serious infection for patients with systemic lupus erythematosus starting glucocorticoids with or without antimalarials. J Rheumatol. 2016;43:1503–1509. doi: 10.3899/jrheum.150671. [DOI] [PubMed] [Google Scholar]
- 4.Marie I, Ménard JF, Hachulla E, Chérin P, Benveniste O, Tiev K, Hatron PY. Infectious complications in polymyositis and dermatomyositis: a series of 279 patients. Semin Arthritis Rheum. 2011;41:48–60. doi: 10.1016/j.semarthrit.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Migita K, Sasaki Y, Ishizuka N, Arai T, Kiyokawa T, Suematsu E, Yoshimura M, Kawabe Y, Matsumura R, Akagawa S, et al. Glucocorticoid therapy and the risk of infection in patients with newly diagnosed autoimmune disease. Medicine (Baltimore) 2013;92:285–293. doi: 10.1097/MD.0b013e3182a72299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swigris JJ, Olson AL, Huie TJ, Fernandez-Perez ER, Solomon J, Sprunger D, Brown KK. Sarcoidosis-related mortality in the United States from 1988 to 2007. Am J Respir Crit Care Med. 2011;183:1524–1530. doi: 10.1164/rccm.201010-1679OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mirsaeidi M, Machado RF, Schraufnagel D, Sweiss NJ, Baughman RP. Racial difference in sarcoidosis mortality in the United States. Chest. 2015;147:438–449. doi: 10.1378/chest.14-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ungprasert P, Carmona EM, Utz JP, Ryu JH, Crowson CS, Matteson EL. Epidemiology of sarcoidosis 1946–2013: a population-based study. Mayo Clin Proc. 2016;91:183–188. doi: 10.1016/j.mayocp.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., III History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costabel U, Hunninghake GW Sarcoidosis Statement Committee; American Thoracic Society; European Respiratory Society; World Association for Sarcoidosis and Other Granulomatous Disorders. ATS/ERS/WASOG statement on sarcoidosis. Eur Respir J. 1999;14:735–737. doi: 10.1034/j.1399-3003.1999.14d02.x. [DOI] [PubMed] [Google Scholar]
- 11.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 12.Migita K, Arai T, Ishizuka N, Jiuchi Y, Sasaki Y, Izumi Y, Kiyokawa T, Suematsu E, Miyamura T, Tsutani H, et al. Rates of serious intracellular infections in autoimmune disease patients receiving initial glucocorticoid therapy. PLoS One. 2013;8:e78699. doi: 10.1371/journal.pone.0078699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baschant U, Tuckermann J. The role of the glucocorticoid receptor in inflammation and immunity. J Steroid Biochem Mol Biol. 2010;120:69–75. doi: 10.1016/j.jsbmb.2010.03.058. [DOI] [PubMed] [Google Scholar]
- 14.De Bosscher K, Haegeman G. Minireview: latest perspectives on antiinflammatory actions of glucocorticoids. Mol Endocrinol. 2009;23:281–291. doi: 10.1210/me.2008-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sweiss NJ, Salloum R, Gandhi S, Alegre ML, Sawaqed R, Badaracco M, Pursell K, Pitrak D, Baughman RP, Moller DR, et al. Significant CD4, CD8, and CD19 lymphopenia in peripheral blood of sarcoidosis patients correlates with severe disease manifestations. PLoS One. 2010;5:e9088. doi: 10.1371/journal.pone.0009088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friou GJ. A study of the cutaneous reactions to oidiomycin, trichophytin, and mumps skin test antigens in patients with sarcoidosis. Yale J Biol Med. 1952;24:533–539. [PMC free article] [PubMed] [Google Scholar]
- 17.Bertrán G, Arzt E, Resnik E, Mosca C, Nahmod V. Inhibition of interferon γ production by peripheral blood mononuclear leukocytes of patients with sarcoidosis: pathogenic implications. Chest. 1992;101:996–999. doi: 10.1378/chest.101.4.996. [DOI] [PubMed] [Google Scholar]
- 18.Goodwin JS, DeHoratius R, Israel H, Peake GT, Messner RP. Suppressor cell function in sarcoidosis. Ann Intern Med. 1979;90:169–173. doi: 10.7326/0003-4819-90-2-169. [DOI] [PubMed] [Google Scholar]
- 19.Mathew S, Bauer KL, Fischoeder A, Bhardwaj N, Oliver SJ. The anergic state in sarcoidosis is associated with diminished dendritic cell function. J Immunol. 2008;181:746–755. doi: 10.4049/jimmunol.181.1.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birnbaum AD, French DD, Mirsaeidi M, Wehrli S. Sarcoidosis in the national veteran population: association of ocular inflammation and mortality. Ophthalmology. 2015;122:934–938. doi: 10.1016/j.ophtha.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.