Abstract
A case of 60-year-old male with acute pulmonary embolism without hypotension but signs of right ventricular dysfunction and elevated cardiac biomarkers is reported in this study. The patient comes under intermediate high-risk category and was successfully thrombolysed with alteplase infused through pulmonary artery catheter. Catheter-directed thrombolysis (CDT) can be considered as much safer and effective alternative to systemic thrombolysis in such patients with lower risk of bleeding. This novel bedside method of pulmonary artery CDT with the advantage of no radiation exposure and real time monitoring of pulmonary artery pressures as an end-point of thrombolysis can be utilized in the near future.
KEY WORDS: Acute pulmonary embolism, pulmonary artery catheter, thrombolysis
INTRODUCTION
Acute pulmonary embolism (PE) is a common life-threatening condition and represents the most serious manifestation of venous thromboembolic disease. Acute PE interferes with both the circulation and gas exchange and right ventricular (RV) failure due to pressure overload is considered as the primary cause of death in severe PE.[1,2,3] The standard treatment is anticoagulation and systemic thrombolysis, while the latter is usually indicated in massive acute PE with improvement in cardiopulmonary hemodynamics. Although systemic thrombolysis is indicated for the treatment of acute PE with hypotension or shock, many patients cannot undergo systemic thrombolysis due to contraindications, and even though patients with acute PE are prescreened for absolute contraindications, the rate of major hemorrhage from systemic thrombolytic administration is approximately 10%–20%.[3,4,5,6] Another category of acute PE patients without hypotension or shock but with signs of RV dysfunction with elevated cardiac biomarkers can also be considered for systemic thrombolysis. Catheter-directed thrombolysis (CDT) can be considered as an alternative life-saving procedure in such patients as it is associated with much lower risk of bleeding. Various catheter intervention techniques have been introduced in clinical practice. Mechanical methods such as thrombus fragmentation, rheolytic thrombectomy, suction thrombectomy, and rotational thrombectomy are indicated for patients with absolute contraindications to thrombolysis whereas, conventional pharmacological CDT and pharmacomechanical thrombolysis with ultrasound enhancement as well as rheolytic thrombectomy are indicated for patients without absolute contraindications to thrombolysis.[7] These methods have improved the outcomes compared to systemic thrombolysis as well as anticoagulation alone with lesser risk of bleeding.[3,8,9,10,11,12,13,14,15,16] However, the mechanical and pharmacomechanical methods require specialized catheters, expertise and are expensive. On the other hand, conventional pharmacological CDT is done in catheterization laboratories under fluoroscopic guidance with repeated pulmonary angiographies adding to cost, radiation exposure, and risk of contrast-induced nephropathy. A bedside method of pharmacological CDT using pulmonary artery catheter with no radiation exposure and real time monitoring of pulmonary artery pressures (PAPs) as an end-point of thrombolysis has been reported in the present study.
