Abstract
Rationale: Spontaneous pneumothorax is a common complication of Birt–Hogg–Dubé syndrome (BHD).
Objectives: The optimal approach to treatment and prevention of BHD-associated spontaneous pneumothorax, and to advising patients with BHD regarding risk of pneumothorax associated with air travel, is not well established.
Methods: Patients with BHD were recruited from the Rare Lung Diseases Clinic Network and the BHD Foundation and surveyed about disease manifestations and air travel experiences.
Results: A total of 104 patients completed the survey. The average age at diagnosis was 47 years, with an average delay from first symptoms of 13 years. Pulmonary cysts were the most frequent phenotypic manifestation of BHD, present in 85% of patients. Spontaneous pneumothorax was the presenting manifestation that led to the diagnosis of BHD in 65% of patients, typically after the second episode (mean, 2.4 episodes). Seventy-nine (76%) of 104 patients had at least one spontaneous pneumothorax during their lifetime, and 82% had multiple pneumothoraces. Among patients with multiple pneumothoraces, 73% had an ipsilateral recurrence, and 48% had a subsequent contralateral spontaneous pneumothorax following a sentinel event. The mean ages at first and second pneumothoraces were 36.5 years (range, 14–63 yr) and 37 years (range, 20–55 yr), respectively. The average number of spontaneous pneumothoraces experienced by patients with a sentinel pneumothorax was 3.6. Pleurodesis was generally performed after the second (mean, 2.4) ipsilateral pneumothorax and reduced the ipsilateral recurrence rate by half. A total of 11 episodes of spontaneous pneumothorax occurred among eight patients either during or within the 24-hour period following air travel, consistent with an air travel–related pneumothorax rate of 8% per patient and 0.12% per flight. Prior pleurodesis reduced the occurrence of a subsequent flight-related pneumothorax.
Conclusions: Spontaneous pneumothorax is an important, recurrent manifestation of pulmonary involvement in patients with BHD, and pleurodesis should be considered following the initial pneumothorax to reduce the risk of recurrent episodes. In general, in patients with BHD, pneumothorax occurs in about 1–2 per 1,000 flights, and the risk is lower among patients with a history of prior pleurodesis.
Keywords: pneumothorax, pleurodesis, air travel, Birt–Hogg–Dubé syndrome
Birt–Hogg–Dubé syndrome (BHD) is an autosomal dominant condition caused by mutations in the folliculin gene (FLCN), a tumor suppressor gene. The characteristic clinical manifestations of BHD include formation of hair follicle tumors (fibrofolliculomas), kidney tumors, and pulmonary cysts (1, 2). Pulmonary cysts are seen in 80 to 100% of patients with BHD (3, 4) and are associated with a risk of spontaneous pneumothorax that is 32-fold higher than in the general population (5). Current literature suggests that spontaneous pneumothorax occurs in 24 to 38% of patients with BHD (3, 6, 7), with a reported recurrence rate of up to 75% (3). Mutations in FLCN are the most common etiology of familial pneumothorax (8). To reduce morbidity, preventive strategies such as pleurodesis have been recommended following the initial spontaneous pneumothorax in patients with BHD (1, 2). However, neither the optimal approach to management of pneumothoraces in BHD nor the efficacy of pleurodesis in preventing recurrent episodes is well established.
Atmospheric pressure changes during the ascent and descent phases of air travel result in expansion and compression of cystic air spaces in the thorax that can lead to cyst rupture and development of pneumothorax. The aim of our study was to evaluate the risk of pneumothorax, the efficacy of pleurodesis, and the safety of air travel in patients with BHD.
Methods
Patient Recruitment
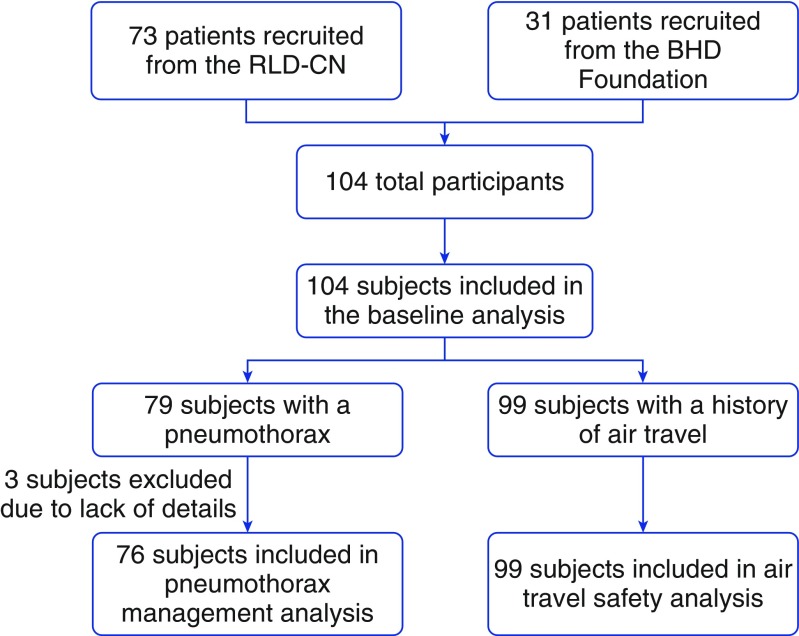
Patients with BHD were recruited from the Rare Lung Diseases Clinic Network, a geographically distributed consortium of clinics that was initially organized by the Lymphangioleiomyomatosis Foundation and adopted for the study of other rare lung diseases by the Rare Lung Diseases Consortium (RLDC). A link to the survey was also posted on the website of the BHD Foundation patient advocacy organization (Figure 1). The study was conducted through the National Institutes of Health (NIH)-supported RLDC. The study protocol was reviewed and approved by the NIH and the University of Cincinnati Institutional Review Board (IRB number 2015-6129).
