Abstract
Integrins are a large family of transmembrane heterodimeric proteins that constitute the main receptors for extracellular matrix components. Integrins were initially thought to be primarily involved in the maintenance of cell adhesion and tissue integrity. However, it is now appreciated that integrins play important roles in many other biological processes such as cell survival, proliferation, differentiation, migration, cell shape and polarity. Lung cells express numerous combinations and permutations of integrin heterodimers. The complexity and diversity of different integrin heterodimers being implicated in different lung diseases present a major challenge for drug development. Here we provide a comprehensive overview of the current knowledge of integrins from studies in cell culture to integrin knockout mouse models and provide an update of results from clinical trials for which integrins are therapeutic targets with a focus on respiratory diseases (asthma, emphysema, pneumonia, lung cancer, pulmonary fibrosis and sarcoidosis).
Keywords: Asthma, emphysema, integrins, lung cancer, lung disease, pulmonary fibrosis, sarcoidosis
Introduction
Respiratory disease is defined as any disease that could impair lung functions. There are many types of respiratory diseases which include: 1) asthma and emphysema that obstruct the airflow of the lung, 2) pneumonia resulting from bacteria and virus infection, 3) lung cancer characterized by the uncontrolled growth and spread of abnormal cells, and 4) pulmonary fibrosis and sarcoidosis which stiffen and scar the lung. Despite the current standard treatments for various respiratory diseases, they remain the third leading killer with one in six deaths in the United States [1]. An estimated 400,000 Americans die from respiratory diseases every year and diseases such as asthma are associated with substantial health impairment and work disability [1].
Integrins are a large family of transmembrane proteins that constitute the main receptors for extracellular matrix (ECM) components. Integrins were initially thought to be primarily involved in the maintenance of cell adhesion and tissue integrity. However, further studies demonstrated that integrins also influence cell survival, proliferation, differentiation, migration, shape, polarity and other biological processes [2-5].
Upon interactions with ECM proteins, integrins activate downstream signaling pathways via direct or functional association with: 1) intracellular adaptors, such as p130Cas and Grb2; 2) cytosolic tyrosine kinases, such as Src family kinase (SFK) and focal adhesion kinase (FAK); 3) growth factor receptors, such as epidermal growth factor receptor (EGFR) and platelet-derived growth factor (PDGF); and 4) cytokine receptors, such as IL-3 receptor to influence cell behaviors [6]. With these functions, integrins are implicated in many pathological processes of human respiratory diseases [7].
Here, we present an up-to-date review on the diverse biological activities of integrins with respect to their involvement in respiratory diseases, in particular asthma, emphysema, lung cancer, pneumonia, pulmonary fibrosis, and sarcoidosis.
Integrin gene family
The integrin family exists as non-covalently linked heterodimers of α- and β-subunits. There are 18 α and 8 β subunits encoded by the human genome. To date, 24 functional integrin receptors have been described via various combinations of the α and β subunits [8]. The ligand binding properties of integrins are defined by the α subunit, while the downstream signaling events are thought to be defined by the β subunit [9]. Many α subunits associate with only a single β subunit, while some α subunits associate with more than one β subunit (for example, αv may assemble with subunit β1, β3, β5, β6 and β8). Some integrins are able to recognize several ECM proteins, while others bind to only one type of ligand. Although in vitro studies have demonstrated considerable functional overlap among the integrin heterodimers, inactivation of individual integrins in mice has produced unique phenotypes (as discussed later). This suggests that the large number of integrins supports an array of unique functions.
Integrins are transmembrane receptors that mediate bi-directional signals through the cell membrane. On the cell surface, integrins exist in inactive, low affinity conformation states. With exception, Minagawa and colleagues showed that integrin αvβ8 adopts a constitutively active, extended-closed headpiece conformation on the cell surface by hydrodynamic, mutational and electron microscopy methods and thus does not fit the conventional models of integrin activation [10]. It is believed that the function of integrin αvβ8 is modulated by the metalloproteolytic cleavage of latent TGF-β [11]. Integrins can signal through the cell membrane in both directions: inside-out signaling and outside-in signaling. The extracellular binding activity of integrins is regulated from the inside of the cell (inside-out signaling). Switching of affinity state of integrins to an active conformation allows for inside-out signaling. In contrast, the signals that are transmitted into the cells are elicited by the binding of ECM proteins on integrins (outside-in signaling) [12]. It is through these signaling activation events that integrins regulate cell attachment, survival, proliferation, cell spreading, differentiation, cytoskeleton organization, cell shape, cell migration, gene expression, tumorigenicity, intracellular pH, and increase in concentration of cytosolic Ca2+ [13]. To activate downstream signaling pathways, integrins assemble signaling complexes termed “integrin adhesome” [14]. There are up to 156 distinct proteins in the integrin adhesome, some of which are fundamental to the adhesion site, while others only transiently associate. The integrin adhesome-associated proteins include talin, paxillin, filamin, integrin-linked kinase (ILK), FAK, p130Cas, SFK and GTPases of the Rho family [14-17].
One of the key signaling events upon integrin ligation is the activation of FAK. FAK is a non-receptor tyrosine kinase with SH2 domain binding sites. Upon integrin activation, integrins cluster and autophosphorylate at position tyrosine 397 (Y397). FAK binds to this phosphorylated site and recruits other proteins containing the SH2 domain. This includes the binding of SFK, leading to increased activation of the FAK-SFK complex which further phosphorylates downstream signaling players. One of the downstream targets of FAK is the Rho family of GTPases, which consists of Rac, Cdc42 and RhoA [18, 19]. Activation of Rac, Cdc42 and Rho A is critical for the organization of the actin cytoskeleton and for the recruitment of other signaling molecules to the focal adhesion site. FAK activation also recruits phosphatidylinositol-3-kinase (PI3K), leading to the activation of Akt [5, 20]. The PI3K-Akt pathway mediates survival signaling via increased Bcl-2 protein expression. In addition, FAK represents a crosstalk point with the growth factor receptor pathway [21]. Studies have shown that the activation of MEK1 and Raf1 via integrin mediated FAK-Src signaling is required for MAPK activation [22, 23]. This suggests that the integrin and growth factor receptor signaling may be integrated via the Ras-Raf-MEK-MAPK pathway to regulate key cellular functions such as cell proliferation.
Integrin expression in the lung
Integrins are expressed in various lung cell types and they may play important roles in regulating lung development. These include branching morphogenesis, epithelial cell polarization and differentiation. To determine the role of specific integrins in lung development, various studies have been done to investigate the expression patterns of integrins in foetal, human and murine lung developments [24-27].
Eight different integrins which include α2β1, α3β1, α5β1, α6β4, α9β1, αvβ5, αvβ6 and αvβ8 are found to be expressed on human airway epithelial cells [28-33]. The expression patterns of these integrins in epithelial cells are different. For example, integrins α3 and α6 are diffusely expressed across the epithelial cells but integrin α2 is only found to be expressed on the branching tips of the cells [27]. Although epithelial cells express integrins α3 and α6, the subcellular distribution of these integrins are developmentally regulated. Young epithelial cells express integrins α3 and α6 pericellularly. As epithelial cells mature, they express integrins α3 and α6 basally [27, 34-36]. This suggests that integrins α3 and α6 might play an important role in lung epithelial maturation, development and basement membrane organization, whereas integrin α2 may be responsible for assembly and extension of epithelial tubule into mesenchyme in branching morphogeneisis. Integrins α2β1 and α3β1 are thought to play key roles in homotypic cell-cell interaction in the epithelium [37-39]. However, the significance of this finding is uncertain since other studies have shown that there are no defects in epithelial cell-cell interactions in either integrin α2- or α3-null mice [40-42]. The integrin α6β4 is found to be important for the maintenance of epithelial integrity. This is evident by integrin α6- or β4-null mice which die soon after birth due to severe skin blistering [43, 44]. The other integrins (α5β1, α9β1 and αvβ5) are not expressed in healthy human epithelial cells but are rapidly induced on these cells upon lung inflammation and injury [11, 32, 45, 46]. Thus, these integrins serve as detectors to allow the lung cells to sense and respond to injury and inflammation in the lung.
Integrins such as α1β1, α2β1, α5β1 and αvβ3 are expressed on lung endothelial cells. Wu and colleagues demonstrated that integrin α1 is stained positively on the airway endothelial cells and large blood vessels while integrin α2 is present on lung mesenchymal cells which mainly constitute the endothelium [27]. Another study showed that integrins α5β1 and αvβ3 are expressed on the mesenchyme of the embryonic lungs [47]. This is supported by another study showing that integrin αvβ3 is extensively evident in the endothelium of alveolar micro-vessels and vascular smooth muscle [48]. Taken together, these studies suggest that the integrins expressed on lung endothelial cells may regulate blood vessel formation and microvascular permeability.
Other integrins such as α2β1, α4β1, α5β1, and α11 are expressed on lung fibroblasts, whilst integrins α5β1, α7β1 and αvβ5 are expressed on airway smooth muscle. Integrins α4β1, α5β1, αEβ7 and β2 are expressed on respiratory T lymphocytes and leukocytes [7, 49-56]. Collectively, all these studies demonstrate that there are differential expression of integrins during lung development and dysregulation of their expression in the lungs may contribute to respiratory diseases.
Integrins and their association with respiratory diseases
Table 1 summarizes the distribution of integrins in various lung cell types and their association with respiratory diseases. Fig. (1) illustrates integrin expressions in various respiratory diseases.
Table 1.
Distribution of integrins in the lungs and their association with respiratory diseases.
| Disease | Integrin | ECM | Cell Type | Remarks | Ref | ||||
|---|---|---|---|---|---|---|---|---|---|
| Asthma | α1β1 | Collagen | Endothelial cell | * Activation of α1β1 and α2β1 on endothelial cells is essential for VEGF-induced angiogenesis in vivo. * Successful early vasculogenesis and angiogenesis require α5β1 integrin-fibronectin interactions in vivo and in vitro. * αvβ3 and αvβ5 are involved in angiogenesis of the lung microvascular bed. |
[57, 108, 130-134] | ||||
| α2β1 | Collagen | ||||||||
| α5β1 | Fibronectin | ||||||||
| αvβ3 | Collagen; Fibronectin; Vitronectin |
||||||||
| αvβ5 | Collagen; Fibronectin; Vitronectin |
||||||||
| α5β1 | Collagen; Fibronectin; Laminin |
Airway smooth muscle | * Airway smooth muscle cells receive strong survival signals from fibronectin, laminin and collagen in vitro. * α5β1 and α7β1 are the transducer of survival signals from ECM. * Airway smooth muscle cells express αvβ5 * αvβ5 contributes to asthmatic airway remodeling via TGFβ activation |
[47, 56, 62] | |||||
| α7β1 | Laminin | ||||||||
| αvβ5 | Collagen; Fibronectin; Vitronectin |
||||||||
| α4β1 | VCAM-1; ICAM-4; Osteopontin |
Eosinophil; Lymphocyte; Monocyte; Macrophage; Basophil; Mast cell |
* Eosinophils transmigrate across VCAM-1-expressing human pulmonary microvascular endothelial cells via α4β1 in vitro. * Osteopontin promotes leukocyte adhesion via α4β1 in vitro. * α4β1 expression correlates with the severity of asthma. |
[61, 116-118] | |||||
| αEβ7 | E-cadherin | T lymphocyte | * Expression is enhanced on lymphocytes in bronchoalveolar lavage fluid of asthma patients. | [55] | |||||
| Emphysema | α9β1; αvβ3; αvβ5 |
Fibulin-5 | Elastic fiber | * Fibulin-5 knockout mice exhibit disorganized elastic fiber system throughout the body. * Fibulin-5 knockout mice have tortuous aorta, severe emphysema and loose skin. * Fibulin-5 and its integrin partners stabilize and organize elastic fiber in the skin, lung and vasculature. |
[135] | ||||
| αvβ6 | TGFβ | Epithelial cell | * β6 knockout mice have marked induction of macrophage metalloelastase (MMP12) that degrades elastin. * β6-null mice develop age-dependent emphysema. * Loss of αvβ6 mediated activation of latent TGFβ leads to age-dependent pulmonary emphysema via alterations of Mmp12 expression. * Functional alteration in the TGFβ activation pathway affects susceptibility to emphysema. |
[64, 65] | |||||