CASE REPORT
A 60-year-old male with no comorbid illness underwent cervical laminectomy for compression myelopathy 3 months back. There was prolonged immobilization for 2 weeks of postsurgery and patient was put on thromboprophylaxis with low molecular weight heparin. After resuming daily routine activities, there were no symptoms for 2 months. The patient has presented with complaints of acute onset of breathlessness, palpitation, syncopal attacks, and bilateral lower limb swelling for 10 days. On physical examination, the patient had an arterial blood pressure (BP) of 120/80 mmHg, a heart rate (HR) of 136 beats/min, respiratory rate (RR) of 28 breaths/min and oxygen saturation (SaO2) of 88% on room air. The arterial blood gas on room air showed a PaO2 of 45.7 mmHg, PaCO2 of 36.4 mmHg, HCO3 of 30.2 mmol, and pH of 7.53 with wide alveolar-arterial gradient 60.3 mmHg (respiratory alkalosis with metabolic alkalosis with hypoxemic respiratory failure). Chest skiagram showed obliteration of pulmonary bay with bilateral prominent pulmonary arteries and hyperlucent left lung [Figure 1]. Electrocardiography revealed sinus tachycardia with right bundle branch block. Two-dimensional echocardiography revealed dilated right atrium and right ventricle, hypokinetic RV wall, dilated inferior vena cava, moderate tricuspid regurgitation (estimated mean PAP = 44 mmHg), and left ventricular ejection fraction of 55%. Contrast-enhanced computed tomography (CT) thorax showed multifocal filling defects noted in the right and left branch of pulmonary artery extending into the descending branch of the left pulmonary artery as well as the bifurcation of right and left pulmonary artery [Figure 2]. Quantitative troponin-I was raised (0.2 pg/ml) and serum N-terminal brain natriuretic peptide level was also raised (5871 pg/ml). Ultrasound Doppler venography showed subacute thrombus in the right distal superficial femoral and right popliteal vein. The diagnosis of intermediate-high risk acute PE was established on the basis of calculated simplified PE severity index score of >1 along with signs of RV dysfunction on echocardiography and elevated cardiac biomarkers in the absence of shock or hypotension. CDT was performed in the Intensive Care Unit (ICU). The patient was prepared and draped in standard sterile fashion while supine on the procedure table. Swan-Ganz catheter (Edwards Lifesciences LLC Irvine, USA) was introduced through the right internal jugular vein. Mean PAP of 39 mmHg was then recorded at baseline. CDT using the flow directed technique, which is a form of pharmacologic thrombolysis in which the fibrinolytic drug infusion is administered through a catheter positioned in the pulmonary artery proximal to the location of pulmonary artery thrombus, was then pursued. The catheter was positioned in the pulmonary artery guided by the pressure readings, and alteplase was administered with nonbolus continuous infusion at 0.5–1 mg/h with initial higher infusion doses with a slower rate of taper (50 mg of alteplase reconstituted in 50 ml of sterile water and diluted with 500 ml of 0.9% normal saline solution to a concentration of 0.1–0.2 mg/ml). Systemic intravenous (IV) heparin was also administered through continuous infusion with an initial dosing rate of 1000 units/h to prevent thrombus propagation and was further titrated to achieve a partial thromboplastin time of 60–80 s by performing coagulation profile every six hourly. The patient was continuously monitored for any evidence of bleeding in an ICU setting during the thrombolytic therapy. The clinical progress of CDT was assessed by evaluating patient's hemodynamic status, SaO2 and mean PAP. Daily chest radiography was performed to ensure catheter positional stability and to enable catheter repositioning as needed. Thrombolysis was continued until mean PAP was normalized (<18 mmHg) or the maximum dose of alteplase (100 mg) was reached. After 3 days of thrombolysis with total dose of alteplase 50 mg, there was a reduction of PAP to 18 mmHg. The patient became hemodynamically stable as vital parameters returned to normal (HR – 94/min, BP – 130/80 mmHg and RR – 20/min). There was also an improvement in oxygenation parameter with PaO2 of 74.4 mmHg as well as SaO2 of 97% on room air and normalization of alveolar-arterial gradient to 21.5 mmHg as shown in Table 1. Serial two-dimensional (2D) echocardiogram after thrombolysis revealed normal right atrium and right ventricle and serum N-terminal pro-b-type natriuretic peptide decreased to 562 pg/ml. After completion of thrombolytic therapy, the pulmonary artery catheter was removed, and hemostasis was achieved at the venous site with manual compression. CT-pulmonary angiography (CTPA) was not performed immediately at the completion of thrombolysis to avoid harmful radiation exposure and contrast-induced nephropathy. The patient was on continuous infusion of heparin for 3 days during thrombolysis followed by intermittent dosing of IV 5000 units three times daily for next 2 days and then switched to oral anticoagulation dabigatran 150 mg twice daily on 6th day of admission and further maintained on a 6-month course of oral anticoagulation after completion of CDT. He was discharged on the 10th day of admission under regular follow-up at three and 6 months of oral anti-coagulation therapy. The findings of serial 2D echocardiogram at each follow-up remained unremarkable after thrombolysis. Follow-up CTPA was also advised after 3 months that revealed near resolution of thrombus at bifurcation of right and left pulmonary artery as shown in Figure 3. Currently, the patient is asymptomatic and under regular follow-up.
