Abstract
Objective:
Osteoporotic fracture is one of the most common health risks and aggravates the quality of life among postmenopausal women worldwide. In this study, osteoporosis-associated protein biomarkers were identified from urine of osteoporotic female Sprague-Dawley rats developed by ovariectomy.
Method:
Four months after the operation, the bone mineral density of the femur of ovariectomized rats was significantly lowered in comparison with that of the sham operated rats. The protein profiles of the urine samples collected from the sham, ovariectomized (OVX) and 2 month-old non-operated (Young) rats were compared by 2-D gel and MS spectrometry.
Results:
Proteins consistently expressed between Young and sham but differentially expressed in OVX rats were selected and identified. One down-regulated 21 kDa protein, superoxide dismutase (SOD), and 1 up-regulated 53-54 kDa protein, alph-1-antitrypsin (A1AT), were selected from urine of the ovariectomized rats by 2-D gel analysis. Further, a total of 30 with 19 up-regulated and 11-down-regulated proteins were selected by LC-MS analysis with more than 2-fold differences in spectral counts. The fact that SOD and A1AT are also listed in the 30 differential proteins suggests that our biomarker isolation procedure suitably represents osteoporosis-associated proteins in urine.
Conclusion:
Supporting the facts, the differential expressions of SOD and A1AT in urine could be validated by Western blotting. These urinary osteoporosis-associated proteins have high potentials to become candidates for non-invasive diagnosis of osteoporosis from urine.
Keywords: 2-D gel, biomarker, mass spectrometry, osteoporosis, ovariectomized rat urine, proteomics
1. INTRODUCTION
Osteoporosis defined as a systemic skeletal disease with increased bone fragility and fracture from low bone mass and micro-architectural deterioration of bone tissue is a common disease associated with aging and menopause, and is a major public health problem in aging population worldwide [1]. Bone homeostasis is dynamically controlled by a balanced bone resorption by osteoclasts, which are originated from myeloid progenitors, and new bone formation by osteoblasts, which are mesenchymal origin and common precursor cell with adipocytes [2]. Many trophic and cell differentiating factors are involved in bone remodeling. One of the factors, estrogen, a sex hormone, governs bone homeostasis by regulating the primary bone remodeling regulators, receptor for activated nuclear factor kappa B and its ligand, and osteoprotegerin system [3].
To date, a large number of histological, biochemical and genetic markers related to osteoporosis have been identified (reviewed in [4, 5]. However, so far, most physicians rely on bone mineral density (BMD) by dual-energy x-ray absorptiometry (DEXA) scanning for diagnosis and treatment of patients with bone fragility, although BMD by itself may not be always an accurate predictor of bone strength. In addition, the clinical potentials of bone turnover markers for monitoring bone fracture risk are limited due to their variability among individuals [6]. Thus, more biomarkers for better diagnosis and prognosis of osteoporosis are necessary, if possible, effective noninvasive biomarkers for the efficient analysis of bone health and fracture risk prediction.
Urine as a filtrate of plasma represents protein contents in the plasma and is easily obtained for noninvasive diagnostic purposes. Biomarker development based on proteome analysis of urine has provided new information and illustrated its potential value for diagnosis, prognosis and prediction of therapeutic response [7]. Advances in mass spectrometry (MS) have dramatically made progresses in identifying and quantifying proteins in urine. Proteomic analysis of urine by MS provides us good chances of developing noninvasive methods to identify novel biomarkers. However, same as in most of biological samples, the variability of human urine proteome among individuals [8] has been one of the obstacles to development of disease-induced protein biomarkers. Many investigators in biomarker development have tried various ways to reduce the variations between subjects (age, race, gender, etc.) and within subjects (time, day, biorhythmic fluctuation, etc.) by handling the samples pooled, less fractionated, and treated in fewer steps for better representation of the total proteome of a population [8-10]. Nevertheless, it is not possible to control the innate problems of the samples of human specimens, which are affected by environmental factors including cultural and dietary differences of the individuals. To circumvent those problems, researchers collect urine samples from model animals, such as rats or mice, which have more homogenous genetic backgrounds, and are grown in same environment with same diets, can provide less variable proteome profiles in urine of the experimental groups.
In this study, we collected urine samples from ovariectomy (OVX)-induced osteoporosis model rats to isolate urinary proteins for osteoporosis biomarker development. From the comparisons with normal and osteoporotic urinary proteins by 2-D gel analysis and mass spectrometry, we found some osteoporosis specific protein biomarkers. For the validation of the identified biomarker proteins, we tested normal and osteoporotic urine by Western blot analysis, and showed that the selected proteins are indeed osteoporosis associated.
The osteoporosis associated proteins identified in this study could be good candidates for biomarkers of non-invasive diagnosis of osteoporosis with a verification of large scale clinical tests.
2. MATERIALS AND METHODs
2.1. Animals
Twenty 8-month old Sprague-Dawley female rats (SLC Inc., Hamamatsu, Japan) were housed at 23˚C and 60% humidity with ad libitum access to food and water at 12/12-h light/dark cycle. After two weeks, to generate ovariectomized (OVX) group, ovariectomy was performed according to the published protocol [11]. Briefly, the anesthesia was established by intraperitoneal injection of 2 mg of Zoletil 50 (Virbac, France)/kg rat and the ovaries were exposed and removed (n=10). The sham-operated group (n=10) was treated in the same way except for the removal of the ovaries. After surgery, each rat was i.p. injected with Penicillin-G (20,000 IU). The rats were further maintained up to 4 months. All animal procedures were followed by the Kyungpook National University (KNU) Animal Care and Use Committee.