| Epithelial injury and lung wound repair | α2β1; α3β1; α5β1; α6β1; αv; β5; β6 |
Laminins; Collagen; Fibronectin; Vitronectin; Fibrinogen |
Epithelial cell | * Increased expression of αv, β5, β6 and α5 is observed at the edges of surface epithelial wounds in an in vivo xenograft model of human bronchial epithelium. * Similar up-regulation of αv, β5 and β6 is also seen in areas of undifferentiated airway from cystic fibrotic patients. * Integrins mediate wound repair on different constitutive ECM in a cultured human airway epithelial cell line. * β1 is required for epithelial cell migration and spreading for wound closure. |
[32, 136] | ||||
| Disease | Integrin | ECM | Cell Type | Remarks | Ref | ||||
| NSCLC | α1 | Collagen | Epithelial cell | * KrasLA2/α1-null mice have decreased incidence of primary lung tumors and longer survival compared with KrasLA2/α1-wildtype mice. * Tumors are smaller, less vascularized, reduced cell proliferation and increased apoptosis. * α1β1 is required to drive the growth of Kras-induced NSCLC. |
[58] | ||||
| α5β1 | Fibronectin | Epithelial cell | * Lung cancer cell lines express high levels of α5. * Node negative NSCLC samples have α5 over-expression. * α5 expression is associated with the differentiation status of the cancer cells and the age of the patients. * The overall survival rate for patients with node-negative and over-expressed α5 NSCLS is lower than patients with tumour of normal α5 expression. * α5β1 over-expression and the lost expression of collagen matrices correlate with laminin metastasis of NSCLC. * Tumor cell survival and invasiveness are promoted by the enhanced expression of α5β1 in NSCLC. |
[63, 137] | |||||
| α11 | Collagen; Fibroblast |
Stromal fibroblast | * Interaction between the collagen matrix and stromal fibroblast via α11 regulates IGF2 expression. * IGF2 enhances NSCLC cell growth. |
[52] | |||||
| αvβ3 | Osteopontin | Epithelial cell | * Promotes lung cancer progression and metastasis by boosting the α5β1-EGFR signaling. | [138] | |||||
| αvβ6 | Fibronectin | Epithelial cell | * Enhances the ability of tumor cells to adhere, migrate, and invade the fibronectin-rich matrix that surrounds NSCLC cells. * Activates the release of active TGFβ from ECM to promote tumor progression and invasion. |
[66, 67] | |||||
| Pneumonia | αEβ7 | E-cadherin | T lymphocyte | * Expression is enhanced on lymphocytes in bronchoalveolar lavage fluid in patients. | [55] | ||||
| β2 | VCAM; ICAM |
Leukocyte | * Synergises with selectin on neutrophil adhesion to endothelial cell. * Aids in extravasation of leukocytes across the vascular endothelium. |
[7] | |||||
| Pulmonary fibrosis | α4β1 | VCAM; ICAM |
Fibroblast | * Loss of α4β1 signaling via PTEN results in a migratory/invasive phenotype of fibrotic lung fibroblasts. | [51] | ||||
| α5β1 | Fibronectin | Fibroblast | * α5β1-fibronectin signaling is required and sufficient to induce lung fibroblast migration/invasion across basement membrane | [51] | |||||
| Pneumocyte; Endothelial cell; Mesenchymal cell; Fibroblast; Myofibroblast |
* α5β1 staining is apparent mainly on fibroblasts and differentiated myofibroblasts after 7 days of bleomycin treatment. * α5β1 plays a key role in the activation, proliferation, differentiation and increased ECM synthesis of these cells during pulmonary fibrogenesis. |
[139] | |||||||
| Disease | Integrin | ECM | Cell Type | Remarks | Ref | ||||
| αvβ6 | Collagen; Fibronectin |
Pneumocyte | * αvβ6 is over-expressed in penumocytes lining the alveolar ducts and alveoli of human lung fibrosis. * Partial inhibition of αvβ6 activation via a blocking antibody inhibits murine TGFβ-mediated pulmonary fibrosis without aggravating inflammation. * αvβ6, an activator of TGFβ, is involved in the pathophysiology of human pulmonary fibrosis. |
[68, 69, 89] | |||||
| αEβ7 | E-cadherin | T lymphocyte | * Expression is enhanced on lymphocytes in bronchoalveolar lavage fluid in patients. | [55] | |||||
| Sarcoidosis | α4β1 | VCAM; ICAM |
Alveolar T lymphocyte; Granuloma lymphocyte |
* α4β1 is over-expressed in human lymphocytes during the active phase of the disease. * Interaction between VCAM-1 and α4β1 is important for the extravasation of lymphocytes to the inflammatory site within the lung. |
[49] | ||||
| α5β1 | Fibronectin | Alveolar T lymphocyte; Granuloma lymphocyte; Epitheloid cell; Fibroblast |
* α5β1 is over-expressed in human lymphocytes, epitheloid cells and fibroblasts during the active phase of the disease. * Interaction is important for cell proliferation, cytokine production, and cell differentiation. * α5β1-fibronectin binding is important for adhesion of leukocytes to the endothelial cells, to migrate into inflammatory sites and to remain at the affected sites via ECM binding. |
[49, 50] | |||||
| α1β1 | Collagen | Alveolar T lymphocyte | * α1β1 is over-expressed in human lymphocytes during the active phase of the disease. * The chronically stimulated lung T cells are compartmentalized on the alveolar epithelial surface and are gradually exchanged with the systemic immune system via α1β1 integrin. |
[49, 140] | |||||
| α6β1 | Laminin | Granuloma lymphocyte | * α6β1 is over-expressed in human lymphocytes during the active phase of the disease. | [49] | |||||
| α2β1 | Collagens | Fibroblast | * α2β1 is over-expressed in human lymphocytes during the active phase of the disease. | [49, 50] | |||||
Fig. (1).
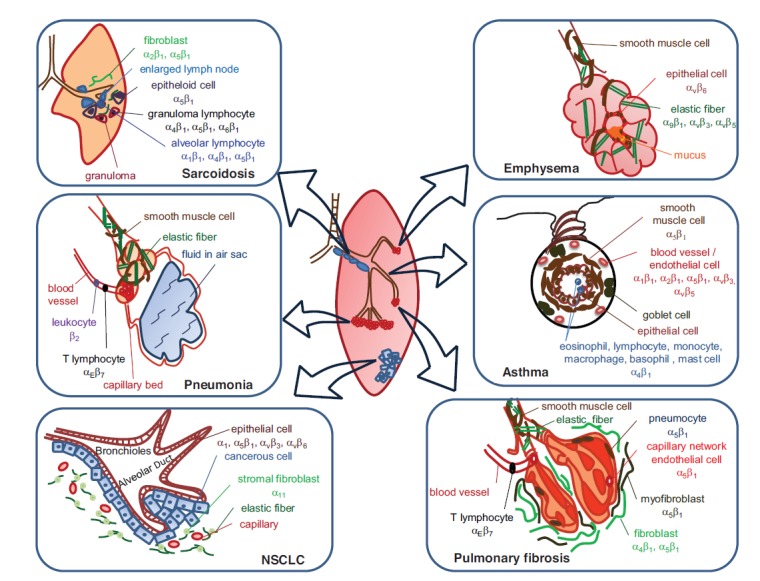
Integrin expressions in various respiratory diseases.
INTEGRIN α1β1 IN ANGIOGENESIS
The integrin α1β1 plays a key role in angiogenesis (formation of new blood vessels) and its expression is elevated in asthma, non-small-cell lung cancer (NSCLC) and sarcoidosis [57-59]. In asthma, angiogenesis constitutes one of the structural changes seen in airway wall remodeling. Activation of integrin α1β1 on endothelial cells is essential for VEGF-induced angiogenesis within the airway [57], thus promoting airway wall remodeling in asthmatic subjects. In NSCLC, the tumors are smaller and less vascularised in KrasLA2/α1-null mice when compared with KrasLA2/ α1 wild-type mice [58]. This suggests that the integrin α1β1 is involved in the growth of Kras-induced NSCLC, potentially by promoting angiogenesis.
INTEGRIN α4β1 IN INFLAMMATION
The integrin α4β1 is expressed on cells in inflammatory diseases such as asthma, sarcoidosis and pulmonary fibrosis. Accumulation of leukocytes, mainly eosinophils in the airway is one of the characteristic features of asthma [60]. The integrin α4β1-expressing eosinophils are able to transmigrate across human pulmonary microvascular VCAM-1-expressing endothelial cells [61]. Sarcoidosis is a chronic lung inflammation characterized by the accumulation of lymphocytes in the lung [7]. The over-expression of integrin α4β1 in human lymphocytes during sarcoidosis aids lymphocyte extravasation to the site of inflammation within the lung [49]. Collectively, these studies suggest that the integrin α4β1 plays an essential role in the recruitment of pro-inflammatory cells to the lung, contributing to inflammatory diseases.
INTEGRIN α5β1 IN SURVIVAL AND ADHESION/ MIGRATION
The integrin α5β1 is associated with asthma, epithelial injury and repair, NSCLC, pulmonary fibrosis and sarcoidosis due to its diverse roles in cell signaling. It primarily regulates the survival and adhesion/ migration signaling pathways. The expression of
integrin α5β1 and its ECM ligand fibronectin are increased in the asthmatic airways [62]. Thus, the integrin mediated survival signaling from ECM proteins may be one of the mechanisms underlying the increased bulk of smooth muscle cells in airway wall remodeling [53]. In epithelial injury and repair, integrin α5β1 is required for epithelial cell migration and spreading for wound closure [32]. In NSCLC, increased expression of integrin α5β1 enhanced tumor cell survival and invasiveness on disrupted collagen matrix [63]. During pulmonary fibrosis, increased numbers of fibroblasts have been attributed to the migration/i nvasion of lung fibroblasts across the basement membrane to the site of wound via integrin α5β1-fibronectin signaling, leading to scarring of the lung [51]. In sarcoidosis, the integrin α5β1-fibronectin signaling is required for lymphocyte adhesion to the endothelial cells and their subsequent migration to the site of inflammation [49, 50]. Taken together, these studies demonstrate that the integrin α5β1 is essential for the survival and adhesion/migration signaling of various cells in respiratory diseases.
INTEGRIN αVβ6 IN TGFβ SIGNALING
The integrin αvβ6 mediates emphysema, NSCLC, pulmonary fibrosis, acute lung injury, influenza infection, epithelial injury and repair due to its ability to activate the TGFβ signaling pathway. Studies on integrin β6 knockout mice suggest that integrin αvβ6 may play a role in emphysema [64, 65]. Loss of integrin αvβ6 leads to the loss of latent TGFβ activation. Since TGFβ inhibits macrophage metalloelastase (MMP12), there is also a marked induction of MMP12 that degrades elastin. Therefore, integrin α6-null mice eventually develop age-dependent emphysema. In NSCLC, integrin αvβ6 activates the release of active TGFβ from the ECM to promote tumor progression and invasion [66, 67]. In patients with pulmonary fibrosis, integrin αvβ6 is over-expressed in pneumocytes lining the alveolar ducts and alveoli [68]. Additionally, integrin αvβ6 are dramatically upregulated in murine-model of bleomycin-induced pulmonary fibrosis [69]. The lungs of bleomycin-induced pulmonary fibrosis mice contained higher percentage and greater intensity of integrin αvβ6-positive epithelial cells than the saline-treated mice [69]. The use of a blocking antibody further revealed that the partial inhibition of integrin αvβ6 activation inhibits murine TGFβ-mediated pulmonary fibrosis [68]. Jenkins and colleagues demonstrated that protease-activated receptor 1 activates TGFβ in an integrin αvβ6-dependent manner via RhoA and Rho kinase signaling [70]. Another group while using lysophosphatidic acid to induce integrin αvβ6-mediated activation of TGFβ showed that Gαq is an important link between integrin αvβ6 to RhoA and Rho kinase signaling [71]. This pathway is a critical step in the development of acute lung injury and fibrosis [70, 71]. Jolly and colleagues also demonstrated that the influenza virus infection led to increased integrin αvβ6-mediated TGFβ activation. The functional consequence of this is epithelial cell apoptosis and increased collagen deposition, which are critical steps in exacerbations of pulmonary fibrosis [72, 73]. Collectively, these studies demonstrate that the integrin αvβ6 is an activator of TGFβ and that functional alterations in the TGFβ signaling pathway increase the susceptibility to emphysema, NSCLC and pulmonary fibrosis.
Integrin 71 in airway wall remodeling
Airway wall remodeling is a prominent feature of asthma that contributes to chronic symptoms [74-76]. Airway wall remodeling is defined by a number of structural changes including increased mass of contractile airway smooth muscle cells, and fibrosis resulting from the accumulation of ECM proteins such as laminin. Increased expression of laminin is associated with asthma [77]. Our laboratory has recently demonstrated that the integrin 71 and its ECM protein laminin maintain and regulate contractile ASM phenotype and cell survival. This contributes to airway hyperresponsiveness and supports the enlargement of muscle bundles encircling the airways, a critical feature of airway wall remodeling [75]. Findings from our study suggests that targeting the laminin-integrin 71 signaling axis may offer new avenues for the development of therapies to reduce dysfunctions associated with contractile ASM cells in asthmatic patients.
INTEGRIN αEβ7 IN RESPIRATORY DISEASES
The expression of the integrin αEβ7 is enhanced on lymphocytes in the bronchoalveolar lavage fluid of patients with asthma, pneumonia and pulmonary fibrosis [55]. The integrin αEβ7 is expressed only on lymphocytes of the intraepithelial phenotype [78, 79]. The only ligand for this integrin is the epithelial E-cadherin. The precise role of integrin αEβ7 in these respiratory diseases is yet unknown but it has been suggested to be involved in the selective retention of lymphocytes in mucosal tissues of the lung during disease progression [55]. Further study in this area is warranted.
Matricellular proteins and their association with integrins in respiratory diseases
Thrombospondin (TSP-1)
TSP-1 is a major constituent of human blood platelets and it functions at the cell surface to bring together membrane proteins and cytokines that regulate the ECM and cellular phenotype [80]. TSP-1 interacts with integrins and is involved in respiratory diseases such as pneumonia, small cell lung carcinoma (SCLC) and pulmonary fibrosis.
Abnormalities were observed in the lungs of TSP-1 deficient mice. There is increased inflammatory cell infiltrates, fibroblastic and epithelial cell hyperplasia and matrix deposition in the lungs of these mice, and hemosiderein-laden macrophages were observed suggesting diffuse alveolar hemorrhage [81]. TSP-1 binds to integrins α4β1 and α5β1 in activated T-cell adhesion [82]. The binding of TSP-1 to integrins may aid in neutrophil adhesion and migration, monocytes chemotaxis, haptotaxis, and diapedesis [83-85]. The aberrant inflammation in TSP-1 deficient mice could be due to defects in recruitment and clearance of inflammatory cells via integrins. This results in pneumonia in the lungs of TSP-1 deficient mice, suggesting that TSP-1 is required in normal lung homoestasis [81].