Figure 1.
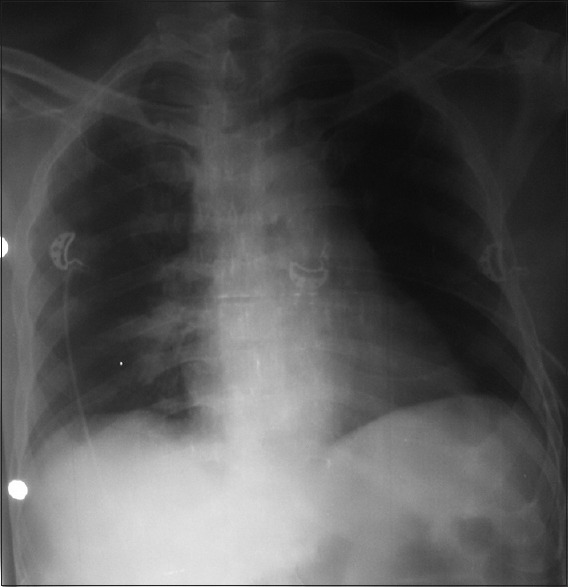
Frontal radiograph of chest shows obliteration of pulmonary bay with bilateral prominent pulmonary arteries
Figure 2.
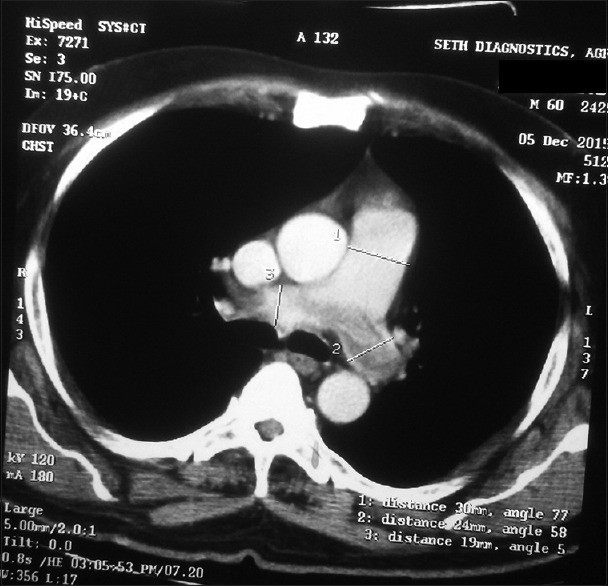
Pretreatment axial contrast enhanced computed tomography image of thorax shows prominent main pulmonary artery (transverse diameter measures 3 cm) with hypodense filling defects in right and left main pulmonary artery suggestive of thrombus
Table 1.
Serial hemodynamic and gas exchange parameters in our patient undergoing catheter guided thrombolytic therapy
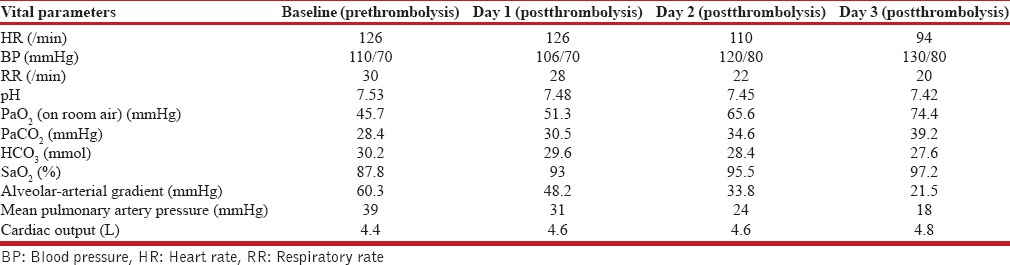
Figure 3.
