Abstract
Context:
Asthma is a chronic inflammatory disorder of the airway with involvement of various cellular populations and release of many inflammatory mediators. Eosinophils and serum immunoglobulin E (IgE) are considered a good marker of airway inflammation in asthma. The correlation of clinical assessment with various markers of airway inflammation in asthma is not well established in the Indian population.
Aims:
This study aims to study the correlation of serum IgE, sputum eosinophil count, and peripheral eosinophil count with clinical severity of Asthma.
Methods:
This is a cross-sectional study involving 76 stable asthmatic patients of 18–60 years of age attending the pulmonary medicine OPD. Spirometry measured at baseline. Participants were categorized according to the GINA criteria based on clinical symptoms and pulmonary function test. Blood samples were collected for peripheral eosinophil count, serum IgE levels, and sputum samples for eosinophil count. All three parameters were compared with severity of asthma. The correlation of sputum eosinophil count, peripheral eosinophil count, and serum IgE with severity of asthma was analyzed by Pearson's Chi-square test, Fisher's exact test, and the correlation coefficient was reported together with standard error of the estimate.
Results:
The mean age of patients in our study was 37.42 years and 56.6% were male. There was a significant inverse correlation between serum IgE levels and predicted forced expiratory volume 1 s (FEV1). Sputum eosinophilia was significantly seen in severe persistent asthma patients (19.7%). There was a significant inverse correlation between sputum eosinophil count and predicted FEV1 and forced vital capacity. We also found there was a significant association between peripheral eosinophil count, sputum eosinophil count, and elevated serum IgE (g100 IU/mL) with severe persistent asthma.
Conclusions:
The assessment of sputum eosinophil count is simple, inexpensive, noninvasive, and direct measurement of airway inflammation. It could be the preferred method in monitoring airway inflammation and guided management in day-to-day practice.
KEY WORDS: Asthma, peripheral eosinophil count, serum immunoglobulin E level, severity of asthma, sputum eosinophil count
INTRODUCTION
Asthma is a chronic respiratory disorder of the airways characterized by bronchial hyper-responsiveness, respiratory symptoms, structural remodeling and reversible, and variable airflow limitation.[1,2] In the developed and developing nation, it is the most common respiratory disorder with evidence suggesting that over the last two decades its prevalence has increased worldwide.[3] Even with increased knowledge and understanding of asthma as a disease, it only remains controllable with proper treatment. The asthma control and severity staging are usually assessed by subjective including clinical assessment and quality of life questionnaires and objective measure such as spirometry, peak expiratory flow rate, and bronchoprovocation testing.[4] Treatment in asthma is currently guided based on clinical assessment and pulmonary function test (PFT). Current guidelines assess the level of asthma severity according to the severity of symptoms, use of rescue medication, asthma exacerbations, and severity of airflow obstruction.[1,2] The correlation between clinical, functional, and various biological markers of airway inflammation in asthma severity is not well established.[5,6,7] Over the last decade, various noninvasive markers for measurement of airways inflammation such as exhaled nitric oxide, sputum differential cytology, and serum proteins such as eosinophilic cationic protein have been used in monitoring of asthma.[8]
In this study, we wanted to evaluate the relationship between asthma severity and various inflammatory markers. There is not much data on the relation between clinical symptoms and functional parameters to biomarkers of airway inflammation. Therefore, this study was done with the intention to find the correlation of sputum and peripheral eosinophil count and serum immunoglobulin E (IgE) with severity of asthma.
Aim and objective
To study the correlation of serum IgE, sputum eosinophil count, and peripheral eosinophil count in assessing the clinical severity of asthma.