In SCLC, the adhesion of cancer cells to TSP-1 was inhibited by heparin (integrin α3β1 inhibitor) [86]. The adhesion of SCLC cells to TSP-1 via integrin α3β1 induced neurite-like outgrowth and inhibited SCLC proliferation [86]. The TSP-1-integrin α3β1-induced neurotypic differentiation of SCLC is further enhanced by epidermal growth factor (EGF), suggesting that TSP-1 and EGF work synergistically to induce differentiation of SCLC via integrin α3β1 [86].
TSP-1 deficient mice are not protected from bleomycin-induced pulmonary fibrosis. These mice manifested higher expression of connective tissue growth factor and collagen deposition compared with wild type control mice [87]. This suggests that TSP-1 may basally suppress connective tissue growth factor and collagen expression. Moreover, TSP-1 deficiency appears to worsen pulmonary fibrosis in response to bleomycin. As TGFβ is involved in pulmonary fibrosis [88] and integrin αvβ6 mediates TGFβ activation [89], TSP-1 may mediate the integrin α6β1-TGFβ activation in the pathogenesis of pulmonary fibrosis.
SPARC
Secreted Protein Acidic and Rich in Cysteine (SPARC) is a matricellular protein that mediates tissue repair and wound healing, and is a known target of TGFβ [90]. SPARC interacts with integrins and is involved in pulmonary fibrosis.
Immunohistochemical staining revealed that SPARC was observed in fibroblast of Masson bodies, fibroblast foci and interstitial fibroblast in pulmonary fibrosis [91]. SPARC binds to collagen III but not fibronectin [92]. Fibroblasts in the interstitial compartment of alveolar walls (collagen III rich) migrate to exudates in alveoli (fibronectin rich) during pulmonary fibrosis. Taken together, this suggests that SPARC may function early in pulmonary fibrosis by aiding fibroblast migration. SPARC regulates the production of integrin in an integrin-linked kinase-dependent manner [93-95]. Barker and colleagues also demonstrated that SPARC modulates integrin-linked kinase activity in fibroblasts [96]. This suggests that migration of fibroblasts from alveolar walls to exudates in alveoli by SPARC may be integrin-dependent. Chang and colleagues showed that fibroblasts in pulmonary fibrosis have higher survival rate [97]. This may be attributed to elevated levels of SPARC which activates the PI3-Akt pathway via integrin, thus leading to glycogen synthase kinase-3β (GSK-3β) inhibition and β-catenin activation. β-catenin activation increased expression of plasminogen activator inhibitor-1 (PAI-1) which suppresses fibroblast apoptosis in pulmonary fibrosis. Therefore, the inhibition of SPARC-induced β-catenin signaling pathway may be a novel therapeutic target for pulmonary fibrosis [97].
Periostin
Periostin is an extracellular matrix protein induced by IL-4 and IL-13 in airway epithelial cells [98]. Its expression is also induced by TGFβ in fibroblasts [98]. Periostin interacts with integrins to mediate allergic lung inflammation, asthma, NSCLC and pulmonary fibrosis.
Allergen-challenged periostin-deficient mice developed reduced eosinophil infiltration in the lung compared with wild type mice [99]. This suggests a direct role of periostin in promoting allergen-induced eosinophil infiltration into the allergic inflammatory lungs. Recently, Takayama and colleagues demonstrated an upregulation of periostin in the lung epithelial cells by IL-13 and in the lungs of allergen-challenged wild-type mice [98]. Furthermore, periostin binds to integrins αvβ3 and αvβ5 to mediate fibroblast or malignant cell migration [100]. This suggests that the integrins expressed on eosinophils may bind to periostin on lung epithelial cells and thereby facilitate eosinophil recruitment to the lung of allergen-challenged mice.
In asthma, periostin deficiency leads to an increase in airway resistance and mucus production [101]. Allergen sensitization and challenge in periostin deficient mice had significantly higher PAS-staining index and Muc5ac and Gob5 expression compared with wild-type mice. This suggests that periostin attenuates mucus production that leads to airway resistance in asthma. Given that periostin binds to integrins αvβ3 and αvβ5 in cancer cells for adhesion and migration [100], it may also bind to integrins α4 and β1/2 which are involved in asthma development and thus downregulate mucus production by repressing mucus production genes such as NF-B. SP-1, and AP-1 [102]. The secretion of periostin is increased in lung fibroblasts in response to IL-4 and IL-13 stimulation, which are novel components of subepithelial fibrosis in bronchial asthma [98]. Secreted periostin binds ECM proteins such as fibronection, tenascin-C and collagen V [98]. Since the ECM proteins induce downstream signaling pathways via integrins, periostin-bound ECM proteins could potentially activate integrins, contributing to features of asthma.
In NSCLC, chemical hypoxia mimicking reagents increase periostin expression. The hypoxia responsive genes TGFα and bFGF induce the upregulation of periostin that in turn activates RTK/PI3K signaling cascade [103]. This signaling cascade confers survival of NSCLC. Bao and colleagues showed that periostin binds to integrin αvβ3 to promote cancer cell metastasis by activating the PI3K/Akt survival signaling pathway [104]. This suggests that periostin may interact with integrins to promote NSCLC survival via PI3K/Akt pathway.
In pulmonary fibrosis, periostin levels are elevated. Immunohistochemical staining showed that periostin stained mainly in areas of active fibrosis in the lung [105]. Okamoto and colleagues detected increased periostin levels in the lung of pulmonary fibrosis patients compared with healthy controls [106]. Periostin knockout mice were also protected from bleomycin-induced pulmonary fibrosis [107]. Collectively, this suggests an important role of periostin in pulmonary fibrosis. Naik and colleagues demonstrated that TGFβ upregulates periostin expression in mesenchymal cells. Periostin in turn regulates ECM protein deposition, mesenchymal cell proliferation and wound closure, which are features of pulmonary fibrosis [105]. This suggests that TGFβ-induced periostin play an important role in the late stages of pulmonary fibrosis. Importantly, when OC-20, an antibody that prevents periostin from binding to integrin, was administered 10 days post-bleomycin treatment in wild type mice, it significantly reduced bleomycin-associated fibrosis [105]. This suggests that periostin binds to integrin to mediate pulmonary fibrosis in the lung.
Phenotypes of integrin knockout mice in relation to the lung
The availability of integrin knockout mice via gene targeting technology has increased our understanding of integrin functions. It reveals the unique functions each integrin have in the lung in vivo, whereby the deletion of a specific integrin results in distinctive phenotypes not shared by null mutations of other integrins. This is in contrast to the overlapping functions of integrins demonstrated in vitro. Table 2 summarizes the phenotypes of integrin knockout mice in relation to the lung.
Table 2.
Effect of integrin knockout in the lung development of mice.
| Integrin Knockout | Phenotype of Mice | Impact on Lung Development | Ref |
|---|---|---|---|
| α1 | * Viable and fertile. * No overt phenotype. Vasculature * Increased collagen synthesis. * Increased Mmp7 and Mmp9 synthesis. * Increased plasma levels of angiostatin. * Implanted tumors show reduced vascularization. Skin * Embryonic fibroblasts fail to spread and migrate on collagen IV and laminin in vitro. * Hypocellular dermis. * Reduced embryonic dermal fibroblast proliferation. |
* Isolated α1-null endothelial cells from the lung show reduced proliferation on substrata. * Role of α1 integrin in agiogenesis is not restricted to tumor endothelial cells only. |
[108, 141-143] |
| α2 | * Viable, normal development and fertile. * No obvious anatomical or histological differences. Vasculature * Display partially defective platelet adhesion to collagen. * Platelets aggregate very slowly in the presence of collagen. * No bleeding anomalies. Mammary Gland * Defects in branching morphogenesis of mammary gland. Immunity * Normal number of mast cells. * Do not support mast cell-mediated inflammatory responses. * Defects in innate immune response to Listeria monocytogenes. |
* No abnormalities. | [144-147] |
| α3 | * Survive to birth but die during the neonatal period. Kidney * Defective branching of the medullary collecting duct despite normal number of nephrons. * Proximal tubule epithelial cells contain excess lysosomes and exhibit microcystic. * Disorganized glomerular basement membrane. Skin * Disorganized basement membrane of the skin. * Skin blistering at the dermal-epidermal junction. Brain * Abnormal layering of the cerebral cortex. * Developing cerebral cortex shows adhesive preference switch from glial cells to neuronal tissues. |
* Decreased bronchus branching. * Large bronchi extend into the periphery. * Reduced maturation of distal bronchiolar. * α3 is important in regulating basement membrane organization and branching morphogenesis. |
[109-111, 148] |
| α4 | * Embryonically lethal. Placenta * Defects in chorroallantois fusion during placental development. Heart * Abnormal development of the cardiac epicardium. * Lack coronary vessels. * Cardiac hemorrhage. Face * Abnormalities in the cranial and facial structure. Lymphatic system * Defects in lymphoid and myeloid lineage development. * Defects in lymphocyte homing to Peyer’s patches. |
* Not determined. | [149-151] |
| Integrin Knockout | Phenotype of Mice | Impact on Lung Development | Ref |
| α5 | * Embryonically lethal. Embroyonic Development * Defects in extraembryonic and embryonic vasculature. * Defects in posterior trunk and yolk sac mesodermal structures. Vasculature * Severe vascular defect. Skeletal Muscle * Develop typical alterations resembling muscular dystrophy. * Reduced adhesion and survival of myoblasts. |
* Signs of thorax muscle degeneration. * α5 maintains muscle integrity. |
[152-155] |
| α6 | * Die shortly after birth. Brain * Abnormal laminar organization of the cerebral cortex. * Ectopic neuroblastic outgrowth on the brain surface. * Altered laminin deposition in the mutant brains. Eye * Abnormal laminar organization of the eye. * Ectopic neuroblastic outgrowth on the eye. Skin * Severe skin blistering. |
* No abnormalities. | [112] |
| α7 | * Viable and fertile. Skeletal Muscle * Develop symptoms of progressive muscular dystrophy soon after birth. * Severe disruption of the myotendinous junctions. Heart * Congenital myopathies. Central Nervous System * Delayed motor milestones. * Impaired axonal regeneration. |
* Not determined. | [156, 157] |
| α8 | * Die soon after birth. Kidney * Profound deficits in kidney morphogenesis. * Reduced branching and growth of the uretic bud. * Mesenchymal cells fail to be recruited into the kidney epithelial structures. Ear * Lacked / malformed stereocilia on hair cells in the utricle. * Difficulty in balancing due to structural defects of the inner ear. |
* Not determined. | [158, 159] |
| α9 | * Born alive but develop bilateral chylothorax. * Despite temporal and spatial expression of α9 on nonlymphatic tissues (epithelium, airway and gut smooth muscle, choroid plexus and liver) during development, its expression was not altered. Bone * Severe neutropenia due to the loss of α9 in bone marrow cells. Lymphatic system * Develop edema and lymphocytic infiltration in the chest wall. * Abnormality in lymphatic development. |
* Respiratory failure caused by an accumulation of large volumes of pleural fluid. * α9 plays a critical role in development of the thoracic duct and other lymphatic vessels. |
[114] |
| Integrin Knockout | Phenotype of Mice | Impact on Lung Development | Ref |
| α10 | * Not reported | ||
| αv | * Embryonically lethal. * Conditional knockout of αv on myofibroblasts in multiple organs Organs * Conditional knockout of αv on myofibroblasts in liver, lung and kidney inhibited fibrosis in these organs Vasculature * Defects in placenta, central nervous system, and gastrointestinal blood vessels. * Exhibit intracerebral and intestinal hemorrhages. * Extensive vasculogenesis and angiogenesis in knockout mice despite the role of αv integrin in vascular development. Mouth * Cleft palates. |
* Not determined. | [160, 161] |
| αL |
Immunity * Impaired lymphocyte recirculation and homotypic interactions. * Reduced leukocyte proliferation in response to mixed lymphocyte reaction and growth factor in vitro despite normal cytotoxic T cell responses. * No rejection of immunogenic tumors grafted into footpads. * No priming responses towards tumor-specific antigen. * Impaired induction of peripheral immune responses but respond to systemic infection. |
* Not determined. | [162-164] |
| αM | * Obesity. Immunity * Impaired phagocytosis, polymorphonuclear leucocytes (PMN) apoptosis, and mast cell development. |
* Not determined. | [165-167] |
| αE |
Immunity * Reduced number of intestinal and vaginal intraepithelial lymphocytes. * Diminished lamina propia T lymphocytes numbers. * Number of T lymphocytes is not altered in Peyer’s patch and spleen. |
* Peribronchial and intrapulmonary T lymphocytes numbers are not reduced. * αE plays a role in generating and maintaining the gut and vaginal T lymphocytes (but not in the lungs). |
[168] |
| αIIb |
Vasculature * Defective platelet function caused by the failure of platelets to bind fibrinogen, to aggregate and to retract fibrin clot. * Lack of fibrinogen in platelet granules. * Increased rebleeding tendency similar to Glanzmann thrombasthenia in humans. |
* Not determined. | [169] |
| αX | * Not reported. | ||
| αD | * Not reported. | ||
| β1 | * Peri-implantation lethality. Embroyonic Development * Inner cell mass deterioration due to the lost of β1 integrin-mediated survival signaling. Heart * Cardiac muscle in the heart becomes smaller and degenerates. * Alterations in the sarcomeric architecture. * Hypertrophic changes in the heart. * Display replacement fibrosis - the formation of fibrous tissues at sites where the cells are atrophied, degenerated and necrotic. Bone * Impaired membranous bone formation, characterized by decreased cortical bone formation and reduced bone mass in cortical and flat bones. |
* Not determined. | [170-174] |
| Integrin Knockout | Phenotype of Mice | Impact on Lung Development | Ref |
| β2 |
Immunity * Impaired leukocyte recruitment. * Develop chronic dermatitis with extensive erosion on face and submandibular region. * Increased neutrophil counts, elevated immunoglobulin levels, splenomegaly, lymphadenopathy and abundant plasma cells in skin, gut, kidney and lymph nodes. * Severe defect in T cell proliferation. * Reduced neutrophil extravasation into infected tissues during irritant dermatitis. |