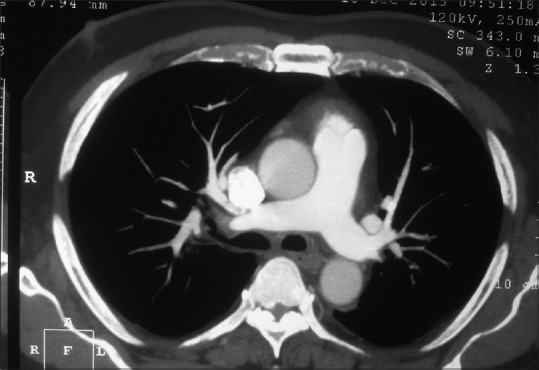
Post intrapulmonary arterial instillation of alteplase axial contrast enhanced computed tomography image of thorax shows dissolution of the thrombus seen within the right and left pulmonary artery previously
DISCUSSION
The objective of CDT is to remove obstructing thrombi from the main pulmonary arteries to facilitate rapid reperfusion leading to RV recovery and improve symptoms and survival.[7] The indication for application of thrombolytic therapy in treatment of patients with intermediate high risk PE is currently considered as controversial.[17,18]
In the current case, doctors were successfully able to normalize PAP and achieve complete resolution of PA clot using alteplase infusion without any bleeding or other complication. The time required for normalization of PA pressures and the dose required for clot dissolution was comparable to that reported in previous studies. However, the dose was half of that required with systemic thrombolysis, probably due to high local concentration of alteplase in the pulmonary circulation. Another advantage of local instillation of alteplase through pulmonary artery is real time measurement of PAPs throughout the period of thrombolysis with a measurable endpoint of normalisation of PA pressures. The entire procedure was performed bedside without any radiation exposure or use of specialized catheter. The major limitations of CDT were the risk of catheter related blood stream infection and prolonged period of thrombolytic therapy (50 h for the infusion). However, there was no evidence of bloodstream infections in the patient. Mechanical and pharmacomechanical CDT are associated with several demerits such as periprocedural hemodynamic deterioration, distal embolization, pulmonary artery perforation, systemic bleeding complications, lung hemorrhage, pericardial tamponade, transient heart block or bradycardia, contrast-induced nephropathy, and access-related complications, including hematoma, pseudoaneurysm, or arteriovenous fistula. Such complications are negligible with the use of Swan-Ganz catheter. Patients with preexisting renal failure can also be considered for pulmonary artery CDT as no IV contrast is used. The safety and efficacy should be validated by conducting large-scale studies for comparing efficacy and safety of pulmonary artery CDT to other methods of CDT, which would be necessary before its inclusion in the existing guidelines.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tapson VF. Acute pulmonary embolism. N Engl J Med. 2008;358:1037–52. doi: 10.1056/NEJMra072753. [DOI] [PubMed] [Google Scholar]
- 2.Lilienfeld DE, Rubin LJ. Mortality from primary pulmonary hypertension in the United States, 1979-1996. Chest. 2000;117:796–800. doi: 10.1378/chest.117.3.796. [DOI] [PubMed] [Google Scholar]
- 3.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: Clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet. 1999;353:1386–9. doi: 10.1016/s0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]
- 4.Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, et al. Management of massive and sub-massive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: A scientific statement from the American Heart Association. Circulation. 2011;123:1788–830. doi: 10.1161/CIR.0b013e318214914f. Available from: https://www.ncbi.nlm.nih.gov/pubmed/21422387 . [DOI] [PubMed] [Google Scholar]