SUBJECTS AND METHODS
This was an institutional cross-sectional study which was conducted in pulmonary medicine outpatient department. All stable asthmatic patients of 18–60 years of age from January 2014 to August 2015 were enrolled in the study. A volunteer-written consent was taken from all the patients before the study. This study was approved by the Institutional Human Ethical Committee. Brief explanation of the procedure is given in Figure 1. Patients with acute exacerbation, clinical features, and spirometry suggestive of chronic obstructive pulmonary disease, those who did not give consent, those who were not able to perform spirometry correctly, patients with history of recent myocardial infarction, and patients on chronic corticosteroid therapy were excluded from the study [Flow Chart 1].
Figure 1.
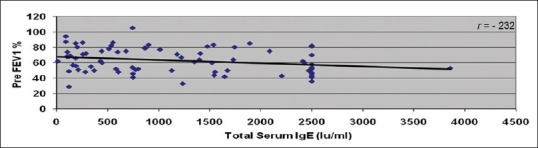
Correlation between total serum immunoglobulin E (IU/ml) and preforced expiratory volume 1 s %
Flow Chart 1.
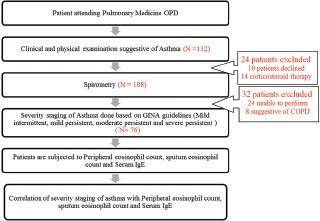
Brief explanation of the procedure
A total of 132 participants were identified and invited to participate in the study, out of which 24 participants were unable to perform spirometry. Based on inclusion and exclusion criteria, 76 participants are enrolled, and all gave consent to participate in the study.
Assessment of severity of asthma
The severity of asthma was assessed according to the GINA criteria.[1] This includes frequency of diurnal and nocturnal symptoms, frequency of short-acting beta 2-agonist used, interference with daily activity and spirometry.
Spirometry
Patients were subjected to PFT with flow sensing MIR Spirobank II and were assessed for postbronchodilator reversibility after administering 200 μg of inhaled salbutamol by repeating the test after 15 min from the baseline.[4] The degree of reversibility in forced expiratory volume 1 s (FEV1) of 12% and 200 ml from the prebronchodilator value was considered as diagnostic for asthma as per GINA guidelines.[4]
Sputum eosinophil
All the study participants were instructed to cough sputum into plastic containers. The sputum was homogenized by adding phosphate-buffered saline (PBS), vortexed for 30 s, and centrifuged for 10 min. We added 0.1% dithiothreitol to the cells in a ratio of 4:1, which was agitated for 20 min to break up the disulfide bonds and disperse the cells. Cells were washed once more with PBS and resuspended. The cell suspension was aspirated and filtered to remove any remaining debris. Supernatant was separated from cell pellet. Sputum sample was transferred to the slide and was distributed thinly and evenly over the slide. Staining was done by hematoxylin and eosin stain and analyzed using microscopy to determine the count for eosinophils.[9] The eosinophil count was then expressed as a percentage as it is more accurate than absolute count.[9] Sputum eosinophil count ≥3% was considered abnormal.
Blood investigations
Under aseptic precaution, 5 ml of blood was taken from medial cubital vein into vacutainers from each patient and measured for peripheral eosinophil count and serum IgE and estimation of eosinophil percentage done by automated analyzer cellenium.