* In pneumonia, the neutrophil emigration in lung section is not reduced compared to wild type mice. * β2 is essential only for dermal neutrophil emigration during skin infection. * Reduced number of dendritic cells in the lung, alveolar wall, large and small airways. * β2 is required for dendritic cell migration into the lung. |
[175-177] |
| β3 | * Viable and fertile. Vasculature * Display cardinal features of Glanzmann thrombasthenia - defective platelet aggregation, impaired clot retraction and increased rebleeding tendency. * Reduced survival and anemia due to postnatal hemorrhage. Placenta * Placental defects leading to fetal mortality despite normal implantation. Bone * Develop osteosclerosis - increased bone mass with age. * Contain more osteoclasts despite increased bone mass. However, osteoclasts are dysfunctional as evidenced by significant hypocalcemia in knockout mice. |
* Not determined. | [178-180] |
| β4 | * Die shortly after birth. * α6 subunit significantly downregulated. Skin * Display pathological phenotype similar to human junctional epidermolysis bullosa. * Skin blistering with severe epidermis and squamous epithelial detachment. * Reduced skin adhesive properties due to the absence of hemidesmosomes. * Epithelial tissues show signs of degeneration and disorganization. |
* Not determined. | [44, 181] |
| β5 | * Develop, grow and reproduce normally. Skin * Harvested keratinocytes demonstrate defects in migration and adhesion on vitronectin in vitro. However, cutaneous wound healing is the same as wild type mice. Eye * Age-related blindness. |
* Protection from increased vascular permeability in a model of acute lung injury. * β5 from pulmonary endothelial cells regulates vascular permeability in acute lung injury. * β5 deletion did not affect adenovirus infection. |
[182] |
| β6 |
Skin * Skin inflammation. * Juvenile baldness due to macrophage infiltration to the dermis of the affected area. |
* Accumulation of activated lymphocytes around conducting airways during lung inflammation. Hence, there is airway hyperresponsiveness to increasing acetylcholine stimulation. * β6 regulates local inflammation of lung epithelial cells. * β6 activates TGFβ, a signaling pathway essential for pulmonary fibrosis. Hence, these mice are protected from lung fibrosis. |
[64, 89] |
| β7 |
Gut associated Lymphoid Tissue (GALT) * Abnormal Peyer’s patches. * Defective migration of T cells to Payer’s patches. * Decreased number of lamina propia intraepithelial lymphocytes. |
* β7 is required for lymphocytes to adhere on vasculature at the site for transmigration into the GALT. | [183] |
| Integrin Knockout | Phenotype of Mice | Impact on Lung Development | Ref |
| β8 |
Vasculature * Defects in placenta, central nervous system and gastrointestinal blood vessels. Mouth * Cleft palate. |
* Not determined. | [184] |
| αvβ6 | * Marked increase in macrophage metalloelastase level (MMP12) | * Loss of αvβ6-mediated activation of latent TGFβ causes age-dependent pulmonary emphysema through alterations in macrophage MMP12 expression. | [65, 89, 185] |
| α6β4 |
Skin * Complete absence of hemidesmosomes. * Skin blistering leading to postnatal death. * Epidermolysis bullosa with congenital pyloric atresia. |
* Not determined. | [186] |
| α5 and αv | * Embryonically lethal. Embroyonic Development * Display severe gastrulation defect with a lack of anterior mesoderm. * Severe amniotic defect similar to fibronectin-null mice. |
* Not determined. | [153] |
| α3 and α6 | * Growth retarded. Limb * Limb abnormalities - syndactyly and fusion of preskeletal elements. * Defects in the apical ectodermal ridge, a limb organizing centre. Brain * Exencephaly - absence of neural tube closure. * Cortical disorganization Kidney * Kidney defects * Structural disorganization and reduced proliferation of the ridge cells. Eye * Eye lamination defects |
* Develop bilateral lung hypoplasia * α3 and α6 integrins are required for structural organization of presumptive ectodermal ridge cells for normal lung development. |
[113, 155, 187] |
| β7 and L-selectin |
Immunity * Suffer from a nearly complete impairment of lymphocyte migration to mesenteric lymph nodes (MLN). * Impaired MLN formation. * T lymphocyte numbers drastically reduced compared to control mice. |
* Not determined. | [188] |
Using integrin α1 knockout mice, Pozzi and colleagues showed that integrin α1 plays an important role in blood vessel formation in the lungs. Tumors implanted into these mice had reduced vascularization (reduced capillary number and size) [108]. This is due to the loss of integrin α1-induced tumor endothelial cell proliferation. In the same study, isolated α1-null endothelial cells from the lung also had reduced proliferation on the substrata. This suggests that the role of integrin α1 in angiogenesis is not restricted to tumor endothelial cells but also in the normal lung endothelial cells.
Kreidberg and colleagues demonstrated that the integrin α3 knockout mice showed severe decrease in bronchial branching and maturation of distal bronchioles [109]. In combination with other organ defects such as kidneys and cerebral cortex, integrin α3 knockout leads to death of mice shortly after birth [109-111]. Although both integrins α3 and α6 are expressed on lung epithelial cells, either integrin α3 or α6 knockout mice have normal lung development without any obvious lung defects [112]. Nonetheless, mice with double homozygous mutant for integrins α3 and α6 develop bilateral lung hypoplasia; a phenomenon characterized by low number and size of bronchopulmonary segments or alveoli in the lung [113]. This defect is not observed in either integrin α3 or α6 knockout mice, suggesting that the integrins α3 and α6 synergize in the normal development of the lung. It also suggests that the lack of either one integrin function may be compensated by the other to prevent bilateral lung hypoplasia.
Huang and colleagues showed that integrin α9 gene knockout mice exhibit increased volumes of pleural fluid, giving rise to bilateral chylothorax and respiratory failure [114]. The integrin α9-null mice also develop edema and lymphocytic infiltration (rich in lymphocytes, triglyceride, and cholesterol) in the chest wall. This study suggests that the integin α9 plays a critical role in the development of the thoracic duct and other lymphatic vessels essential for lung development.
Overall, studies in integrin knockout mice provided evidence that integrins promote respiratory disease progression and regulation of inflammation. The integrin β5 knockout mice were protected from increased vascular permeability in a model of acute lung injury [115]. This may be attributed to decreased angiogenesis resulting from the loss of integrin β5 on the pulmonary endothelial cells. The integrin αvβ6 knockout mice showed age-dependent pulmonary emphysema due to the loss of TGFβ activation, leading to increased Mmp12 levels in the integrin αvβ6-null mice and promoting emphysema development [65]. Integrin β6 knockout mice also had severe lung inflammation (elevated lymphocyte accumulation in the conducting airways) and exhibited airway hyperresponsiveness to acetylcholine, a hallmark of asthma [64]. An enhanced lung inflammation observed in integrin β6 knockout mice suggests the role of integrin αvβ6 in downregulating inflammatory responses [89]. In this study, integrin β6 knockout mice are protected from bleomycin-induced pulmonary fibrosis, a model that is critically dependent on TGFβ signaling. This protection against pulmonary fibrosis is not due to the downregulation of inflammatory response to bleomycin [89]. This suggests that integrin αvβ6 plays an important role in the development of pulmonary fibrosis.
Integrins in clinical trials
The diverse roles of integrins in the pathogenesis of respiratory diseases have placed them as potential targets for therapeutic interventions. Table 3 summarizes the integrin antagonists that are currently in the development for the treatment of respiratory diseases such as asthma and NSCLC.
Table 3.
Targeted integrins in clinical trials.
| Targeted Integrin | Drug Name | Clinical Stage | Remarks | Respiratory Disease Target | Ref |
|---|---|---|---|---|---|
| α4β1 | Valategrast (R411) | Terminated at Phase II | * Attenuates cellular trafficking of activated CD4+ T cells and eosinophils in lung airways mediated by α4-VCAM-1 interactions. * Terminated after clarification of the regulatory framework for this class of compounds. |
Asthma | [9] |
| IVL745 | Terminated at Phase II | * Reduces the percentage of eosinophils in sputum. * Inhibits antigen-induced eosinophil and lymphocyte accumulation. * Prevents eosinophil extravasations. * Terminated because there is no statistical significant of IVL745 treatment on the early and late asthmatic responses, methacholine hyperresponsiveness, symptoms and exhaled nitric oxide compared with placebo. |
Asthma | [9, 120] | |
| Bio-1211 | Terminated at Phase II | * Reduces the recruitment of VLA-4 expressing cells- eosinophils, lymphocytes, metachromatic staining cells and neutrophils to the airways. * Interferes with inflammatory cell degranulation. * Terminated due to the lack of drug efficacy in asthmatic patients. |
Asthma | [189, 190] | |
| GW-559090X | Terminated at Phase II | * Inhibits eosinophil recruitment and allergen induced airway hyperresponsiveness in rat and guinea pig models of ovalbumin-induced lung inflammation. * Terminated because it does not reduce the early and late asthmatic response in a population of atopic asthmatic patients. * Not effective in allergen-induced airway hyperresponsiveness or exhaled nitric oxide levels of asthmatic patients. |
Asthma | [191] | |
| HMR 1031 | Terminated at Phase II | * Substantially reduces allergen-induced airway inflammatory and airway hyperresponsiveness in sensitized mice and sheep respectively. * Terminated due to lack of effect in subjects with atopic asthma. |
Asthma | [192] | |
| TR14035 | Phase I | * Dual antagonist for α4β1/α4β7-mediated leukocyte cell adhesion. * Suppresses airway hyperrespoinsiveness to 5-HT and reduces the number of eosinophils, neutrophils, lymphocytes and macrophages in bonchoalveolar lavage fluid of sensitized Brown Norway rats. * Blocks VCAM/VLA-4 interactions and allergen-induced airway responses in a sheep model of asthma. * This drug was reported to be in Phase I clinical trial for asthma. Since this drug is no longer listed under GSK’s pipeline, the status of the current development of this drug is unknown. |
Asthma | [193, 194] | |
| α4β1; α4β7 |
Vitaxin II applied in combination with cancer therapy | Phase I | * Ongoing study with no data reported yet. | NSCLC | [19] |
| α5β1 | Volociximab | Phase II | * Exhibit encouraging safety profiles in Phase I clinical trial. * Ongoing study with no data reported yet. |
NSCLC | [129] |
| Targeted Integrin | Drug Name | Clinical Stage | Remarks | Respiratory Disease Target | Ref |
| αvβ3 | ACDCRGDCFC peptide conjugated with antimicrobial synthetic peptide (KLAKLAK)2 |
Preclinical | * ACDCRGDCFC - a targeting domain to guide the “homing’ proapoptotic peptide to the targeted cells and allow internalization. * (KLAKLAK)2 - a proapoptotic domain designed to be non-toxic outside the cells but toxic when internalized into targeted cells by the disruption of mitochondrial membranes. * Significant tumor-reducing effect. * Able to lower the lung metastasis burden. |
NSCLC | [127] |
| αvβ3; αvβ5 |
Cilengitide | Phase II | * Exhibit encouraging safety profiles in Phase I clinical trial. * Ongoing study with no data reported yet. |
NSCLC | [128, 195] |
Currently, only the integrin α4β1 is being targeted for the treatment of inflammation in asthma. This is because integrin α4β1 recruits leukocytes into the airway and exacerbates inflammation via VCAM and ICAM interactions [61, 116-118]. Integrin antagonists such as Valategrast (R411), IVL745, Bio 1211, GW-559090X, HMR 1031 and TR14035 target these interactions [119, 120]. Nonetheless, they have failed to be effective during clinical trials. It is unclear whether the failure is due to the route of administration, bioavailability of the drugs, or attributed to functional compensation by the other integrins. Such integrins include integrin α5β1, which is found to be important for lymphocytes migration into the lung during sarcoidosis [49, 50].
Besides using integrin antagonists to treat inflammation in asthma, other aspects of asthma such as airway wall remodeling should also be considered. The formation of tortuous blood vessels and accumulation of smooth muscle bulks are common structural changes in asthmatic airways [121]. These processes are mainly regulated by integrins such as integrin α1β1 that increases angiogenesis and integrin α5β1 that mediates survival signals to airway smooth muscle cells [53]. Hence, drug therapies targeting the integrins α1β1 and α5β1 are warranted for the treatment of asthma. Airway wall remodeling is characterized by inflammation and fibrosis (increased deposition of ECM proteins) that deteriorates lung function. TGFβ, a pleiotropic cytokine that drives the inflammation and matrix deposition is implicated in asthmatic and COPD airway remodeling [122-124]. The activation of TGFβ is integrin αvβ5- and αvβ8-dependent and these two integrins have been shown to be expressed on airway smooth muscle cells (αvβ5) and airway fibroblasts (αvβ8) and their expression are increased in asthma and COPD [10, 56, 125]. Hence, integrins αvβ5 and αvβ8 represent attractive targets for the treatment of airway remodeling in asthma and COPD.
Besides utilizing integrin antagonists, integrin inhibitors have been conjugated to cytotoxic drugs. This is done by synthetic chemistry or by coupling the inhibitors to biomacromolecules via DNA recombination technology or fusion protein technology to facilitate drug delivery into the targeted cells, such as cancerous cells in NSCLC and leukocytes in asthma [126]. For example, the ACDCRGDCFC peptide is conjugated with the antimicrobial synthetic peptide (KLAKLAK)2 and targets the integrins αvβ3 and αvβ6 in NSCLC [127]. ACDCRGDCFC is a targeting domain for integrins αvβ3 and αvβ6, which homes the proapoptotic peptide to the targeted cells for further internalization. (KLAKLAK)2 is a proapoptotic domain. This conjugation is designed to be toxic only when internalized into the cells because it specifically disrupts mitochondrial membranes. It shows significant tumour reducing effect and reduced lung metastasis burden in preclinical trials [127].