- 5.Fiumara K, Kucher N, Fanikos J, Goldhaber SZ. Predictors of major hemorrhage following fibrinolysis for acute pulmonary embolism. Am J Cardiol. 2006;97:127–9. doi: 10.1016/j.amjcard.2005.07.117. [DOI] [PubMed] [Google Scholar]
- 6.Konstantinides S, Geibel A, Heusel G, Heinrich F, Kasper W. Management Strategies and Prognosis of Pulmonary Embolism- Trial Investigators. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med. 2002;347:1143–50. doi: 10.1056/NEJMoa021274. [DOI] [PubMed] [Google Scholar]
- 7.Engelberger RP, Kucher N. Catheter-based reperfusion treatment of pulmonary embolism. Circulation. 2011;124:2139–44. doi: 10.1161/CIRCULATIONAHA.111.023689. [DOI] [PubMed] [Google Scholar]
- 8.Liu P, Meneveau N, Schiele F, Bassan JP. Predictors of long-term clinical outcome of patients with acute massive pulmonary embolism after thrombolytic therapy. Chin Med J (Engl) 2003;116:503–9. [PubMed] [Google Scholar]
- 9.Kline JA, Steuerwald MT, Marchick MR, Hernandez-Nino J, Rose GA. Prospective evaluation of right ventricular function and functional status 6 months after acute submassive pulmonary embolism: Frequency of persistent or subsequent elevation in estimated pulmonary artery pressure. Chest. 2009;136:1202–10. doi: 10.1378/chest.08-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melanson SE, Laposata M, Camargo CA, Jr, Chen AA, Tung R, Krauser D, et al. Combination of D-dimer and amino-terminal pro-B-type natriuretic Peptide testing for the evaluation of dyspneic patients with and without acute pulmonary embolism. Arch Pathol Lab Med. 2006;130:1326–9. doi: 10.5858/2006-130-1326-CODAAP. [DOI] [PubMed] [Google Scholar]
- 11.Kuo WT, Gould MK, Louie JD, Rosenberg JK, Sze DY, Hofmann LV. Catheter-directed therapy for the treatment of massive pulmonary embolism: Systematic review and meta-analysis of modern techniques. J Vasc Interv Radiol. 2009;20:1431–40. doi: 10.1016/j.jvir.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Becattini C, Agnelli G, Salvi A, Grifoni S, Pancaldi LG, Enea I, et al. Bolus tenecteplase for right ventricle dysfunction in hemodynamically stable patients with pulmonary embolism. Thromb Res. 2010;125:e82–6. doi: 10.1016/j.thromres.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Konstantinides S, Tiede N, Geibel A, Olschewski M, Just H, Kasper W. Comparison of alteplase versus heparin for resolution of major pulmonary embolism. Am J Cardiol. 1998;82:966–70. doi: 10.1016/s0002-9149(98)00513-x. [DOI] [PubMed] [Google Scholar]
- 14.Engelberger RP, Kucher N. Ultrasound-assisted thrombolysis for acute pulmonary embolism: A systematic review. Eur Heart J. 2014;35:758–64. doi: 10.1093/eurheartj/ehu029. [DOI] [PubMed] [Google Scholar]
- 15.Kucher N, Boekstegers P, Müller OJ, Kupatt C, Beyer-Westendorf J, Heitzer T, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014;129:479–86. doi: 10.1161/CIRCULATIONAHA.113.005544. [DOI] [PubMed] [Google Scholar]
- 16.Gaba RC, Gundavaram MS, Parvinian A, Knuttinen MG, Minocha J, Owens CA, et al. Efficacy and safety of flow-directed pulmonary artery catheter thrombolysis for treatment of sub-massive pulmonary embolism. AJR. 2014;202:1355–60. doi: 10.2214/AJR.13.11366. [DOI] [PubMed] [Google Scholar]
- 17.Lankeit M, Konstantinides S. Thrombolytic therapy for submassive pulmonary embolism. Best Pract Res Clin Haematol. 2012;25:379–89. doi: 10.1016/j.beha.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3033–69. doi: 10.1093/eurheartj/ehu283. [DOI] [PubMed] [Google Scholar]