The total IgE levels were measured using Elecsys IgE II reagent kit in cobas e411 analyzers. The Elecsys IgE II assay uses monoclonal antibodies specifically directed against IgE. The reagents in the kit have been assembled into a ready for use unit that cannot be separated. The test is performed based on sandwich principle. Total IgE levels more than 100 IU/mL were taken as abnormal.[10,11]
RESULTS
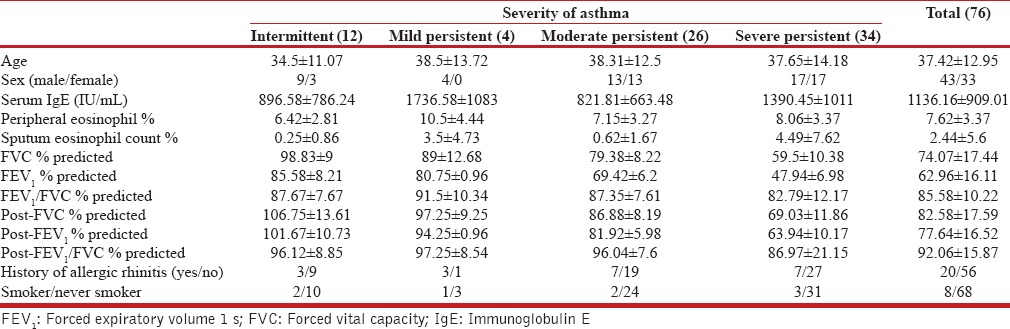
A total number 76 cases were enrolled for the study. The demographic, baseline clinical, and functional characteristics are enumerated in Table 1. The mean age of patients in the present study was 37.42 years. Of the 76 patients in our study, 43 (56.6%) were male, and 33 (43.4%) were female. Out of 76 cases, 34 (44.7%) of the study population had severe persistent asthma.
Table 1.
Demographic, baseline clinical, and functional characteristic data
Among 76 patients in our study, 73 (96.1%) patients had abnormal serum IgE levels. However, we did not observe a dose–response relationship between severity of asthma and serum IgE levels. We observed a statistically significant inverse correlation between total serum IgE levels and predicted FEV1 (P = 0.04), which was, however, a weak correlation (r = −0.23).
Among 76 patients, 20 (26.3%) patients had abnormal sputum eosinophil count. We observed an abnormal sputum eosinophil count and severity of asthma had statistically significant (P = 0.004) [Figure 2]. Again, as in total IgE, we did not observe a dose–response relationship between elevated sputum eosinophil counts and asthma severity. We observed that there is a statistically significant inverse correlation between sputum eosinophil count and predicted forced expiratory volume in 1 s (P = 0.004) [Figure 3] and the predicted forced vital capacity (FVC) (P = 0.011). However, as in total IgE, the correlation was weak. More than half of the patients with severe asthma had normal sputum eosinophil counts suggesting that there could be specific phenotypes of asthma with elevated sputum eosinophils, which may not be a common feature among all asthmatics.
Figure 2.
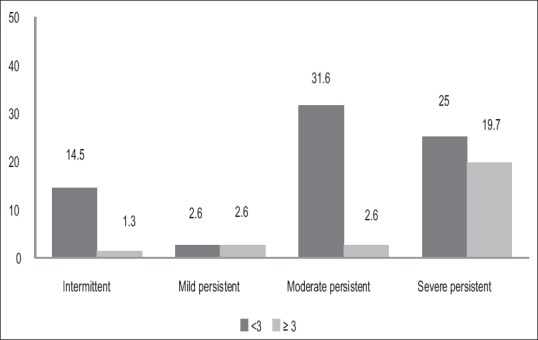
Comparison between sputum eosinophil count (%) and severity of asthma
Figure 3.
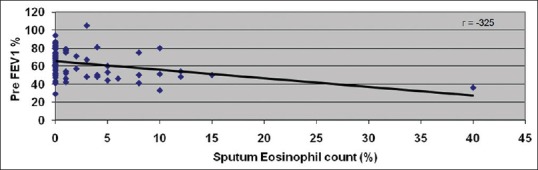
Correlation between sputum eosinophil count (%) and preforced expiratory volume 1 s %
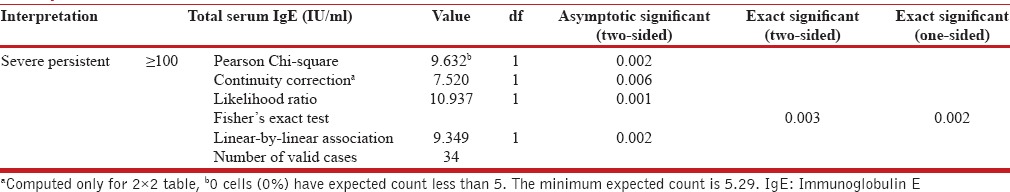
Chi-square test comparing peripheral eosinophil count, sputum eosinophil, and serum IgE with the severity of asthma shows significant association between peripheral eosinophil count and sputum eosinophil when serum IgE ≥100 IU/mL and severe persistent asthma [Table 2]. In our study, 44 (58%) patients had increase peripheral eosinophil count. Among 76 cases, 20 (26.3%) patients had a history of allergic rhinitis. We observed there was no significant correlation between peripheral eosinophil count and lung function test (r = −095).