Some integrin antagonists are applied in combination with the standard anti-cancer therapies. One such example is Vitaxin II which targets the integrin αvβ3 in NSCLC. It is currently under Phase I clinical trial [19]. Other integrin antagonists that are currently under Phase II clinical trials for NSCLC are Cilengitide and Volociximab. Cilengitide targets the integrins αvβ3 and αvβ5 whereas Volociximab targets the integrin α5β1. Both drugs have anti-angiogenic effect in NSCLC and showed encouraging safety profiles in Phase I trials [128, 129].
Currently, there are only 3 integrins (αvβ3, αvβ5 and α5β1) targeted in NSCLC. These integrins are targeted mainly for their anti-angiogenic function in tumors. Given that other integrins (α1, α11 and αvβ6) are also involved in NSCLC disease progression, antagonists against these integrins should be considered for therapeutic development [52, 58, 66, 67].
The study of integrin antagonists should also be extended to other lung diseases such as emphysema, epithelial injury and repair, pulmonary fibrosis, pneumonia and sarcoidosis, whereby integrins play important roles (Table 1).
Conclusion
We have reviewed and highlighted the importance of integrins in mediating various respiratory disease progressions. Integrins are promising therapeutic targets. Nonetheless, the complexity and diversity of integrins present a major challenge for drug development. Recent advances in the understanding of the integrin function and their signaling mechanisms using the gene knockout technology have aided in the development of integrin antagonists. This is coupled with the possibility of altering integrin signaling in respiratory diseases. Collectively, drugs targeting integrins hold great potential for the treatment of respiratory diseases in the future.
ACKNOWLEDGEMENTS
TT received funding support from the Singapore Ministry of Health’s National Medical Research Council under its IRG scheme (NMRC/1215/2009).
Abbreviations
- ECM
Extracellular matrix
- EGFR
Epidermal growth factor receptor
- FAK
Focal adhesion kinase
- GALT
Gut associated lymphoid tissues
- ILK
Integrin-linked kinase
- MLN
Mesenteric lymph nodes
- MMP12
Macrophage metalloelastase
- NSCLC
Non-small cell lung cancer
- NSCLC
non-small cell lung cancer
- PDGF
Platelet-derived growth factor
- PI3K
Phosphatidylinositol-3-kinase
- PMN
Polymorphonuclear leucocytes
- SFK
Src family kinase
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Miniño A.M., Heron M.P., Murphy S.L., et al. Deaths: final data for 2004. Natl. Vital Stat. Rep. 2007;55(19):1–119. [PubMed] [Google Scholar]
- 2.Hynes R.O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 3.Hood J.D., Cheresh D.A. Role of integrins in cell invasion and migration. Nat. Rev. Cancer. 2002;2(2):91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 4.Humphries M.J. Integrin structure. Biochem. Soc. Trans. 2000;28(4):311–339. [PubMed] [Google Scholar]
- 5.Giancotti F.G., Ruoslahti E. Integrin signaling. Science. 1999;285(5430):1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 6.Cabodi S, Di Stefano P. Integrins and signal transduction. Adv. Exp. Med. Biol. 2010;674:43–54. doi: 10.1007/978-1-4419-6066-5_5. [DOI] [PubMed] [Google Scholar]
- 7.Bazan-Socha S., Bukiej A., Marcinkiewicz C., et al. Integrins in pulmonary inflammatory diseases. Curr. Pharm. Des. 2005;11(7):893–901. doi: 10.2174/1381612053381710. [DOI] [PubMed] [Google Scholar]
- 8.van der Flier A., Sonnenberg A. Function and interactions of integrins. Cell Tissue Res. 2001;305(3):285–298. doi: 10.1007/s004410100417. [DOI] [PubMed] [Google Scholar]
- 9.Cox D., Brennan M., Moran N. Integrins as therapeutic targets: lessons and opportunities. Nat. Rev. Drug Discov. 2010;9(10):804–820. doi: 10.1038/nrd3266. [DOI] [PubMed] [Google Scholar]
- 10.Minagawa S., Lou J., Seed R.I., et al. Selective targeting of TGF-β activation to treat fibroinflammatory airway disease. Sci. Transl. Med. 2014;6(241):241ra79. doi: 10.1126/scitranslmed.3008074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mu D., Cambier S., Fjellbirkeland L., et al. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J. Cell Biol. 2002;157(3):493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giancotti F., Ruoslahti E. Integrin signaling. Science. 1999;285(5430):1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 13.Ross R. Molecular and mechanical synergy: cross-talk between integrins and growth factor receptors. Cardiovasc. Res. 2004;63(3):81–90. doi: 10.1016/j.cardiores.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 14.Zaidel-Bar R., Itzkovitz S., Ma'ayan A., et al. Functional atlas of the integrin adhesome. Nat. Cell Biol. 2007;9(8):858–867. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harburger D.S., Calderwood D.A. Integrin signalling at a glance. J. Cell Sci. 2009;122(Pt 2):159–163. doi: 10.1242/jcs.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Legate K.R., Wickstrom S.A., Fassler R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 2009;23(4):397–418. doi: 10.1101/gad.1758709. [DOI] [PubMed] [Google Scholar]
- 17.Welsh C.F., Assoian R.K. A growing role for Rho family GTPases as intermediaries in growth factor- and adhesion-dependent cell cycle progression. Biochim. Biophys. Acta. 2000;1471(1):M21–M29. doi: 10.1016/s0304-419x(00)00016-0. [DOI] [PubMed] [Google Scholar]
- 18.Hanks S.K., Calalb M.B., Harper M.C., et al. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc. Natl. Acad. Sci. USA. 1992;89(18):8487–8491. doi: 10.1073/pnas.89.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huveneers S., Truong H., Danen H.J. Integrins: signaling, disease, and therapy. Int. J. Radiat. Biol. 2007;83(11-12):743–751. doi: 10.1080/09553000701481808. [DOI] [PubMed] [Google Scholar]
- 20.Kim S.H., Turnbull J., Guimond S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J. Endocrinol. 2011;209(2):139–151. doi: 10.1530/JOE-10-0377. [DOI] [PubMed] [Google Scholar]
- 21.Kim S.H. Antagonistic effect of EGF on FAK phosphorylation/dephosphorylation in a cell. Cell Biochem. Funct. 2008;26(5):539–547. doi: 10.1002/cbf.1457. [DOI] [PubMed] [Google Scholar]
- 22.Slack-Davis J.K., Eblen S.T., Zecevic M., et al. PAK1 phosphorylation of MEK1 regulates fibronectin-stimulated MAPK activation. J. Cell Biol. 2003;162(2):281–291. doi: 10.1083/jcb.200212141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edin M.L., Juliano R.L. Raf-1 serine 338 phosphorylation plays a key role in adhesion-dependent activation of extracellular signal-regulated kinase by epidermal growth factor. Mol. Cell. Biol. 2005;25(11):4466–4475. doi: 10.1128/MCB.25.11.4466-4475.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheppard D. Functions of pulmonary epithelial integrins: from development to disease. Physiol. Rev. 2003;83(3):673–686. doi: 10.1152/physrev.00033.2002. [DOI] [PubMed] [Google Scholar]
- 25.Coraux C., Delplanque A., Hinnrasky J., et al. Distribution of integrins during human fetal lung development. J. Histochem. Cytochem. 1998;46(7):803–810. doi: 10.1177/002215549804600703. [DOI] [PubMed] [Google Scholar]
- 26.Wagner T.E., Frevert C.W., Herzog E.L., et al. Expression of the integrin subunit alpha8 in murine lung development. J. Histochem. Cytochem. 2003;51(10):1307–1315. doi: 10.1177/002215540305101008. [DOI] [PubMed] [Google Scholar]
- 27.Wu J.E., Santoro S.A. Differential expression of integrin alpha subunits supports distinct roles during lung branching morphogenesis. Dev. Dyn. 1996;206(2):169–181. doi: 10.1002/(SICI)1097-0177(199606)206:2<169::AID-AJA6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 28.Cambier S., Mu D.Z., O'Connell D., et al. A role for the integrin alphavbeta8 in the negative regulation of epithelial cell growth. Cancer Res. 2000;60(24):7084–7093. [PubMed] [Google Scholar]
- 29.Damjanovich L., Albelda S.M., Mette S.A., et al. Distribution of integrin cell adhesion receptors in normal and malignant lung tissue. Am. J. Respir. Cell Mol. Biol. 1992;6(2):197–206. doi: 10.1165/ajrcmb/6.2.197. [DOI] [PubMed] [Google Scholar]
- 30.Mette S.A., Pilewski J., Buck C.A., et al. Distribution of integrin cell adhesion receptors on normal bronchial epithelial cells and lung cancer cells in vitro and in vivo. Am. J. Respir. Cell Mol. Biol. 1993;8(5):562–572. doi: 10.1165/ajrcmb/8.5.562. [DOI] [PubMed] [Google Scholar]
- 31.Palmer E.L., Ruegg C., Ferrando R., et al. Sequence and tissue distribution of the integrin alpha 9 subunit, a novel partner of beta 1 that is widely distributed in epithelia and muscle. J. Cell Biol. 1993;123(5):1289–1297. doi: 10.1083/jcb.123.5.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pilewski J.M., Latoche J.D., Arcasoy S.M., et al. Expression of integrin cell adhesion receptors during human airway epithelial repair in vivo. Am. J. Physiol. 1997;273(1 Pt 1):L256–L263. doi: 10.1152/ajplung.1997.273.1.L256. [DOI] [PubMed] [Google Scholar]
- 33.Weinacker A., Ferrando R., Elliott M., et al. Distribution of integrins alpha v beta 6 and alpha 9 beta 1 and their known ligands, fibronectin and tenascin, in human airways. Am. J. Respir. Cell Mol. Biol. 1995;12(5):547–556. doi: 10.1165/ajrcmb.12.5.7537970. [DOI] [PubMed] [Google Scholar]
- 34.Korhonen M., Ylänne J., Laitinen L., et al. The alpha 1-alpha 6 subunits of integrins are characteristically expressed in distinct segments of developing and adult human nephron. J. Cell Biol. 1990;111(3):1245–1254. doi: 10.1083/jcb.111.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kadoya Y., Kadoya K., Durbeej M., et al. Antibodies against domain E3 of laminin-1 and integrin alpha 6 subunit perturb branching epithelial morphogenesis of submandibular gland, but by different modes. J. Cell Biol. 1995;129(2):521–534. doi: 10.1083/jcb.129.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sorokin L., Sonnenberg A., Aumailley M., et al. Recognition of the laminin E8 cell-binding site by an integrin possessing the alpha 6 subunit is essential for epithelial polarization in developing kidney tubules. J. Cell Biol. 1990;111(3):1265–1273. doi: 10.1083/jcb.111.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carter W.G., Wayner E.A., Bouchard T.S., et al. The role of integrins alpha 2 beta 1 and alpha 3 beta 1 in cell-cell and cell-substrate adhesion of human epidermal cells. J. Cell Biol. 1990;110(4):1387–1404. doi: 10.1083/jcb.110.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sriramarao P., Steffner P., Gehlsen K.R. Biochemical evidence for a homophilic interaction of the alpha 3 beta 1 integrin. J. Biol. Chem. 1993;268(29):22036–22041. [PubMed] [Google Scholar]
- 39.Symington B.E., Takada Y., Carter W.G. Interaction of integrins alpha 3 beta 1 and alpha 2 beta 1: potential role in keratinocyte intercellular adhesion. J. Cell Biol. 1993;120(2):523–535. doi: 10.1083/jcb.120.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tenchini M.L., Adams J.C., Gilberty C., et al. Evidence against a major role for integrins in calcium-dependent intercellular adhesion of epidermal keratinocytes. Cell Adhes. Commun. 1993;1(1):55–66. doi: 10.3109/15419069309095681. [DOI] [PubMed] [Google Scholar]
- 41.Weitzman J.B., Chen A., Hemler M.E. Investigation of the role of beta 1 integrins in cell-cell adhesion. J. Cell Sci. 1995;108(Pt 11):3635–3644. doi: 10.1242/jcs.108.11.3635. [DOI] [PubMed] [Google Scholar]
- 42.Weitzman J.B., Pasqualini R., Takada Y., et al. The function and distinctive regulation of the integrin VLA-3 in cell adhesion, spreading, and homotypic cell aggregation. J. Biol. Chem. 1993;268(12):8651–8657. [PubMed] [Google Scholar]
- 43.Georges-Labouesse E., Messaddeq N., Yehia G., et al. Absence of integrin alpha 6 leads to epidermolysis bullosa and neonatal death in mice. Nat. Genet. 1996;13(3):370–373. doi: 10.1038/ng0796-370. [DOI] [PubMed] [Google Scholar]
- 44.van der Neut R., Krimpenfort P., Calafat J., et al. Epithelial detachment due to absence of hemidesmosomes in integrin beta 4 null mice. Nat. Genet. 1996;13(3):366–369. doi: 10.1038/ng0796-366. [DOI] [PubMed] [Google Scholar]
- 45.Eto K., Huet C., Tarui T., et al. Functional classification of ADAMs based on a conserved motif for binding to integrin alpha 9beta 1: implications for sperm-egg binding and other cell interactions. J. Biol. Chem. 2002;277(20):17804–17810. doi: 10.1074/jbc.M200086200. [DOI] [PubMed] [Google Scholar]