Figure 1.
Flow diagram depicting the subject recruitment and selection for our study. BHD = Birt–Hogg–Dubé syndrome; RLD-CN = Rare Lung Diseases Clinic Network.
Survey Design
The survey consisted of 50 questions that addressed patient demographics, clinical manifestations of BHD, management of pneumothoraces, and details about air travel. Patients signed a written informed consent form prior to taking the survey and had the option of revealing their identity. Prior to distribution of the survey, test questionnaires were distributed to two patients with BHD and multiple lay personnel to enhance clarity, reduce ambiguity, and refine the questions and instructions.
Because retrospective distinction between persistent and recurrent pneumothorax can be problematic, all ipsilateral pneumothoraces reported within the same month were considered as one episode. If a given pneumothorax was treated with more than one intervention (e.g., chest tube followed by chemical pleurodesis followed by surgical pleurodesis), the most aggressive intervention (e.g., in this case, surgical pleurodesis) was listed as the treatment of record for that event.
For the purpose of our study, a pneumothorax that happened either during an air flight or within 24 hours after a flight was defined as flight-related pneumothorax. Authors of previous reports have commented on the likelihood of symptom delay and a late presentation of spontaneous pneumothorax following air travel (9). A recent survey of patients with BHD conducted in the Netherlands defined any pneumothorax that happened within 1 month of air travel as flight-related pneumothorax (10). We chose to limit our definition to 24 hours to minimize the confusion between a true flight-related pneumothorax and an unrelated spontaneous pneumothorax that happened to occur a few days after air travel.
Data Collection and Analysis
Study data were collected and managed using Research Electronic Data Capture (REDCap) tools hosted at the University of Cincinnati (11). REDCap is a secure, web-based application designed to support data capture for research studies and provides audit trails for tracking data manipulation, as well as automated export procedures to help with data analysis. De-identified data were stored in a password-protected format at the Data Management and Coordinating Center at the University of South Florida as part of the NIH-supported RLDC. Statistical significance was set at P < 0.05 and determined by the chi-square test and the z-statistic, as appropriate. Data analysis was performed using SAS for Windows version 9.4 software (SAS Institute, Cary, NC).
Results
Baseline Characteristics
A total of 104 patients completed the survey. The majority of the respondents (86 [83%] of 104) were female. Ninety-nine (94%) of the 104 respondents were white, and the remainder were Asian. The median age of patients at the time of completing this survey was 50 years (interquartile range, 39–61 yr). Some patients skipped certain questions of the survey, and thus the denominator for some calculations is different from the total sample size. We have specified the denominator for each calculation to provide clarity. Spontaneous pneumothorax was the presenting manifestation of BHD in 65% of the patients (63 of 97), followed by skin lesions (37%), pulmonary cysts (23%), and renal tumors (9%). In 79% of the patients (82 of 104), the diagnosis of BHD was confirmed by genetic analysis revealing a pathogenic FLCN mutation, and in the remaining 21% of patients, the diagnosis was established on the basis of clinical context, which included a combination of pulmonary cysts and skin biopsy showing the characteristic hair follicle tumors of BHD, fibrofolliculomas, or trichodiscomas. On average, the diagnosis of BHD was delayed by 13 years (range, 0–48 yr) from the time of symptom onset (Table 1).
Table 1.
Baseline characteristics and patient demographics
| Variables | Data |
|---|---|
| Total respondents | 104 |
| Males/females | 18/86 |
| Average age at time of survey | 50 yr |
| Average age at diagnosis of BHD | 47 yr (range, 5–79 yr) |
| Average delay between symptom onset and diagnosis | 13 yr (range, 0–48 yr) |
| Most common disease manifestation of BHD | Pulmonary cysts (85%) |
| Prevalence of skin lesions | 71% |
| Prevalence of kidney tumors | 33% |
Definition of abbreviation: BHD = Birt–Hogg–Dubé syndrome.
Disease Manifestations
Pulmonary
Pulmonary cysts were the most common disease manifestation, being present in 85% of the patients (88 of 104). Symptomatic dyspnea was reported by 50% of the patients (52 of 104). The majority (90%) of the patients characterized their dyspnea as mild in nature (Medical Research Council dyspnea scale grade 1 or 2). Only 4 (4%) of 104 patients reported needing supplemental oxygen. Two of these four patients used supplemental oxygen only while sleeping, and the remaining two patients required supplemental oxygen 24 hours per day. Other pulmonary symptoms included episodic chest pain (66%) and cough (47%). Twenty-nine percent of patients (30 of 104) carried a concomitant diagnosis of asthma, and 20% routinely used bronchodilators. Sixty percent of the patients in this study (62 of 104) were lifetime nonsmokers, 28% (29 of 104) were former smokers, and 12% (12 of 104) were current smokers. Among the patients with a smoking history (active and former), the median tobacco use was 7.5 pack-years (interquartile range, 2.5–20 pack-years).