Table 2.
Correlation of asthma severity with peripheral eosinophil count, sputum eosinophil count, and serum IgE by Chi-square test
DISCUSSION
Asthma is a complex chronic respiratory disorder characterized by airway hyperresponsiveness and variable airflow obstruction. It is a Type 1 hypersensitivity reaction. Several methods of assessing airway inflammation have been proposed in literature. Noninvasive methods of assessment of airway inflammation are much safer, easier, and a convenient tool for monitoring in patients, especially those with more severe asthma.
Correlation of sputum eosinophil count and severity of asthma
We found higher percentage of sputum eosinophil count in 26.3% cases of the study population that included predominantly moderate and severe persistent asthma. We observed that Manise et al.[12] in their study reported almost similar distribution.
We observed high sputum eosinophil count (>3) was significantly seen in more patients with severe persistent asthma (19.7%) though more than half of them had normal sputum eosniophils. Similar results were observed in various studies.[13,14,15,16,17] On the other hand, Gibson et al.[18] and Palomino et al.[19] have reported conflicting results. In our study, there was no significant difference in sputum eosinophil level in intermittent and moderate persistent asthma, and we did not observe a dose–response relationship between asthma severity and proportion of patients with higher sputum eosinophilia, suggesting an asthma phenotype with sputum eosinophilia which may be seen in any asthma severity. Various authors also have reported similar findings.[15,20] The importance of identifying this phenotype of asthma with elevated sputum eosinophilia could be related to steroid responsiveness, which future studies should demonstrate in the Indian population. In our present study, though there was a significant inverse correlation between sputum eosinophil count and predicted forced expiratory volume in 1 s (P = 0.011) and predicted FVC (P = 0.015), the correlation has been weak. Various studies have also reported significant correlation between sputum eosinophil count and predicted FEV1 (P < 0.05).[15,21,22,23,24]
Correlation of serum immunoglobulin E and severity of asthma
We found that 96.1% of study population had levels of total serum IgE of >100 IU/ml. This is in concurrence with the observation made by various authors.[12,19,25] A similar distribution was reported by Kartasamita et al.[26]
A study in the general population in South India showed the mean total serum IgE to be much higher than the Western population at 522.19 IU/ml.[27] We observed very high levels of total serum IgE in our asthmatic population as well with a mean of 1136.16 IU/ml. We have not included controls from the general population to assess the mean total IgE levels in the general population. Certain populations in the world from Asia (including India) and Africa are very high IgE producers. These high levels of IgE are hypothesized to be protective against various insults, such as detoxification and neutralization of venoms, expulsion of ectoparasites, and degradation of xenobiotics.[28]
In our study, we observed higher levels of serum IgE in all patients of mild persistent (n = 4) and severe persistent asthma (n = 34). The observed distribution had no significant correlation between serum IgE and severity of asthma. Similar results were obtained by various authors.[19,29] On the contrary, some authors have documented positive correlation between total serum IgE and severity of asthma.[30,31]
We also observed that mean value of serum IgE in mild persistent asthma was higher when compared with other group of asthma severity staging. This could probably due to associated history of allergic rhinitis in 3 out of 4 patients in mild persistent group.