- 46.Busk M., Pytela R., Sheppard D. Characterization of the integrin alpha v beta 6 as a fibronectin-binding protein. J. Biol. Chem. 1992;267(9):5790–5796. [PubMed] [Google Scholar]
- 47.Roman J., Little C.W., McDonald J.A. Potential role of RGD-binding integrins in mammalian lung branching morphogenesis. Development. 1991;112(2):551–558. doi: 10.1242/dev.112.2.551. [DOI] [PubMed] [Google Scholar]
- 48.Singh B., Fu C., Bhattacharya J. Vascular expression of the alpha(v)beta(3)-integrin in lung and other organs. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;278(1):L217–L226. doi: 10.1152/ajplung.2000.278.1.L217. [DOI] [PubMed] [Google Scholar]
- 49.Berlin M., Lundahl J., Skold C.M., et al. The lymphocytic alveolitis in sarcoidosis is associated with increased amounts of soluble and cell-bound adhesion molecules in bronchoalveolar lavage fluid and serum. J. Intern. Med. 1998;244(4):333–340. doi: 10.1046/j.1365-2796.1998.00378.x. [DOI] [PubMed] [Google Scholar]
- 50.Shigehara K., Shijubo N., Hirasawa M., et al. Immunolocalization of extracellular matrix proteins and integrins in sarcoid lymph nodes. Virchows Arch. 1998;433(1):55–61. doi: 10.1007/s004280050216. [DOI] [PubMed] [Google Scholar]
- 51.White E.S., Thannickal V.J., Carskadon S.L., et al. Integrin alpha4beta1 regulates migration across basement membranes by lung fibroblasts: a role for phosphatase and tensin homologue deleted on chromosome 10. Am. J. Respir. Crit. Care Med. 2003;168(4):436–442. doi: 10.1164/rccm.200301-041OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu C.Q., Popova S.N., Brown E.R., et al. Integrin alpha 11 regulates IGF2 expression in fibroblasts to enhance tumorigenicity of human non-small-cell lung cancer cells. Proc. Natl. Acad. Sci. USA. 2007;104(28):11754–11759. doi: 10.1073/pnas.0703040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freyer A., Johnson S., Hall I. Effects of growth factors and extracellular matrix on survival of human airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 2001;25(5):569–576. doi: 10.1165/ajrcmb.25.5.4605. [DOI] [PubMed] [Google Scholar]
- 54.Tran T., Ens-Blackie K., Rector E.S., et al. Laminin-binding integrin alpha7 is required for contractile phenotype expression by human airway myocytes. Am. J. Respir. Cell Mol. Biol. 2007;37(6):668–680. doi: 10.1165/rcmb.2007-0165OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lohmeyer J., Friedrich J., Grimminger F., et al. Expression of mucosa-related integrin alphaEbeta7 on alveolar T cells in interstitial lung diseases. Clin. Exp. Immunol. 1999;116(2):340–346. doi: 10.1046/j.1365-2249.1999.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tatler A.L., John A.E., Jolly L., et al. Integrin αvβ5-mediated TGF-β activation by airway smooth muscle cells in asthma. J. Immunol. 2011;187(11):6094–6107. doi: 10.4049/jimmunol.1003507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Senger D.R., Claffey K.P., Benes J.E., et al. Angiogenesis promoted by vascular endothelial growth factor: regulation through alpha1beta1 and alpha2beta1 integrins. Proc. Natl. Acad. Sci. USA. 1997;94(25):13612–13617. doi: 10.1073/pnas.94.25.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Macias-Perez I., Borza C., Chen X., et al. Loss of integrin alpha1beta1 ameliorates Kras-induced lung cancer. Cancer Res. 2008;68(15):6127–6135. doi: 10.1158/0008-5472.CAN-08-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berlin M., Lundahl J., Sköld C.M., et al. The lymphocytic alveolitis in sarcoidosis is associated with increased amounts of soluble and cell-bound adhesion molecules in bronchoalveolar lavage fluid and serum. J. Intern. Med. 1998;244(4):333–340. doi: 10.1046/j.1365-2796.1998.00378.x. [DOI] [PubMed] [Google Scholar]
- 60.Ohashi Y., Motojima S., Fukuda T., et al. Airway hyperresponsiveness, increased intracellular spaces of bronchial epithelium, and increased infiltration of eosinophils and lymphocytes in bronchial mucosa in asthma. Am. Rev. Respir. Dis. 1992;145(6):1469–1476. doi: 10.1164/ajrccm/145.6.1469. [DOI] [PubMed] [Google Scholar]
- 61.Nagata M., Yamamoto H., Tabe K., et al. Eosinophil transmigration across VCAM-1-expressing endothelial cells is upregulated by antigen-stimulated mononuclear cells. Int. Arch. Allergy Immunol. 2001;125(Suppl. 1):7–11. doi: 10.1159/000053844. [DOI] [PubMed] [Google Scholar]
- 62.Hocking D.C. Fibronectin matrix deposition and cell contractility: implications for airway remodeling in asthma. Chest. 2002;122(6) Suppl.:275S–278S. doi: 10.1378/chest.122.6_suppl.275s. [DOI] [PubMed] [Google Scholar]
- 63.Han J.Y., Kim H.S., Lee S.H., et al. Immunohistochemical expression of integrins and extracellular matrix proteins in non-small cell lung cancer: correlation with lymph node metastasis. Lung Cancer. 2003;41(1):65–70. doi: 10.1016/s0169-5002(03)00146-6. [DOI] [PubMed] [Google Scholar]
- 64.Huang X.Z., Wu J.F., Cass D., et al. Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J. Cell Biol. 1996;133(4):921–928. doi: 10.1083/jcb.133.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morris D.G., Huang X., Kaminski N., et al. Loss of integrin alpha(v)beta6-mediated TGF-beta activation causes Mmp12-dependent emphysema. Nature. 2003;422(6928):169–173. doi: 10.1038/nature01413. [DOI] [PubMed] [Google Scholar]
- 66.Sheppard D. Roles of alphav integrins in vascular biology and pulmonary pathology. Curr. Opin. Cell Biol. 2004;16(5):552–557. doi: 10.1016/j.ceb.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 67.Wipff P.J., Rifkin D.B., Meister J.J., et al. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J. Cell Biol. 2007;179(6):1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Horan G.S., Wood S., Ona V., et al. Partial inhibition of integrin alpha(v)beta6 prevents pulmonary fibrosis without exacerbating inflammation. Am. J. Respir. Crit. Care Med. 2008;177(1):56–65. doi: 10.1164/rccm.200706-805OC. [DOI] [PubMed] [Google Scholar]
- 69.John A.E., Luckett J.C., Tatler A.L., et al. Preclinical SPECT/CT imaging of αvβ6 integrins for molecular stratification of idiopathic pulmonary fibrosis. J. Nucl. Med. 2013;54(12):2146–2152. doi: 10.2967/jnumed.113.120592. [DOI] [PubMed] [Google Scholar]
- 70.Jenkins R.G., Su X., Su G., et al. Ligation of protease-activated receptor 1 enhances alpha(v)beta6 integrin-dependent TGF-beta activation and promotes acute lung injury. J. Clin. Invest. 2006;116(6):1606–1614. doi: 10.1172/JCI27183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu M.Y., Porte J., Knox A.J., et al. Lysophosphatidic acid induces alphavbeta6 integrin-mediated TGF-beta activation via the LPA2 receptor and the small G protein G alpha(q). Am. J. Pathol. 2009;174(4):1264–1279. doi: 10.2353/ajpath.2009.080160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jolly L., Stavrou A., Vanderstoken G., et al. Influenza promotes collagen deposition via αvβ6 integrin-mediated transforming growth factor β activation. J. Biol. Chem. 2014;289(51):35246–35263. doi: 10.1074/jbc.M114.582262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Konishi K., Gibson K.F., Lindell K.O., et al. Gene expression profiles of acute exacerbations of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2009;180(2):167–175. doi: 10.1164/rccm.200810-1596OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Benayoun L., Druilhe A., Dombret M., et al. Airway structural alterations selectively associated with severe asthma. Am. J. Respir. Crit. Care Med. 2003;167(10):1360–1368. doi: 10.1164/rccm.200209-1030OC. [DOI] [PubMed] [Google Scholar]
- 75.Tran T., Teoh C.M., Tam J.K., et al. Laminin drives survival signals to promote a contractile smooth muscle phenotype and airway hyperreactivity. FASEB J. 2013;27(10):3991–4003. doi: 10.1096/fj.12-221341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lambert R.K., Wiggs B.R., Kuwano K., et al. Functional significance of increased airway smooth muscle in asthma and COPD. J. Appl. Physiol. 1993;74(6):2771–2781. doi: 10.1152/jappl.1993.74.6.2771. [DOI] [PubMed] [Google Scholar]
- 77.Christie P.E., Jonas M., Tsai C.H., et al. Increase in laminin expression in allergic airway remodelling and decrease by dexamethasone. Eur. Respir. J. 2004;24(1):107–115. doi: 10.1183/09031936.04.00013303. [DOI] [PubMed] [Google Scholar]
- 78.Rihs S., Walker C., Virchow J.C., Jr, et al. Differential expression of alpha E beta 7 integrins on bronchoalveolar lavage T lymphocyte subsets: regulation by alpha 4 beta 1-integrin crosslinking and TGF-beta. Am. J. Respir. Cell Mol. Biol. 1996;15(5):600–610. doi: 10.1165/ajrcmb.15.5.8918367. [DOI] [PubMed] [Google Scholar]
- 79.Leckie M.J., Jenkins G.R., Khan J., et al. Sputum T lymphocytes in asthma, COPD and healthy subjects have the phenotype of activated intraepithelial T cells (CD69+ CD103+). Thorax. 2003;58(1):23–29. doi: 10.1136/thorax.58.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lawler J. The functions of thrombospondin-1 and-2. Curr. Opin. Cell Biol. 2000;12(5):634–640. doi: 10.1016/s0955-0674(00)00143-5. [DOI] [PubMed] [Google Scholar]
- 81.Lawler J., Sunday M., Thibert V., et al. Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. J. Clin. Invest. 1998;101(5):982–992. doi: 10.1172/JCI1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yabkowitz R., Dixit V.M., Guo N., et al. Activated T-cell adhesion to thrombospondin is mediated by the alpha 4 beta 1 (VLA-4) and alpha 5 beta 1 (VLA-5) integrins. J. Immunol. 1993;151(1):149–158. [PubMed] [Google Scholar]
- 83.Mansfield P.J., Boxer L.A., Suchard S.J. Thrombospondin stimulates motility of human neutrophils. J. Cell Biol. 1990;111(6 Pt 2):3077–3086. doi: 10.1083/jcb.111.6.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mansfield P.J., Suchard S.J. Thrombospondin promotes chemotaxis and haptotaxis of human peripheral blood monocytes. J. Immunol. 1994;153(9):4219–4229. [PubMed] [Google Scholar]
- 85.Huber A.R., Ellis S., Johnson K.J., et al. Monocyte diapedesis through an in vitro vessel wall construct: inhibition with monoclonal antibodies to thrombospondin. J. Leukoc. Biol. 1992;52(5):524–528. doi: 10.1002/jlb.52.5.524. [DOI] [PubMed] [Google Scholar]
- 86.Guo N., Templeton N.S., Al-Barazi H., et al. Thrombospondin-1 promotes alpha3beta1 integrin-mediated adhesion and neurite-like outgrowth and inhibits proliferation of small cell lung carcinoma cells. Cancer Res. 2000;60(2):457–466. [PubMed] [Google Scholar]
- 87.Ezzie M.E., Piper M.G., Montague C., et al. Thrombospondin-1-deficient mice are not protected from bleomycin-induced pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2011;44(4):556–561. doi: 10.1165/rcmb.2009-0019OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sheppard D. Transforming growth factor beta: a central modulator of pulmonary and airway inflammation and fibrosis. Proc. Am. Thorac. Soc. 2006;3(5):413–417. doi: 10.1513/pats.200601-008AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Munger J.S., Huang X., Kawakatsu H., et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96(3):319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 90.Clark C.J., Sage E.H. A prototypic matricellular protein in the tumor microenvironment--where there's SPARC, there's fire. J. Cell. Biochem. 2008;104(3):721–732. doi: 10.1002/jcb.21688. [DOI] [PubMed] [Google Scholar]
- 91.Kuhn C., Mason R.J. Immunolocalization of SPARC, tenascin, and thrombospondin in pulmonary fibrosis. Am. J. Pathol. 1995;147(6):1759–1769. [PMC free article] [PubMed] [Google Scholar]
- 92.Sage H., Vernon R.B., Funk S.E., et al. SPARC, a secreted protein associated with cellular proliferation, inhibits cell spreading in vitro and exhibits Ca+2-dependent binding to the extracellular matrix. J. Cell Biol. 1989;109(1):341–356. doi: 10.1083/jcb.109.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Francki A., Bradshaw A.D., Bassuk J.A., et al. SPARC regulates the expression of collagen type I and transforming growth factor-beta1 in mesangial cells. J. Biol. Chem. 1999;274(45):32145–32152. doi: 10.1074/jbc.274.45.32145. [DOI] [PubMed] [Google Scholar]
- 94.Gruber H.E., Sage E.H., Norton H.J., et al. Targeted deletion of the SPARC gene accelerates disc degeneration in the aging mouse. J. Histochem. Cytochem. 2005;53(9):1131–1138. doi: 10.1369/jhc.5A6687.2005. [DOI] [PubMed] [Google Scholar]