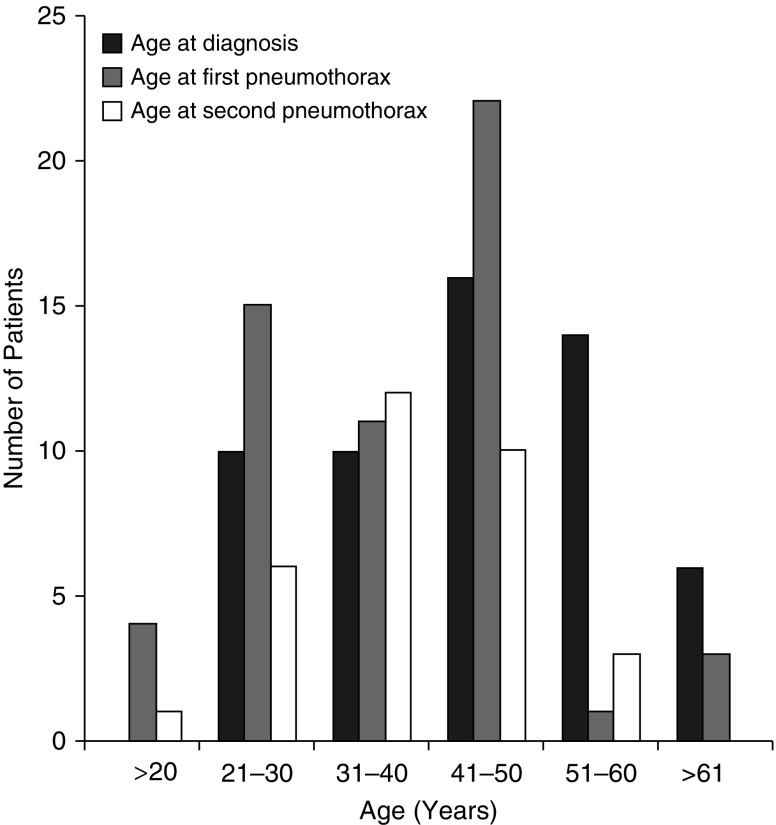
Seventy-six percent of patients in this study (79 of 104) had experienced at least one pneumothorax during their lifetime. The average age at first spontaneous pneumothorax was 36.5 years (range, 14–63 yr), and the average age at second spontaneous spontaneous pneumothorax was 37 years (range, 20–55 yr). Spontaneous pneumothorax was the presenting manifestation that led to the diagnosis of BHD in 65% of patients, after a mean of about two episodes (mean, 2.4; range, 0–7) (Table 2). Figure 2 demonstrates the relationship between age at diagnosis and age at first and second spontaneous pneumothoraces in our cohort.
Table 2.
Pneumothorax rates among patients with Birt–Hogg–Dubé syndrome
| Variables | Data |
|---|---|
| Total number of pneumothoraces (confirmed by chest imaging) | 285 |
| Number of patients with at least one SP | 79 (76%) |
| Number of patients who provided details about their pneumothoraces | 76 |
| Number of patients with multiple (>1) pneumothoraces | 62 (82% of patients with a sentinel SP) |
| Among patients with more than one pneumothorax, number of patients with a recurrent (ipsilateral) SP | 45 (73%) |
| Among patients with more than one pneumothorax, number of patients with a contralateral SP | 30 (48%) |
| Number of patients with a simultaneous bilateral SP | 4 (5%) |
| Patients with pneumothorax as presenting symptom of BHD | 63 (65%) |
| Average age at development of first SP | 36.5 yr (range, 14–63) |
| Average number of pneumothoraces per patient | 3.6 (range, 1 to >8) |
| Average number of pneumothoraces before diagnosis of BHD | 2.4 (range, 0–7) |
| Average number of pneumothoraces prior to undergoing pleurodesis (chemical or surgical) | 2.4 (range, 1–7) |
| Ipsilateral recurrence rate following chemical pleurodesis | 10 (30%) of 33 |
| Ipsilateral recurrence rate following surgical pleurodesis | 19 (35%) of 54 |
Definition of abbreviations: BHD = Birt–Hogg–Dubé syndrome; SP = spontaneous pneumothorax.
Figure 2.
Histogram depicting age at first and second pneumothorax and age at diagnosis of patients with Birt–Hogg–Dubé syndrome. Most patients developed a first and second pneumothorax between the ages of 21 and 50 years, usually before the diagnosis of Birt–Hogg–Dubé syndrome was established.
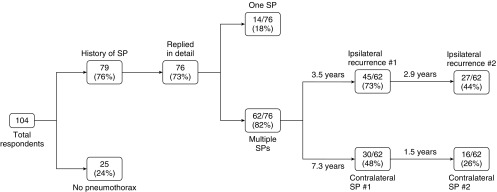
The majority of patients (62 [82%] of 76) with a sentinel pneumothorax experienced at least one additional episode of spontaneous pneumothorax, either an ipsilateral recurrence (45 [73%] of 62) or a contralateral spontaneous pneumothorax (30 [48%] of 62). Among patients with spontaneous pneumothoraces, four patients (5%) reported a total of five episodes of simultaneous bilateral spontaneous pneumothorax. Bilateral spontaneous pneumothorax occurred once in three patients and twice in one patient.