Elevated total IgE may probably be a nonspecific reaction secondary to airway inflammation in asthmatics.[32] It may be proposed that relative higher IgE levels confined at the site of local inflammation and serum levels may not always necessarily reflect the level in lungs. It is also known that IgE binds to mast cells with relatively high affinity. This could provide a possible explanation as why circulating IgE may not yield an conclusive evidence of the severity of inflammation.[33] In our present study, we observed there was a significant inverse correlation between serum IgE and predicted forced expiratory volume in 1 s, which, however, was a weak correlation. Various authors have reported similar findings that the higher serum IgE levels had significantly lower FEV1< 60% predicted (P < 0.05).[34,35,36,37,38]
Correlation of peripheral eosinophil count and severity of asthma
In our study, we observed increased peripheral eosinophil count in 57.9% of the study population. We observed wide difference in peripheral eosinophil percentages among various group of asthma severity staging. This is in concurrence with the observation made by Palomino et al.[19] We conclude that there is no correlation between peripheral eosinophil levels and asthma severity or lung functions.
One of the reasons could be due to elevation of peripheral eosinophil count in other conditions such as allergic rhinitis, parasitic infestation which may not be related to asthma severity.[39] Eosinophils are present in the intravascular space for a brief period only.[40] One of the hypotheses could be influx of peripheral blood eosinophils rapidly into the tissues at the site of local inflammation suggests that the relationship between peripheral blood eosinophil count with airway inflammation may be transitory.[41]
Correlation of sputum eosinophil count, peripheral eosinophil count, and serum immunoglobulin E with severity of asthma
We found a significant association between peripheral eosinophil count, sputum eosinophil count, and serum IgE (g100 IU/mL) with severe persistent asthma. Khadadah et al.[42] in their study reported positive correlation between total blood eosinophil counts, serum total IgE levels, and eosinophilic cationic protein.
In our present study of 76 asthmatics selected as a convenient sampling from the hospital, 44.7% patients had severe persistent asthma, 34.2% patients had moderate persistent asthma, 5.3% patients had mild persistent asthma, and 15.8% patients had intermittent asthma. In our study, 10.8% of patients had smoking history (current or ex-smoker). The effect of smoking on serum IgE has been reported in adult patients.[43] It was not possible to assess the impact of smoking on serum IgE levels, due to the relatively smaller number of smokers in various groups of asthma severity.
This study has few important limitations: (1) The evaluation of allergic bronchopulmonary aspergillosis was not done in patients with high serum IgE. (2) Asthmatic patients other than eosinophilic phenotype were not evaluated. (3) Patients with increased level of eosinophila were not evaluated for parasitic infestation. (4) The patients were recruited from the hospital clinic. (5) We did not recruit equal numbers of patients in the four groups of asthma severity. Very small numbers in mild intermittent and mild persistent asthma influenced our ability to compare across groups for a dose–response relationship. (6) Normal age-matched controls from the general population were not included in the study. (7) We did not use an ultrasonic nebulizer for sputum induction in the evaluation of sputum eosinophilia and only spontaneously generated sputum was used. (8) We did not repeat sputum eosinophilia to assess the repeatability of our measurements.