- 95.Bradshaw A.D., Puolakkainen P., Dasgupta J., et al. SPARC-null mice display abnormalities in the dermis characterized by decreased collagen fibril diameter and reduced tensile strength. J. Invest. Dermatol. 2003;120(6):949–955. doi: 10.1046/j.1523-1747.2003.12241.x. [DOI] [PubMed] [Google Scholar]
- 96.Barker T.H., Baneyx G., Cardó-Vila M., et al. SPARC regulates extracellular matrix organization through its modulation of integrin-linked kinase activity. J. Biol. Chem. 2005;280(43):36483–36493. doi: 10.1074/jbc.M504663200. [DOI] [PubMed] [Google Scholar]
- 97.Chang W., Wei K., Jacobs S.S., et al. SPARC suppresses apoptosis of idiopathic pulmonary fibrosis fibroblasts through constitutive activation of beta-catenin. J. Biol. Chem. 2010;285(11):8196–8206. doi: 10.1074/jbc.M109.025684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takayama G., Arima K., Kanaji T., et al. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J. Allergy Clin. Immunol. 2006;118(1):98–104. doi: 10.1016/j.jaci.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 99.Blanchard C., Mingler M.K., McBride M., et al. Periostin facilitates eosinophil tissue infiltration in allergic lung and esophageal responses. Mucosal Immunol. 2008;1(4):289–296. doi: 10.1038/mi.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gillan L., Matei D., Fishman D.A., et al. Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5) integrins and promotes cell motility. Cancer Res. 2002;62(18):5358–5364. [PubMed] [Google Scholar]
- 101.Sehra S., Yao W., Nguyen E.T., et al. Periostin regulates goblet cell metaplasia in a model of allergic airway inflammation. J. Immunol. 2011;186(8):4959–4966. doi: 10.4049/jimmunol.1002359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thai P., Loukoianov A., Wachi S., et al. Regulation of airway mucin gene expression. Annu. Rev. Physiol. 2008;70:405–429. doi: 10.1146/annurev.physiol.70.113006.100441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ouyang G., Liu M., Ruan K., et al. Upregulated expression of periostin by hypoxia in non-small-cell lung cancer cells promotes cell survival via the Akt/PKB pathway. Cancer Lett. 2009;281(2):213–219. doi: 10.1016/j.canlet.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 104.Bao S., Ouyang G., Bai X., et al. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell. 2004;5(4):329–339. doi: 10.1016/s1535-6108(04)00081-9. [DOI] [PubMed] [Google Scholar]
- 105.Naik P.K., Bozyk P.D., Bentley J.K., et al. Periostin promotes fibrosis and predicts progression in patients with idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012;303(12):L1046–L1056. doi: 10.1152/ajplung.00139.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Okamoto M., Hoshino T., Kitasato Y., et al. Periostin, a matrix protein, is a novel biomarker for idiopathic interstitial pneumonias. Eur. Respir. J. 2011;37(5):1119–1127. doi: 10.1183/09031936.00059810. [DOI] [PubMed] [Google Scholar]
- 107.Uchida M., Shiraishi H., Ohta S., et al. Periostin, a matricellular protein, plays a role in the induction of chemokines in pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2012;46(5):677–686. doi: 10.1165/rcmb.2011-0115OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pozzi A., Moberg P.E., Miles L.A., et al. Elevated matrix metalloprotease and angiostatin levels in integrin alpha 1 knockout mice cause reduced tumor vascularization. Proc. Natl. Acad. Sci. USA. 2000;97(5):2202–2207. doi: 10.1073/pnas.040378497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kreidberg J.A., Donovan M.J., Goldstein S.L., et al. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 1996;122(11):3537–3547. doi: 10.1242/dev.122.11.3537. [DOI] [PubMed] [Google Scholar]
- 110.DiPersio C.M., Hodivala-Dilke K.M., Jaenisch R., et al. alpha3beta1 Integrin is required for normal development of the epidermal basement membrane. J. Cell Biol. 1997;137(3):729–742. doi: 10.1083/jcb.137.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Anton E.S., Kreidberg J.A., Rakic P. Distinct functions of alpha3 and alpha(v) integrin receptors in neuronal migration and laminar organization of the cerebral cortex. Neuron. 1999;22(2):277–289. doi: 10.1016/s0896-6273(00)81089-2. [DOI] [PubMed] [Google Scholar]
- 112.Georges-Labouesse E., Mark M., Messaddeq N., et al. Essential role of alpha 6 integrins in cortical and retinal lamination. Curr. Biol. 1998;8(17):983–986. doi: 10.1016/s0960-9822(98)70402-6. [DOI] [PubMed] [Google Scholar]
- 113.De Arcangelis A., Mark M., Kreidberg J., et al. Synergistic activities of alpha3 and alpha6 integrins are required during apical ectodermal ridge formation and organogenesis in the mouse. Development. 1999;126(17):3957–3968. doi: 10.1242/dev.126.17.3957. [DOI] [PubMed] [Google Scholar]
- 114.Huang X.Z., Wu J.F., Ferrando R., et al. Fatal bilateral chylothorax in mice lacking the integrin alpha9beta1. Mol. Cell. Biol. 2000;20(14):5208–5215. doi: 10.1128/mcb.20.14.5208-5215.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Su G., Hodnett M., Wu N., et al. Integrin alphavbeta5 regulates lung vascular permeability and pulmonary endothelial barrier function. Am. J. Respir. Cell Mol. Biol. 2007;36(3):377–386. doi: 10.1165/rcmb.2006-0238OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kikuchi M., Tachimoto H., Nutku E., et al. Phorbol esters alter alpha4 and alphad integrin usage during eosinophil adhesion to VCAM-1. Cell Commun. Adhes. 2003;10(3):119–128. [PubMed] [Google Scholar]
- 117.Bayless K.J., Meininger G.A., Scholtz J.M., et al. Osteopontin is a ligand for the alpha4beta1 integrin. J. Cell Sci. 1998;111(Pt 9):1165–1174. doi: 10.1242/jcs.111.9.1165. [DOI] [PubMed] [Google Scholar]
- 118.Bocchino V., Bertorelli G., D'Ippolito R., et al. The increased number of very late activation antigen-4-positive cells correlates with eosinophils and severity of disease in the induced sputum of asthmatic patients. J. Allergy Clin. Immunol. 2000;105(1 Pt 1):65–70. doi: 10.1016/s0091-6749(00)90179-9. [DOI] [PubMed] [Google Scholar]
- 119.Cox D., Brennan M., Moran N. Integrins as therapeutic targets: lessons and opportunities. Nat. Rev. Drug Discov. 2013;9(10):804–820. doi: 10.1038/nrd3266. [DOI] [PubMed] [Google Scholar]
- 120.Norris V., Choong L., Tran D., et al. Effect of IVL745, a VLA-4 antagonist, on allergen-induced bronchoconstriction in patients with asthma. J. Allergy Clin. Immunol. 2005;116(4):761–767. doi: 10.1016/j.jaci.2005.04.045. [DOI] [PubMed] [Google Scholar]
- 121.Davies D.E., Wicks J., Powell R.M., et al. Airway remodeling in asthma: new insights. J. Allergy Clin. Immunol. 2003;111(2):215–225. doi: 10.1067/mai.2003.128. [DOI] [PubMed] [Google Scholar]
- 122.McMillan S.J., Xanthou G., Lloyd C.M. Manipulation of allergen-induced airway remodeling by treatment with anti-TGF-beta antibody: effect on the Smad signaling pathway. J. Immunol. 2005;174(9):5774–5780. doi: 10.4049/jimmunol.174.9.5774. [DOI] [PubMed] [Google Scholar]
- 123.Churg A., Zhou S., Wang X., et al. The role of interleukin-1beta in murine cigarette smoke-induced emphysema and small airway remodeling. Am. J. Respir. Cell Mol. Biol. 2009;40(4):482–490. doi: 10.1165/rcmb.2008-0038OC. [DOI] [PubMed] [Google Scholar]
- 124.Podowski M., Calvi C., Metzger S., et al. Angiotensin receptor blockade attenuates cigarette smoke-induced lung injury and rescues lung architecture in mice. J. Clin. Invest. 2012;122(1):229–240. doi: 10.1172/JCI46215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kitamura H., Cambier S., Somanath S., et al. Mouse and human lung fibroblasts regulate dendritic cell trafficking, airway inflammation, and fibrosis through integrin αvβ8-mediated activation of TGF-β. J. Clin. Invest. 2011;121(7):2863–2875. doi: 10.1172/JCI45589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang Z., Chui W.K., Ho P.C. Integrin targeted drug and gene delivery. Expert Opin. Drug Deliv. 2010;7(2):159–171. doi: 10.1517/17425240903468696. [DOI] [PubMed] [Google Scholar]
- 127.Ellerby H.M., Arap W., Ellerby L.M., et al. Anti-cancer activity of targeted pro-apoptotic peptides. Nat. Med. 1999;5(9):1032–1038. doi: 10.1038/12469. [DOI] [PubMed] [Google Scholar]
- 128.Reardon D.A., Neyns B., Weller M., et al. Cilengitide: an RGD pentapeptide ανβ3 and ανβ5 integrin inhibitor in development for glioblastoma and other malignancies. Future Oncol. 2011;7(3):339–354. doi: 10.2217/fon.11.8. [DOI] [PubMed] [Google Scholar]
- 129.Kuwada S.K. Drug evaluation: Volociximab, an angiogenesis-inhibiting chimeric monoclonal antibody. Curr. Opin. Mol. Ther. 2007;9(1):92–98. [PubMed] [Google Scholar]
- 130.Francis S.E., Goh K.L., Hodivala-Dilke K., et al. Central roles of alpha5beta1 integrin and fibronectin in vascular development in mouse embryos and embryoid bodies. Arterioscler. Thromb. Vasc. Biol. 2002;22(6):927–933. doi: 10.1161/01.atv.0000016045.93313.f2. [DOI] [PubMed] [Google Scholar]
- 131.Sudhakar A., Sugimoto H., Yang C., et al. Human tumstatin and human endostatin exhibit distinct antiangiogenic activities mediated by alpha v beta 3 and alpha 5 beta 1 integrins. Proc. Natl. Acad. Sci. USA. 2003;100(8):4766–4771. doi: 10.1073/pnas.0730882100. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 132.Pedchenko V., Zent R., Hudson B.G. Alpha(v)beta3 and alpha(v)beta5 integrins bind both the proximal RGD site and non-RGD motifs within noncollagenous (NC1) domain of the alpha3 chain of type IV collagen: implication for the mechanism of endothelia cell adhesion. J. Biol. Chem. 2004;279(4):2772–2780. doi: 10.1074/jbc.M311901200. [DOI] [PubMed] [Google Scholar]
- 133.Marneros A.G., Olsen B.R. The role of collagen-derived proteolytic fragments in angiogenesis. Matrix Biol. 2001;20(5-6):337–345. doi: 10.1016/s0945-053x(01)00151-2. [DOI] [PubMed] [Google Scholar]
- 134.Marcinkiewicz C., Weinreb P.H., Calvete J.J., et al. Obtustatin: a potent selective inhibitor of alpha1beta1 integrin in vitro and angiogenesis in vivo. Cancer Res. 2003;63(9):2020–2023. [PubMed] [Google Scholar]
- 135.Nakamura T., Lozano P.R., Ikeda Y., et al. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature. 2002;415(6868):171–175. doi: 10.1038/415171a. [DOI] [PubMed] [Google Scholar]
- 136.White S.R., Dorscheid D.R., Rabe K.F., et al. Role of very late adhesion integrins in mediating repair of human airway epithelial cell monolayers after mechanical injury. Am. J. Respir. Cell Mol. Biol. 1999;20(4):787–796. doi: 10.1165/ajrcmb.20.4.3318. [DOI] [PubMed] [Google Scholar]
- 137.Adachi M., Taki T., Higashiyama M., et al. Significance of integrin alpha5 gene expression as a prognostic factor in node-negative non-small cell lung cancer. Clin. Cancer Res. 2000;6(1):96–101. [PubMed] [Google Scholar]
- 138.Caccavari F., Valdembri D., Sandri C., et al. Integrin signaling and lung cancer. Cell Adhes. Migr. 2010;4(1):124–129. doi: 10.4161/cam.4.1.10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wu H., Zhang Y., Xu Z., et al. The expression of integrin alpha5beta1 and transforming growth factor-beta in pulmonary fibrosis of rat. Zhonghua Bing Li Xue Za Zhi. 1999;28(6):427–431. [PubMed] [Google Scholar]
- 140.Saltini C., Hemler M.E., Crystal R.G. T lymphocytes compartmentalized on the epithelial surface of the lower respiratory tract express the very late activation antigen complex VLA-1. Clin. Immunol. Immunopathol. 1988;46(2):221–233. doi: 10.1016/0090-1229(88)90185-7. [DOI] [PubMed] [Google Scholar]
- 141.Gardner H., Kreidberg J., Koteliansky V., et al. Deletion of integrin alpha 1 by homologous recombination permits normal murine development but gives rise to a specific deficit in cell adhesion. Dev. Biol. 1996;175(2):301–313. doi: 10.1006/dbio.1996.0116. [DOI] [PubMed] [Google Scholar]
- 142.Pozzi A., Wary K.K., Giancotti F.G., et al. Integrin alpha1beta1 mediates a unique collagen-dependent proliferation pathway in vivo. J. Cell Biol. 1998;142(2):587–594. doi: 10.1083/jcb.142.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gardner H.A. Integrin signaling in fibrosis and scleroderma. Curr. Rheumatol. Rep. 1999;1(1):28–33. doi: 10.1007/s11926-999-0021-5. [DOI] [PubMed] [Google Scholar]
- 144.Holtkotter O., Nieswandt B., Smyth N., et al. Integrin alpha 2-deficient mice develop normally, are fertile, but display partially defective platelet interaction with collagen. J. Biol. Chem. 2002;277(13):10789–10794. doi: 10.1074/jbc.M112307200. [DOI] [PubMed] [Google Scholar]