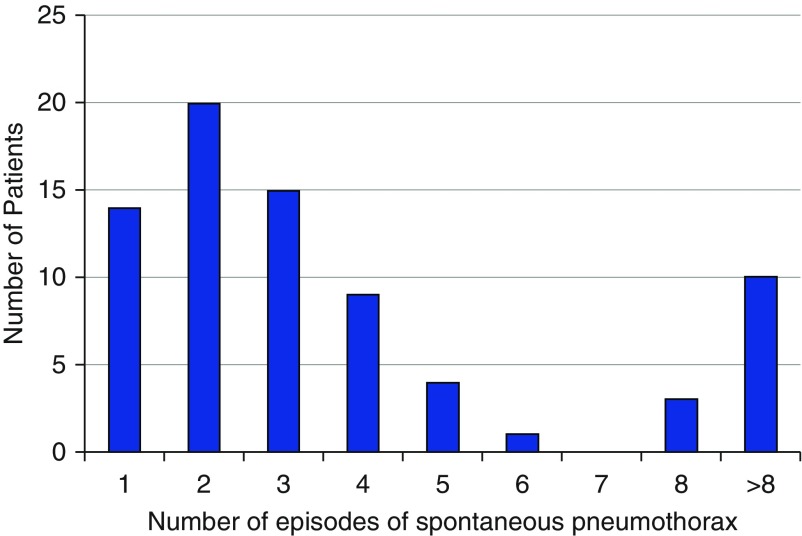
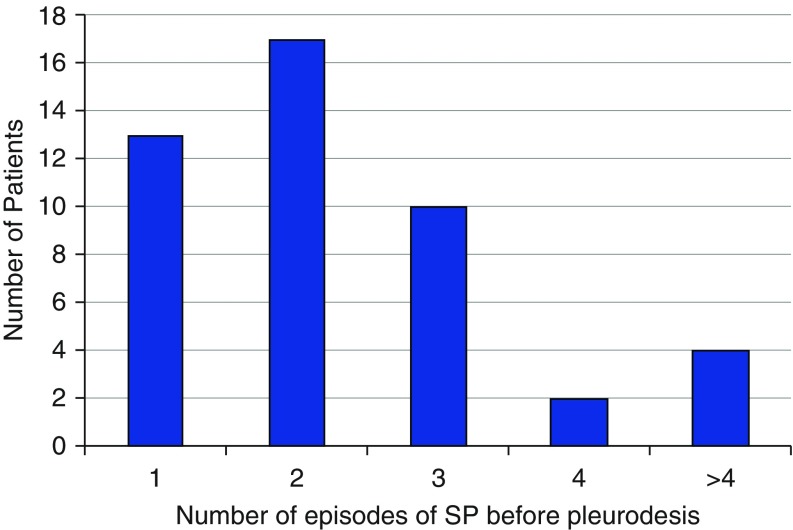
The number of recurrent episodes varied significantly among patients (Figure 3), with an average of 3.6 subsequent episodes per patient following a sentinel spontaneous pneumothorax. Figure 4 demonstrates the breakdown of recurrent pneumothoraces in our cohort.
Figure 3.
Number of spontaneous pneumothoraces per patient.
Figure 4.
Incidence and recurrence of ipsilateral and contralateral pneumothorax in patients with Birt–Hogg–Dubé syndrome. SP = spontaneous pneumothorax.
Spontaneous pneumothorax was diagnosed by chest radiography in the majority of the patients, with computed tomography (CT) used as a diagnostic modality for the first episode in only eight patients (10%). Among patients who underwent chest CT for diagnosis of spontaneous pneumothorax, CT was employed after a mean of 2.75 episodes of spontaneous pneumothorax (range, 1–8). Smoking status had no impact on the pneumothorax rate in our study.
Sixty-two percent (47 of 76) of patients in our cohort had undergone at least one pleurodesis to prevent recurrent pneumothorax. Among patients with a history of pleurodesis, 62% had undergone unilateral pleurodesis, and 38% had undergone bilateral pleurodesis. Pleurodesis (chemical or surgical) was performed in 28% of the patients following their first episode of spontaneous pneumothorax, 37% of patients following a second episode, and 21% of patients after a third episode (Figure 5).
Figure 5.
Number of episodes of spontaneous pneumothoraces experienced by patients prior to ipsilateral pleurodesis. SP = spontaneous pneumothorax.
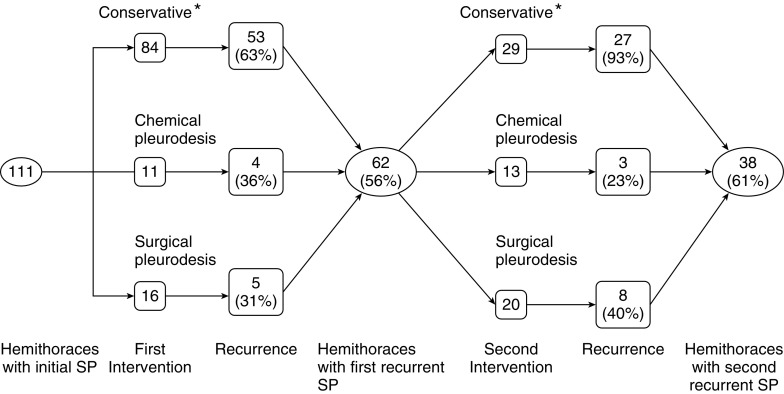
Pleurodesis was only partially effective at reducing the frequency of recurrent episodes of spontaneous pneumothorax (P = 0.02) (Figure 6). Managed conservatively (without pleurodesis), the ipsilateral recurrence rate of spontaneous pneumothorax was 63%. Following pleurodesis, 33% of patients (15 of 46) had an ipsilateral recurrence. A similar conclusion regarding the efficacy of pleurodesis was also reached by another group in a smaller study and reported in abstract form (12). There was no significant difference in the efficacy of chemical (30%) versus surgical pleurodesis (35%) in reducing pneumothorax recurrence rates (Table 2).
Figure 6.
Effectiveness of treatment of pneumothorax in patients with Birt–Hogg–Dubé syndrome. Each hemithorax was considered separately. The highest recurrence rates of pneumothorax occurred with conservative therapy, but surgical and chemical pleurodesis procedures also frequently failed. After the first recurrence episode, almost all patients experienced another pneumothorax if managed conservatively. *Includes observation, simple aspiration, and tube thoracostomy without pleurodesis. SP = spontaneous pneumothorax.