CONCLUSION
Eosinophilic inflammation is a characteristic feature of asthma. Assessment of sputum eosinophil count is simple, inexpensive, noninvasive, and direct measurement of airway inflammation that could help to identify specific phenotypes in asthma that could be more steroid responsive, which needs to be demonstrated in future studies. It could be the preferred method in monitoring airway inflammation and guided management in day-to-day practice.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Global Initiative for Asthma (GINA), National Heart, Lung and Blood Institute (NHLBI) Global Strategy for Asthma Management and Prevention. Bethesda (MD): Global Initiative for Asthma (GINA), National Heart, Lung and Blood Institute (NHLBI); 2006. [Last accessed on 2014 Jul 17]. Available from: http://www.ginasthma.com . [Google Scholar]
- 2.Guidelines for the Diagnosis and Management of Asthma. NIH Publication No. 97-4051A. Bethesda, MD: National Institutes of Health; 1997. National Heart, Lung, and Blood Institute. [Google Scholar]
- 3.World Health Organization. Global Surveillance, Prevention and Control of Chronic Respiratory Diseases: A Comprehensive Approach. World Health Organization; 2007. [Last accessed on 2015 Jul 15]. Available from: http://www.who.int/gard/publications/GARD%20Book%202007.pdf . [Google Scholar]
- 4.From the Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2015. [Last accessed on 2015 Jul 09]. Available from: http://www.ginasthma.org/
- 5.Sandeep T, Roopakala MS, Silvia CR, Chandrashekara S, Rao M. Evaluation of serum immunoglobulin E levels in bronchial asthma. Lung India. 2010;27:138–40. doi: 10.4103/0970-2113.68312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subrahmanyam RM, Srikantaiah C, Krishna P, Delphine Silvia CR, Thirunavukkarasu S, Devi K, et al. Can bronchial asthma be classified based on the immunological status? Lung India. 2011;28:110–3. doi: 10.4103/0970-2113.80323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Susmita J, Vijayalakshmi V, Latha GS, Murthy KJ. Combination of allergens in specific immunotherapy for IgE mediated allergies. Lung India. 2007;24:3. [Google Scholar]
- 8.Cianchetti S, Bacci E, Ruocco L, Bartoli ML, Ricci M, Pavia T, et al. Granulocyte markers in hypertonic and isotonic saline-induced sputum of asthmatic subjects. Eur Respir J. 2004;24:1018–24. doi: 10.1183/09031936.04.00139503. [DOI] [PubMed] [Google Scholar]
- 9.Djukanovic R, Sterk PJ, Fahy JV, Hargreave FE. Standardised methodology of sputum induction and processing. Eur Respir J Suppl. 2002;37:1s–2s. doi: 10.1183/09031936.02.00000102. [DOI] [PubMed] [Google Scholar]
- 10.Kumar Y. Evaluation of serum immunoglobulins IgG, IgA, IgM and total IgE in chronic alcoholics: A community-based study. Immunochem Immunopathol. 2015;1:1. [Google Scholar]
- 11.Razi E, Moosavi GA. Serum total IgE levels and total eosinophil counts: Relationship with treatment response in patients with acute asthma. J Bras Pneumol. 2010;36:23–8. doi: 10.1590/s1806-37132010000100006. [DOI] [PubMed] [Google Scholar]
- 12.Manise M, Holtappels G, Van Crombruggen K, Schleich F, Bachert C, Louis R. Sputum IgE and cytokines in asthma: Relationship with sputum cellular profile. PLoS ONE. 2013;8:e58388. doi: 10.1371/journal.pone.0058388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujimoto K, Kubo K, Matsuzawa Y, Sekiguchi M. Eosinophil cationic protein levels in induced sputum correlate with the severity of bronchial asthma. Chest. 1997;112:1241–7. doi: 10.1378/chest.112.5.1241. [DOI] [PubMed] [Google Scholar]
- 14.Ronchi MC, Piragino C, Rosi E, Stendardi L, Tanini A, Galli G, et al. Do sputum eosinophils and ECP relate to the severity of asthma? Eur Respir J. 1997;10:1809–13. doi: 10.1183/09031936.97.10081809. [DOI] [PubMed] [Google Scholar]