- 145.Chen J., Diacovo T.G., Grenache D.G., et al. The alpha(2) integrin subunit-deficient mouse: a multifaceted phenotype including defects of branching morphogenesis and hemostasis. Am. J. Pathol. 2002;161(1):337–344. doi: 10.1016/s0002-9440(10)64185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Edelson B.T., Li Z., Pappan L.K., et al. Mast cell-mediated inflammatory responses require the alpha 2 beta 1 integrin. Blood. 2004;103(6):2214–2220. doi: 10.1182/blood-2003-08-2978. [DOI] [PubMed] [Google Scholar]
- 147.McCall-Culbreath K.D., Li Z., Zutter M.M. Crosstalk between the alpha2beta1 integrin and c-met/HGF-R regulates innate immunity. Blood. 2008;111(7):3562–3570. doi: 10.1182/blood-2007-08-107664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Menko A.S., Kreidberg J.A., Ryan T.T., et al. Loss of alpha3beta1 integrin function results in an altered differentiation program in the mouse submandibular gland. Dev. Dyn. 2001;220(4):337–349. doi: 10.1002/dvdy.1114. [DOI] [PubMed] [Google Scholar]
- 149.Yang J.T., Rayburn H., Hynes R.O. Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development. 1995;121(2):549–560. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]
- 150.Arroyo A.G., Yang J.T., Rayburn H., et al. Differential requirements for alpha4 integrins during fetal and adult hematopoiesis. Cell. 1996;85(7):997–1008. doi: 10.1016/s0092-8674(00)81301-x. [DOI] [PubMed] [Google Scholar]
- 151.Arroyo A.G., Taverna D., Whittaker C.A., et al. In vivo roles of integrins during leukocyte development and traffic: insights from the analysis of mice chimeric for alpha 5, alpha v, and alpha 4 integrins. J. Immunol. 2000;165(8):4667–4675. doi: 10.4049/jimmunol.165.8.4667. [DOI] [PubMed] [Google Scholar]
- 152.Yang J.T., Rayburn H., Hynes R.O. Embryonic mesodermal defects in alpha 5 integrin-deficient mice. Development. 1993;119(4):1093–1105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
- 153.Yang J.T., Bader B.L., Kreidberg J.A., et al. Overlapping and independent functions of fibronectin receptor integrins in early mesodermal development. Dev. Biol. 1999;215(2):264–277. doi: 10.1006/dbio.1999.9451. [DOI] [PubMed] [Google Scholar]
- 154.Goh K.L., Yang J.T., Hynes R.O. Mesodermal defects and cranial neural crest apoptosis in alpha5 integrin-null embryos. Development. 1997;124(21):4309–4319. doi: 10.1242/dev.124.21.4309. [DOI] [PubMed] [Google Scholar]
- 155.Miner J.H., Li C. Defective glomerulogenesis in the absence of laminin alpha5 demonstrates a developmental role for the kidney glomerular basement membrane. Dev. Biol. 2000;217(2):278–289. doi: 10.1006/dbio.1999.9546. [DOI] [PubMed] [Google Scholar]
- 156.Mayer U., Saher G., Fassler R., et al. Absence of integrin alpha 7 causes a novel form of muscular dystrophy. Nat. Genet. 1997;17(3):318–323. doi: 10.1038/ng1197-318. [DOI] [PubMed] [Google Scholar]
- 157.Hayashi Y.K., Chou F.L., Engvall E., et al. Mutations in the integrin alpha7 gene cause congenital myopathy. Nat. Genet. 1998;19(1):94–97. doi: 10.1038/ng0598-94. [DOI] [PubMed] [Google Scholar]
- 158.Muller U., Wang D., Denda S., et al. Integrin alpha8beta1 is critically important for epithelial-mesenchymal interactions during kidney morphogenesis. Cell. 1997;88(5):603–613. doi: 10.1016/s0092-8674(00)81903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Littlewood Evans A., Muller U. Stereocilia defects in the sensory hair cells of the inner ear in mice deficient in integrin alpha8beta1. Nat. Genet. 2000;24(4):424–428. doi: 10.1038/74286. [DOI] [PubMed] [Google Scholar]
- 160.Bader B.L., Rayburn H., Crowley D., et al. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell. 1998;95(4):507–519. doi: 10.1016/s0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- 161.Henderson N.C., Arnold T.D., Katamura Y., et al. Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat. Med. 2013;19(12):1617–1624. doi: 10.1038/nm.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Schmits R., Kundig T.M., Baker D.M., et al. LFA-1-deficient mice show normal CTL responses to virus but fail to reject immunogenic tumor. J. Exp. Med. 1996;183(4):1415–1426. doi: 10.1084/jem.183.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shier P., Ngo K., Fung-Leung W.P. Defective CD8+ T cell activation and cytolytic function in the absence of LFA-1 cannot be restored by increased TCR signaling. J. Immunol. 1999;163(9):4826–4832. [PubMed] [Google Scholar]
- 164.Berlin-Rufenach C., Otto F., Mathies M., et al. Lymphocyte migration in lymphocyte function-associated antigen (LFA)-1-deficient mice. J. Exp. Med. 1999;189(9):1467–1478. doi: 10.1084/jem.189.9.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Coxon A., Rieu P., Barkalow F.J., et al. A novel role for the beta 2 integrin CD11b/CD18 in neutrophil apoptosis: a homeostatic mechanism in inflammation. Immunity. 1996;5(6):653–666. doi: 10.1016/s1074-7613(00)80278-2. [DOI] [PubMed] [Google Scholar]
- 166.Rosenkranz A.R., Coxon A., Maurer M., et al. Impaired mast cell development and innate immunity in Mac-1 (CD11b/CD18, CR3)-deficient mice. J. Immunol. 1998;161(12):6463–6467. [PubMed] [Google Scholar]
- 167.Dong Z.M., Gutierrez-Ramos J.C., Coxon A., et al. A new class of obesity genes encodes leukocyte adhesion receptors. Proc. Natl. Acad. Sci. USA. 1997;94(14):7526–7530. doi: 10.1073/pnas.94.14.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Schon M.P., Arya A., Murphy E.A., et al. Mucosal T lymphocyte numbers are selectively reduced in integrin alpha E (CD103)-deficient mice. J. Immunol. 1999;162(11):6641–6649. [PubMed] [Google Scholar]
- 169.Tronik-Le Roux D., Roullot V., Poujol C., et al. Thrombasthenic mice generated by replacement of the integrin alpha(IIb) gene: demonstration that transcriptional activation of this megakaryocytic locus precedes lineage commitment. Blood. 2000;96(4):1399–1408. [PubMed] [Google Scholar]
- 170.Fassler R., Meyer M. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 1995;9(15):1896–1908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- 171.Stephens L.E., Sutherland A.E., Klimanskaya I.V., et al. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995;9(15):1883–1895. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
- 172.Hirsch E., Iglesias A., Potocnik A.J., et al. Impaired migration but not differentiation of haematopoietic stem cells in the absence of beta1 integrins. Nature. 1996;380(6570):171–175. doi: 10.1038/380171a0. [DOI] [PubMed] [Google Scholar]
- 173.Potocnik A.J., Brakebusch C., Fassler R. Fetal and adult hematopoietic stem cells require beta1 integrin function for colonizing fetal liver, spleen, and bone marrow. Immunity. 2000;12(6):653–663. doi: 10.1016/s1074-7613(00)80216-2. [DOI] [PubMed] [Google Scholar]
- 174.Keller R.S., Shai S.Y., Babbitt C.J., et al. Disruption of integrin function in the murine myocardium leads to perinatal lethality, fibrosis, and abnormal cardiac performance. Am. J. Pathol. 2001;158(3):1079–1090. doi: 10.1016/S0002-9440(10)64055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Scharffetter-Kochanek K., Lu H., Norman K., et al. Spontaneous skin ulceration and defective T cell function in CD18 null mice. J. Exp. Med. 1998;188(1):119–131. doi: 10.1084/jem.188.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mizgerd J.P., Kubo H., Kutkoski G.J., et al. Neutrophil emigration in the skin, lungs, and peritoneum: different requirements for CD11/CD18 revealed by CD18-deficient mice. J. Exp. Med. 1997;186(8):1357–1364. doi: 10.1084/jem.186.8.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schneeberger E.E., Vu Q., LeBlanc B.W., et al. The accumulation of dendritic cells in the lung is impaired in CD18-/- but not in ICAM-1-/- mutant mice. J. Immunol. 2000;164(5):2472–2478. doi: 10.4049/jimmunol.164.5.2472. [DOI] [PubMed] [Google Scholar]
- 178.Hodivala-Dilke K.M., McHugh K.P., Tsakiris D.A., et al. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J. Clin. Invest. 1999;103(2):229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.McHugh K.P., Hodivala-Dilke K., Zheng M.H., et al. Mice lacking beta3 integrins are osteosclerotic because of dysfunctional osteoclasts. J. Clin. Invest. 2000;105(4):433–440. doi: 10.1172/JCI8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zimmerman D., Jin F., Leboy P., et al. Impaired bone formation in transgenic mice resulting from altered integrin function in osteoblasts. Dev. Biol. 2000;220(1):2–15. doi: 10.1006/dbio.2000.9633. [DOI] [PubMed] [Google Scholar]
- 181.Dowling J., Yu Q.C., Fuchs E. Beta4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J. Cell Biol. 1996;134(2):559–572. doi: 10.1083/jcb.134.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Huang X., Griffiths M., Wu J., et al. Normal development, wound healing, and adenovirus susceptibility in beta5-deficient mice. Mol. Cell. Biol. 2000;20(3):755–759. doi: 10.1128/mcb.20.3.755-759.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wagner N., Lohler J., Kunkel E.J., et al. Critical role for beta7 integrins in formation of the gut-associated lymphoid tissue. Nature. 1996;382(6589):366–370. doi: 10.1038/382366a0. [DOI] [PubMed] [Google Scholar]
- 184.Zhu J., Motejlek K., Wang D., et al. beta8 integrins are required for vascular morphogenesis in mouse embryos. Development. 2002;129(12):2891–2903. doi: 10.1242/dev.129.12.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ding Z.M., Babensee J.E., Simon S.I., et al. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J. Immunol. 1999;163(9):5029–5038. [PubMed] [Google Scholar]
- 186.Pulkkinen L., Uitto J. Mutation analysis and molecular genetics of epidermolysis bullosa. Matrix Biol. 1999;18(1):29–42. doi: 10.1016/s0945-053x(98)00005-5. [DOI] [PubMed] [Google Scholar]
- 187.Miner J.H., Cunningham J., Sanes J.R. Roles for laminin in embryogenesis: exencephaly, syndactyly, and placentopathy in mice lacking the laminin alpha5 chain. J. Cell Biol. 1998;143(6):1713–1723. doi: 10.1083/jcb.143.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wagner N., Lohler J., Tedder T.F., et al. L-selectin and beta7 integrin synergistically mediate lymphocyte migration to mesenteric lymph nodes. Eur. J. Immunol. 1998;28(11):3832–3839. doi: 10.1002/(SICI)1521-4141(199811)28:11<3832::AID-IMMU3832>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 189.Lin K., Ateeq H.S., Hsiung S.H., et al. Selective, tight-binding inhibitors of integrin alpha4beta1 that inhibit allergic airway responses. J. Med. Chem. 1999;42(5):920–934. doi: 10.1021/jm980673g. [DOI] [PubMed] [Google Scholar]
- 190.Abraham W.M., Gill A., Ahmed A., et al. A small-molecule, tight-binding inhibitor of the integrin alpha(4)beta(1) blocks antigen-induced airway responses and inflammation in experimental asthma in sheep. Am. J. Respir. Crit. Care Med. 2000;162(2 Pt 1):603–611. doi: 10.1164/ajrccm.162.2.9911061. [DOI] [PubMed] [Google Scholar]
- 191.Ravensberg A.J., Luijk B., Westers P., et al. The effect of a single inhaled dose of a VLA-4 antagonist on allergen-induced airway responses and airway inflammation in patients with asthma. Allergy. 2006;61(9):1097–1103. doi: 10.1111/j.1398-9995.2006.01146.x. [DOI] [PubMed] [Google Scholar]
- 192.Diamant Z., Kuperus J., Baan R., et al. Effect of a very late antigen-4 receptor antagonist on allergen-induced airway responses and inflammation in asthma. Clin. Exp. Allergy. 2005;35(8):1080–1087. doi: 10.1111/j.1365-2222.2005.02296.x. [DOI] [PubMed] [Google Scholar]
- 193.Sircar I., Gudmundsson K.S., Martin R., et al. Synthesis and SAR of N-benzoyl-L-biphenylalanine derivatives: discovery of TR-14035, a dual alpha(4)beta(7)/alpha(4)beta(1) integrin antagonist. Bioorg. Med. Chem. 2002;10(6):2051–2066. doi: 10.1016/s0968-0896(02)00021-4. [DOI] [PubMed] [Google Scholar]
- 194.Cortijo J., Sanz M.J., Iranzo A., et al. A small molecule, orally active, alpha4beta1/alpha4beta7 dual antagonist reduces leukocyte infiltration and airway hyper-responsiveness in an experimental model of allergic asthma in Brown Norway rats. Br. J. Pharmacol. 2006;147(6):661–670. doi: 10.1038/sj.bjp.0706658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Beekman K.W., Colevas A.D., Cooney K., et al. Phase II evaluations of cilengitide in asymptomatic patients with androgen-independent prostate cancer: scientific rationale and study design. Clin. Genitourin. Cancer. 2006;4(4):299–302. doi: 10.3816/CGC.2006.n.012. [DOI] [PubMed] [Google Scholar]