Air travel
Ninety-six percent of the patients (99 of 104) in our cohort had flown at least once in their lifetime. The number of flights per patient varied from 1 to 2,000, with a median of 25 (interquartile range, 10–51). The number of lifetime flights taken by all members of our cohort was approximately 8,920, but excluding the four highly frequent travelers with more than 1,000 flights each, the total number of flights in the remaining patients was 3,720. There was no difference in the average number of flights taken by patients with a history of spontaneous pneumothorax as compared with patients without a spontaneous pneumothorax.
Patients frequently experienced constitutional symptoms during air travel, including, in decreasing order of frequency, chest pressure (52%), anxiety (50%), headache (31%), shortness of breath (28%), chest pain (28%), nausea (20%), fatigue (7%), oxygen desaturation by handheld pulse oximetry (4%), and peripheral cyanosis (2%). The proportion of patients who experienced chest pain and/or dyspnea during air travel was similar among patients with a history of spontaneous pneumothorax and patients without a spontaneous pneumothorax.
A total of 11 episodes of spontaneous pneumothorax occurred among eight patients either during or within 24 hours of air travel (Table 3), consistent with a flight-related pneumothorax risk of 8% per patient and 0.12% per flight (11 of 8,920). Excluding the four outliers with more than 1,000 flights, the pneumothorax risks were 7.4% per patient and 0.27% per flight. Three (27%) of these 11 episodes were the first episodes of spontaneous pneumothorax, and the remaining 8 episodes represented recurrent spontaneous pneumothoraces. Among these eight patients who had a recurrent flight-related spontaneous pneumothorax, the majority (7 [87.5%] of 8) had not undergone prior pleurodesis and one had experienced a recurrent flight-related spontaneous pneumothorax after having undergone pleurodesis. There was a statistically significant difference between these proportions (P = 0.017), suggesting that prior pleurodesis may reduce the rate of subsequent flight-related pneumothorax. Table 4 provides a detailed description of the flight-related pneumothoraces.
Table 3.
Flight-related pneumothorax rates among patients with Birt–Hogg–Dubé syndrome
| Variables | Data |
|---|---|
| Total number of flights taken | 8,920 |
| Number of pneumothoraces either during or within 24 h after a flight | 11 |
| Number of unique patients with a pneumothorax either during or within 24 h after a flight | 8 |
| Total number of patients who had ever flown | 99 |
| Air travel–related pneumothorax risk per patient | 8% |
| Air travel–related pneumothorax risk per flight | 0.12% |
Table 4.
Details regarding patients with a flight-related pneumothorax
| Pneumothorax Number | Sex | Age at Time of Flight-related SP | Prior SP, Yes/No (n) | Flight Phase at Symptom Onset | Mode of Confirmation | Management | In-Flight Treatment | Flight Duration | Symptoms 24–48 h Prior to Boarding Flight, Yes/No | Smoking History (Pack-Years) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 49 | N | After landing | CxR | Chest tube | None | >3 h | N | Former (10) |
| 2 | F | 51 | Y (2) | After landing | CT | Surgical pleurodesis | None | >3 h | N | Former (10) |
| 3 | F | 32 | Y (1) | After landing | CxR | Chest tube | None | 1–3 h | N | Former (20) |
| 4 | M | 25 | N | After landing | CxR | Chest tube | None | 1–3 h | N | Never |
| 5 | F | 33 | Y (1) | After landing | CxR | Observation | None | >3 h | N | Former (1) |
| 6 | M | NA | Y (4) | Descent | CxR | Chest tube | None | >3 h | N | Never |
| 7 | M | NA | Y (6) | After landing | CxR | Observation | None | 1–3 h | N | Never |
| 8 | F | 46 | N | After landing | CxR | Chest tube | None | >3 h | N | Former (20) |
| 9 | F | 51 | Y (2) | After landing | CT | Surgical pleurodesis | None | >3 h | N | Former (20) |
| 10 | F | 38 | Y (3) | After landing | CxR | Chemical pleurodesis | None | 1–3 h | Y | Never |
| 11 | M | 46 | Y (1) | Ascent | CxR | Surgical pleurodesis | Oxygen | >3 h | N | Never |
Definition of abbreviations: CT = computed tomography; CxR = chest radiography; SP = spontaneous pneumothorax.
Pneumothorax numbers 1 and 2, 6 and 7, and 8 and 9 belong to the same patients. Eight patients experienced 11 flight-related pneumothoraces.
Twenty-four percent (23 of 96) of patients changed their flight frequency after the diagnosis of BHD was established, with 70% avoiding air travel altogether and the remaining 30% significantly reducing their frequency of air travel. The reasons for avoiding air travel were varied and included personal assessment of risk of air travel (50%), physician’s advice (28%), reasons other than BHD (18%), and a history of spontaneous pneumothorax (4%). The recommendations that patients were given by caregivers regarding the safety of air travel after spontaneous pneumothorax were variable. More than half (56%) of the patients were given no specific recommendations regarding the safety of future air travel, 3% were told it is safe to fly 1 week following pneumothorax, 11% were told it is safe to fly 1 month after pneumothorax, 21% were told it is safe to fly more than 1 month after pneumothorax, and 9% of patients were advised against any future air travel.