- 15.Bandyopadhyay A, Roy P, Saha K, Chakraborty S, Jash D, Saha D. Usefulness of induced sputum eosinophil count to assess severity and treatment outcome in asthma patients. Lung India. 2013;30:117–23. doi: 10.4103/0970-2113.110419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duncan CJ, Lawrie A, Blaylock MG, Douglas JG, Walsh GM. Reduced eosinophil apoptosis in induced sputum correlates with asthma severity. Eur Respir J. 2003;22:484–90. doi: 10.1183/09031936.03.00109803a. [DOI] [PubMed] [Google Scholar]
- 17.Lemière C, Ernst P, Olivenstein R, Yamauchi Y, Govindaraju K, Ludwig MS, et al. Airway inflammation assessed by invasive and noninvasive means in severe asthma: Eosinophilic and noneosinophilic phenotypes. J Allergy Clin Immunol. 2006;118:1033–9. doi: 10.1016/j.jaci.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Gibson PG, Girgis-Gabardo A, Morris MM, Mattoli S, Kay JM, Dolovich J, et al. Cellular characteristics of sputum from patients with asthma and chronic bronchitis. Thorax. 1989;44:693–9. doi: 10.1136/thx.44.9.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palomino AL, Bussamra MH, Saraiva-Romanholo BM, Martins MA, Nunes Mdo P, Rodrigues JC. Induced sputum in children and adolescents with asthma: Safety, clinical applicability and inflammatory cells aspects in stable patients and during exacerbation. J Pediatr (Rio J) 2005;81:216–24. [PubMed] [Google Scholar]
- 20.Bartoli ML, Bacci E, Carnevali S, Cianchetti S, Dente FL, Di Franco A, et al. Clinical assessment of asthma severity partially corresponds to sputum eosinophilic airway inflammation. Respir Med. 2004;98:184–93. doi: 10.1016/j.rmed.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Pin I, Gibson PG, Kolendowicz R, Girgis-Gabardo A, Denburg JA, Hargreave FE, et al. Use of induced sputum cell counts to investigate airway inflammation in asthma. Thorax. 1992;47:25–9. doi: 10.1136/thx.47.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Louis R, Lau LC, Bron AO, Roldaan AC, Radermecker M, Djukanovic R. The relationship between airways inflammation and asthma severity. Am J Respir Crit Care Med. 2000;161:9–16. doi: 10.1164/ajrccm.161.1.9802048. [DOI] [PubMed] [Google Scholar]
- 23.Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, et al. Asthma exacerbations and sputum eosinophil counts: A randomised controlled trial. Lancet. 2002;360:1715–21. doi: 10.1016/S0140-6736(02)11679-5. [DOI] [PubMed] [Google Scholar]
- 24.ten Brinke A, Zwinderman AH, Sterk PJ, Rabe KF, Bel EH. Factors associated with persistent airflow limitation in severe asthma. Am J Respir Crit Care Med. 2001;164:744–8. doi: 10.1164/ajrccm.164.5.2011026. [DOI] [PubMed] [Google Scholar]
- 25.Ige OM, Falade AG, Arinola OG. Atopy is a risk factor for adult asthma in urban community of Southwestern Nigeria. Lung India. 2012;29:114–9. doi: 10.4103/0970-2113.95301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kartasamita CB, Rosmayudi O, Demedts M. Total serum IgE and eosinophil count in children with and without a history of asthma, wheezing, or atopy in an urban community in Indonesia.The Respiratory Disease Working Group. J Allergy Clin Immunol. 1994;94(6 Pt 1):981–8. doi: 10.1016/0091-6749(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 27.Mahesh PA, Wong GW, Ogorodova L, Potts J, Leung TF, Fedorova O, et al. Prevalence of food sensitization and probable food allergy among adults in India: The EuroPrevall INCO study. Allergy. 2016;71:1010–9. doi: 10.1111/all.12868. [DOI] [PubMed] [Google Scholar]
- 28.Palm NW, Rosenstein RK, Medzhitov R. Allergic host defences. Nature. 2012;484:465–72. doi: 10.1038/nature11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davila I, Valero A, Entrenas LM, Valveny N, Herráez L. SIGE Study Group. Relationship between serum total IgE and disease severity in patients with allergic asthma in Spain. J Investig Allergol Clin Immunol. 2015;25:120–7. [PubMed] [Google Scholar]
- 30.Borish L, Chipps B, Deniz Y, Gujrathi S, Zheng B, Dolan CM. TENOR Study Group. Total serum IgE levels in a large cohort of patients with severe or difficult-to-treat asthma. Ann Allergy Asthma Immunol. 2005;95:247–53. doi: 10.1016/S1081-1206(10)61221-5. [DOI] [PubMed] [Google Scholar]
- 31.de Marco R, Marcon A, Jarvis D, Accordini S, Almar E, Bugiani M, et al. Prognostic factors of asthma severity: A 9-year international prospective cohort study. J Allergy Clin Immunol. 2006;117:1249–56. doi: 10.1016/j.jaci.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Burrows B, Martinez FD, Cline MG, Lebowitz MD. The relationship between parental and children's serum IgE and asthma. Am J Respir Crit Care Med. 1995;152(5 Pt 1):1497–500. doi: 10.1164/ajrccm.152.5.7582283. [DOI] [PubMed] [Google Scholar]
- 33.Husain AN, Kumar V. The lung. In: Kumar V, Abbas AK, Fausto N, editors. Robbins and Cotran Pathologic Basis of Disease. 7th ed. Philadelphia: W.B. Saunders; 2007. pp. 711–72. [Google Scholar]
- 34.Endoh N, Ichinose M, Takahashi T, Miura M, Kageyama N, Mashito Y, et al. Relationship between cholinergic airway tone and serum immunoglobulin E in human subjects. Eur Respir J. 1998;12:71–4. doi: 10.1183/09031936.98.12010071. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka A, Jinno M, Hirai K, Miyata Y, Mizuma H, Yamaguchi M, et al. Longitudinal increase in total IgE levels in patients with adult asthma: An association with poor asthma control. Respir Res. 2014;15:144. doi: 10.1186/s12931-014-0144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naqvi M, Choudhry S, Tsai HJ, Thyne S, Navarro D, Nazario S, et al. Association between IgE levels and asthma severity among African American, Mexican, and Puerto Rican patients with asthma. J Allergy Clin Immunol. 2007;120:137–43. doi: 10.1016/j.jaci.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 37.Anupama N, Sharma MV, Nagaraja HS, Bhat MR. The serum immunoglobulin E level reflects the severity of bronchial asthma. Thai J Physiol Sci. 2005;18:35–40. [Google Scholar]
- 38.Tollerud DJ, O'Connor GT, Sparrow D, Weiss ST. Asthma, hay fever, and phlegm production associated with distinct patterns of allergy skin test reactivity, eosinophilia, and serum IgE levels.The Normative Aging Study. Am Rev Respir Dis. 1991;144:776–81. doi: 10.1164/ajrccm/144.4.776. [DOI] [PubMed] [Google Scholar]
- 39.Sampson AP. Eosinophils: Provokers or bystanders in asthma? Clin Exp Allergy Rev. 2001;1:73–6. [Google Scholar]
- 40.Spector SL, Tan RA. Is a single blood eosinophil count a reliable marker for “eosinophilic asthma?”. J Asthma. 2012;49:807–10. doi: 10.3109/02770903.2012.713428. [DOI] [PubMed] [Google Scholar]
- 41.Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, et al. Eosinophils: Biological properties and role in health and disease. Clin Exp Allergy. 2008;38:709–50. doi: 10.1111/j.1365-2222.2008.02958.x. [DOI] [PubMed] [Google Scholar]
- 42.Khadadah M, Onadeko BO, Ezeamuzie CI, Mustafa HT, Marouf R, Sugathan TN. The association of skin test reactivity, total serum IgE levels, and peripheral blood eosinophilia with asthma in Kuwait. J Asthma. 2000;37:481–8. doi: 10.3109/02770900009055474. [DOI] [PubMed] [Google Scholar]
- 43.Beeh KM, Ksoll M, Buhl R. Elevation of total serum immunoglobulin E is associated with asthma in nonallergic individuals. Eur Respir J. 2000;16:609–14. doi: 10.1034/j.1399-3003.2000.16d07.x. [DOI] [PubMed] [Google Scholar]