Discussion
The results of our analysis demonstrate that spontaneous pneumothorax is an important phenotypic manifestation of pulmonary involvement in BHD and is characterized by frequent recurrences. In our cohort, greater than 75% of patients with BHD experienced at least one spontaneous pneumothorax, with approximately 80% chance of recurrence. Patients with a sentinel pneumothorax typically experienced three recurrent spontaneous pneumothoraces following their initial event. Pleurodesis reduced the recurrence rates by half compared with conservative management. Thus, we recommend that pleurodesis should be performed following the initial spontaneous pneumothorax, especially given that BHD is generally nonprogressive and does not typically result in the need for lung transplant (which can be more complicated in patients with prior pleurodesis).
Occurrence of an in-flight pneumothorax in the general population is a rare event, with no events reported in multiple large studies investigating the safety of air travel (13–15). However, patients with diffuse cystic lung diseases may be at higher risk of development of in-flight spontaneous pneumothorax owing to cyst expansion related to atmospheric pressure changes.
The risk of development of spontaneous pneumothorax related to air travel in patients with lymphangioleiomyomatosis (LAM) has been investigated in two different studies (16, 17). Both showed that it is quite safe for most women with LAM to travel by air, with a flight-related pneumothorax rate of 1 to 2% per flight. However, a significant proportion of patients with self-reported air travel–related pneumothorax had symptoms consistent with a spontaneous pneumothorax prior to boarding, suggesting that the actual rate of in-flight pneumothorax among patients with LAM is somewhat lower (16). These results have been used to counsel patients with BHD about the safety of air travel in the absence of disease-specific data (1, 2).
Our study indicates that patients with BHD have a risk of spontaneous pneumothorax during flight that is most likely less than 1%, which, although low, is still likely higher than in most other pulmonary diseases. We also found that patients with BHD with a history of pleurodesis are less likely to experience a flight-related pneumothorax than patients without a history of pleurodesis. Patients with BHD should be informed about the risks of pneumothorax and the benefits of pleurodesis, educated regarding the signs and symptoms of a spontaneous pneumothorax, and counseled to seek medical evaluation prior to boarding an airplane if they have new and unexplained chest pain or shortness of breath.
We also found that patients experienced a considerable amount of anxiety related to the risk of spontaneous pneumothorax during air travel, which may be partially rooted in insufficient counseling by caregivers. Approximately half of the patients in our study experienced chest pressure related to air travel, along with a variety of other nonspecific symptoms, and one-fourth changed their flight frequency after a diagnosis of BHD, with most deciding to abandon flying altogether. Anticipatory counseling regarding the general safety of air travel for patients with BHD may help them with symptom management and improve their quality of life.
Another question that is important to patients is when they can return to flying after pneumothorax. Evidenced-based recommendations are not available, but most sources cite air travel restrictions of between 1 and 4 weeks after resolution of pneumothorax (13, 18). The advice that patients in our study received reflects the lack of consensus regarding this question, varying from “it is safe to fly in 1 week” to “I advise against all future flying.” Of note, over half the patients were given no clear recommendations regarding the safety and timing of future air travel.
There is a remarkable delay in establishing the diagnosis of BHD; on average, the interval between onset of symptoms and diagnosis in our study was 13 years (range, 0–48 yr). The general lack of knowledge about this rare disease in the medical and lay communities is likely responsible for the protracted diagnostic delay in most patients with BHD, as it is in patients with LAM and pulmonary Langerhans cell histiocytosis. As a strategy to partially rectify this situation, we have advanced a financial argument that high-resolution CT for underlying LAM, BHD, and pulmonary Langerhans cell histiocytosis in patients who present with an apparent primary spontaneous pneumothorax is cost-effective (19). We believe that earlier deployment of chest CT, as opposed to the typical practice of waiting until a recurrent episode before scanning, will lead to earlier diagnosis and cost-saving interventions such as pleurodesis.
The pneumothorax recurrence and pleurodesis efficacy results for BHD in our study are remarkably similar to those seen in patients with LAM. LAM is a rare, diffuse cystic lung disease seen almost exclusively in women and is characterized by the proliferation of abnormal smooth muscle cells in the lungs leading to progressive destruction of the lung parenchyma (20). A survey-based analysis of 193 patients with LAM revealed a spontaneous pneumothorax rate of approximately 70%, with a 30% pleurodesis failure rate (21). Other similarities between BHD and LAM with regard to pneumothoraces are summarized in Table 5. The reason that post-pleurodesis pneumothorax is more common in BHD and LAM may relate to the subpleural localization of cysts (22–24), which may be more prone to rupture through the visceral pleura and/or limit apposition and durable fusion of the visceral and parietal pleural surfaces with pleurodesis procedures.
Table 5.
Comparative features of pneumothorax incidence and recurrence rates between Birt–Hogg–Dubé syndrome and lymphangioleiomyomatosis
| Feature | BHD | LAM |
|---|---|---|
| Average age of development of spontaneous pneumothorax | 37 yr | 35 yr |
| Proportion of patients who develop a spontaneous pneumothorax | 76% | 66% |
| Patients with spontaneous pneumothorax as presenting disease manifestation | 65% | 82% |
| Average number of pneumothoraces per patient with history of sentinel spontaneous pneumothorax | 3.6 | 3.2 |
| Average number of spontaneous pneumothoraces prior to diagnosis | 2.4 | 2.2 |
| Ipsilateral recurrence rate of spontaneous pneumothorax following a sentinel event | 73% | 71% |
| Recurrence rate of spontaneous pneumothorax following conservative treatment of first spontaneous pneumothorax | 63% | 66% |
| Recurrence rate of spontaneous pneumothorax following conservative treatment of second spontaneous pneumothorax | 93% | 60% |
| Recurrence rate of spontaneous pneumothorax following chemical pleurodesis | 30% | 27% |
| Recurrence rate of spontaneous pneumothorax following surgical pleurodesis | 35% | 32% |
Definition of abbreviations: BHD = Birt–Hogg–Dubé syndrome; LAM = lymphangioleiomyomatosis.
Data from Reference 21.
The sex bias of respondents, with a ratio of 1:5, is unexplained; there is no known sex predilection of BHD or spontaneous pneumothorax in patients with BHD. It is tempting to speculate that there could be some commonalities between the remarkable sex bias in LAM and the apparent female predominance in our BHD population because both diseases involve the mechanistic target of rapamycin pathway. It is also possible that referrals to RLDC clinics are biased toward women with cystic lung disease who were initially sent for evaluation of possible LAM. The lack of sex bias in other BHD cohorts (3) points toward the possibility of selection bias rather than a true biologic sex effect. Polling BHD renal or dermatologic cohorts for spontaneous pneumothorax may help to clarify any sex predilection as well as the overall prevalence of pneumothorax in various populations.
Our goal was to survey the management and outcomes of spontaneous pneumothoraces in patients with BHD, especially with regard to the efficacy of pleurodesis and the safety of air travel. Our hope is that these data will enable clinicians and patients to make more informed decisions regarding the management of spontaneous pneumothoraces.
Limitations
Our study has several important limitations. The overall prevalence of spontaneous pneumothorax (76%) was substantially higher than previously reported in the literature (24 to 38%) (3, 6, 7). This likely represents an ascertainment bias because a significant proportion of the patients were recruited from pulmonary clinics, a BHD population in which spontaneous pneumothorax is almost certainly overrepresented among all BHD manifestations. Also, the study’s premise of assessing safety of air travel may be more attractive to patients with a prior history of pulmonary problems, such as spontaneous pneumothorax.
The results obtained in this study are based largely on recall, often of events that happened years or even decades earlier. Whereas recall of major medical events such as flight-related pneumothorax or prior pleurodesis is probably quite reliable, the same is not true of more minor events. For example, the number of flights taken is at best a rough estimate.
Last, our study is not informative with respect to risk factors that are predictive of pneumothorax in individuals with BHD. Researchers in prior studies have identified features such as cyst size, cyst number, and cyst volume that are associated with increased spontaneous pneumothorax risk in BHD (3). It is worth noting that spontaneous pneumothoraces also occur in patients with BHD without radiologically apparent cysts (24).
Conclusions
Spontaneous pneumothorax is one of the most common phenotypic manifestations of BHD and has an extremely high rate of recurrence. Pleurodesis should be considered following the initial spontaneous pneumothorax to reduce future events. The risk of a flight-related pneumothorax in patients with BHD is less than 1% per flight and even lower for patients with a history of pleurodesis. Patients should be advised not to fly if they have new-onset chest pain or dyspnea prior to boarding.
Supplementary Material
Footnotes
Supported by National Institutes of Health grants U54HL127672 and 1 UL1TR001425-01.
Author Contributions: N.G.: had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; N.G., E.J.K., E.P.H., and F.X.M.: devised the study questionnaire; S.E.-C., S.V., and M.G.D.: helped in patient recruitment; N.G. and L.E.J.: performed the data analysis; and all authors: contributed substantially to the writing and editing of the manuscript.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Gupta N, Seyama K, McCormack FX. Pulmonary manifestations of Birt-Hogg-Dubé syndrome. Fam Cancer. 2013;12:387–396. doi: 10.1007/s10689-013-9660-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta N, Vassallo R, Wikenheiser-Brokamp KA, McCormack FX. Diffuse cystic lung disease: part II. Am J Respir Crit Care Med. 2015;192:17–29. doi: 10.1164/rccm.201411-2096CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toro JR, Pautler SE, Stewart L, Glenn GM, Weinreich M, Toure O, Wei MH, Schmidt LS, Davis L, Zbar B, et al. Lung cysts, spontaneous pneumothorax, and genetic associations in 89 families with Birt-Hogg-Dubé syndrome. Am J Respir Crit Care Med. 2007;175:1044–1053. doi: 10.1164/rccm.200610-1483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furuya M, Tanaka R, Koga S, Yatabe Y, Gotoda H, Takagi S, Hsu YH, Fujii T, Okada A, Kuroda N, et al. Pulmonary cysts of Birt-Hogg-Dubé syndrome: a clinicopathologic and immunohistochemical study of 9 families. Am J Surg Pathol. 2012;36:589–600. doi: 10.1097/PAS.0b013e3182475240. [DOI] [PubMed] [Google Scholar]
- 5.Zbar B, Alvord WG, Glenn G, Turner M, Pavlovich CP, Schmidt L, Walther M, Choyke P, Weirich G, Hewitt SM, et al. Risk of renal and colonic neoplasms and spontaneous pneumothorax in the Birt-Hogg-Dubé syndrome. Cancer Epidemiol Biomarkers Prev. 2002;11:393–400. [PubMed] [Google Scholar]
- 6.Toro JR, Wei MH, Glenn GM, Weinreich M, Toure O, Vocke C, Turner M, Choyke P, Merino MJ, Pinto PA, et al. BHD mutations, clinical and molecular genetic investigations of Birt-Hogg-Dubé syndrome: a new series of 50 families and a review of published reports. J Med Genet. 2008;45:321–331. doi: 10.1136/jmg.2007.054304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Houweling AC, Gijezen LM, Jonker MA, van Doorn MB, Oldenburg RA, van Spaendonck-Zwarts KY, Leter EM, van Os TA, van Grieken NC, Jaspars EH, et al. Renal cancer and pneumothorax risk in Birt-Hogg-Dubé syndrome; an analysis of 115 FLCN mutation carriers from 35 BHD families. Br J Cancer. 2011;105:1912–1919. doi: 10.1038/bjc.2011.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiu HT, Garcia CK. Familial spontaneous pneumothorax. Curr Opin Pulm Med. 2006;12:268–272. doi: 10.1097/01.mcp.0000230630.73139.f0. [DOI] [PubMed] [Google Scholar]
- 9.Postmus PE, Johannesma PC, Menko FH, Paul MA. In-flight pneumothorax: diagnosis may be missed because of symptom delay. Am J Respir Crit Care Med. 2014;190:704–705. doi: 10.1164/rccm.201404-0698LE. [DOI] [PubMed] [Google Scholar]
- 10.Johannesma PC, van de Beek I, van der Wel JW, Paul MA, Houweling AC, Jonker MA, van Waesberghe JH, Reinhard R, Starink TM, van Moorselaar RJ, et al. Risk of spontaneous pneumothorax due to air travel and diving in patients with Birt-Hogg-Dubé syndrome. Springerplus. 2016;5:1506. doi: 10.1186/s40064-016-3009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johannesma PC, Jonker M, van der Wal T, van Waesberghe JH, van Moorselaar J, Menko F, Postmus P. Management of spontaneous pneumothorax in patients with or without Birt-Hogg-Dubé syndrome [abstract] Eur Respir J. 2014;44(Suppl 58):P752. [Google Scholar]
- 13.Hu X, Cowl CT, Baqir M, Ryu JH. Air travel and pneumothorax. Chest. 2014;145:688–694. doi: 10.1378/chest.13-2363. [DOI] [PubMed] [Google Scholar]
- 14.Peterson DC, Martin-Gill C, Guyette FX, Tobias AZ, McCarthy CE, Harrington ST, Delbridge TR, Yealy DM. Outcomes of medical emergencies on commercial airline flights. N Engl J Med. 2013;368:2075–2083. doi: 10.1056/NEJMoa1212052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sand M, Bechara FG, Sand D, Mann B. Surgical and medical emergencies on board European aircraft: a retrospective study of 10189 cases. Crit Care. 2009;13:R3. doi: 10.1186/cc7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pollock-BarZiv S, Cohen MM, Downey GP, Johnson SR, Sullivan E, McCormack FX. Air travel in women with lymphangioleiomyomatosis. Thorax. 2007;62:176–180. doi: 10.1136/thx.2006.058537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taveira-DaSilva AM, Burstein D, Hathaway OM, Fontana JR, Gochuico BR, Avila NA, Moss J. Pneumothorax after air travel in lymphangioleiomyomatosis, idiopathic pulmonary fibrosis, and sarcoidosis. Chest. 2009;136:665–670. doi: 10.1378/chest.08-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmedzai S, Balfour-Lynn IM, Bewick T, Buchdahl R, Coker RK, Cummin AR, Gradwell DP, Howard L, Innes JA, Johnson AO, et al. British Thoracic Society Standards of Care Committee. Managing passengers with stable respiratory disease planning air travel: British Thoracic Society recommendations. Thorax. 2011;66(Suppl 1):i1–i30. doi: 10.1136/thoraxjnl-2011-200295. [DOI] [PubMed] [Google Scholar]
- 19.Gupta N, Langenderfer D, McCormack FX, Schauer DP, Eckman MH. Chest computed tomographic image screening for cystic lung diseases in patients with spontaneous pneumothorax is cost-effective. Ann Am Thorac Soc. 2017;14:17–25. doi: 10.1513/AnnalsATS.201606-459OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta N, Vassallo R, Wikenheiser-Brokamp KA, McCormack FX. Diffuse cystic lung disease: part I. Am J Respir Crit Care Med. 2015;191:1354–1366. doi: 10.1164/rccm.201411-2094CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Almoosa KF, Ryu JH, Mendez J, Huggins JT, Young LR, Sullivan EJ, Maurer J, McCormack FX, Sahn SA. Management of pneumothorax in lymphangioleiomyomatosis: effects on recurrence and lung transplantation complications. Chest. 2006;129:1274–1281. doi: 10.1378/chest.129.5.1274. [DOI] [PubMed] [Google Scholar]
- 22.Tobino K, Hirai T, Johkoh T, Kurihara M, Fujimoto K, Tomiyama N, Mishima M, Takahashi K, Seyama K. Differentiation between Birt-Hogg-Dubé syndrome and lymphangioleiomyomatosis: quantitative analysis of pulmonary cysts on computed tomography of the chest in 66 females. Eur J Radiol. 2012;81:1340–1346. doi: 10.1016/j.ejrad.2011.03.039. [DOI] [PubMed] [Google Scholar]
- 23.Gupta N, Meraj R, Tanase D, James LE, Seyama K, Lynch DA, Akira M, Meyer CA, Ruoss SJ, Burger CD, et al. Accuracy of chest high-resolution computed tomography in diagnosing diffuse cystic lung diseases. Eur Respir J. 2015;46:1196–1199. doi: 10.1183/13993003.00570-2015. [DOI] [PubMed] [Google Scholar]
- 24.Onuki T, Goto Y, Kuramochi M, Inagaki M, Bhunchet E, Suzuki K, Tanaka R, Furuya M. Radiologically indeterminate pulmonary cysts in Birt-Hogg-Dubé syndrome. Ann Thorac Surg. 2014;97:682–685. doi: 10.1016/j.athoracsur.2013.05.120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.