2.2. Plasma Estrogen (E2) Level
Blood samples (up to 0.2 mL) were withdrawn from the tail aorta in heparinized syringes and centrifuged for 20 min at RT. Plasma E2 concentration was determined by using a commercial kit (Diagnostic Products, Los Angeles, CA). All samples and standards were measured in duplicate and repeated twice.
2.3. Measurement of Bone Mineral Density by Dual Energy X-ray Absorptiometry (DEXA)
Femurs were extracted from euthanized rats, cleansed soft tissue, and frozen at − 20 °C until analyzed. For the areal bone mineral density (areal BMD), femurs were slowly thawed and immersed in PBS and positioned in the anterior posterior view on the specimen table. The total area (cm2), bone mineral content (g), and areal BMD (g/cm2) were measured in the whole femur by dual-energy X-ray absorptiometry (DEXA) with a software version 2.0 (Lunar PIXImus2, Madison, WI, USA) according to the manufacturer’s instructions.
2.4. Protein Sample Preparation and 2-DE
Urine samples were collected from individual rats using metabolic cages for 24 h in a bottle containing 2 mL deionized water with 2X protease inhibitor cocktail (Complete-Mini EDTA-Free, Roche, Indianapolis, USA). Then, for a batch of protein extraction, each urine sample equivalent to 2 mL of the original urine was pooled and kept on ice until use. The urinary proteins were prepared by following Oh’s method [8] with slight modification of treating the samples with 2 mM EDTA as described previously [12]. Briefly, the urine samples treated with 2 mM EDTA were dialyzed (MWCO 10000) against 50 vol-umes of deionized water at 4 oC with four changes in 24 h. The dialyzed urine was concentrated by lyophilization. The lyophilized urine sample was dissolved in 5 mL of deionized water, and further dialysed two times against 500 mL of dis-tilled water at 4 oC for 24 h in order to remove any salts that could interfere with the subsequent focusing process in IEF. The dialyzed urinary proteins were precipitated with 10% (mg/mL) trichloroacetic acid. The protein precipitates were collected as pellets by centrifugation and washed thoroughly for 3 times with 100% ethanol, and finally with 70% (mL/mL) ethanol, and then dried. The urinary proteins were dissolved in rehydration buffer containing 7 M urea, 2 M thiourea, 4% (mg/mL) CHAPS, 1 M DTT and 0.4% (mL/mL) of an ampholyte, Biotyte 3/10 (Biolyte, 3/10, Bio-Rad, Herculus, CA, USA). The protein samples in rehydra-tion buffer were applied to immobilized pH gradient strips (pH 4-7, 17 cm; Bio-Rad, Herculus, CA USA) for the first dimensional isoelectric focusing and separated on second dimensional SDS-PAGE. To subtract simply aging related differential urinary proteins, a comparison set of pooled urines from eight 7-week old (Young) rats and sham oper-ated rats were analyzed on 2-D gels. The differential proteins from the comparison between Young and sham were se-lected and deleted from the list of the differential proteins from sham and OVX comparison set. The comparisons were performed in periods of 2 months and 4 months. The consistently increased or decreased protein spots at 4-month period were selected as osteoporosis associated biomarker proteins and subjected for the mass spectrometric analysis for the identification of the proteins.
2.5. Mass Spectrometry Analysis and Protein Identification
The spots of interest were manually excised, washed with deionized water and destained using 50 mM ammonium bicarbonate/acetonitrile (6:4, mL/mL) with vigorous shaking. Digestion was carried out by adding sequencing grade modified trypsin (Promega, Madison, WI, USA) onto the dried gel pieces and incubated overnight at 37 °C. The peptides were extracted with extraction buffer (60% (mL/mL) ACN in water and 0.1% (mg/mL) TFA) after the digestion, and were dried with the aid of vacuum drier. From the concentrated peptide extract, 1 μL was taken and mixed with 1 μL of the matrix solution (10% (mg/mL) CHCA in 50% (mL/mL) methanol and 0.1% TFA containing internal standards such as bradykinin, angiotensin and neurotensin) on a target MALDI plate.
The acquired mass spectra were analyzed by Mascot from Matrix Science ( http://www.matrixscience.com ) and MS-Fit from Protein Prospector ( http://prospector.ucsf.edu/ ).
2.6. One Dimensional Gel Electrophoresis and Coomassie Brilliant Blue Staining
The urine proteins from the rats of three groups, sham, OVX and young, prepared by the above mentioned method for 2-D gel analysis and dissolved in reducing SDS sample buffer (2% (mg/mL) SDS, 10% (mL/mL) glycerol, 0.005% (mg/mL) bromophenol blue, and 5% (mL/mL) β-mercaptoethanol in 63 mM Tris-HCl, pH 6.8) were separated by 10% SDS-PAGE. After gel electrophoresis, the gel was slightly stained with Coomassie Brilliant Blue G-250 (0.06% (mg/mL) Coomassie Brilliant Blue G-250, 3% (mL/mL) perchloric acid) for 1 hr and destained overnight with deionized water, and then, each lane was cut into 10 slices.
In-gel Digestion with Trypsin—Each of the gel slices from three different urine samples was cut into pieces (1-mm cubes) and in-gel digested with trypsin. The tryptic digested peptides were extracted from the gel pieces as described by Soskic and Godovac-Zimmermann [13]. Briefly, the gel pieces were washed with 50 mM NH4HCO3 and 50% (mL/mL) CH3CN in 50 mM NH4HCO3 to remove SDS and Coomassie Blue dye. After destaining, 5 mM dithiothreitol/25 mM NH4HCO3 was added to the tube to cover the gel pieces for reduction, and incubated at 60 oC for 30 min. Then, the gel pieces were dehydrated with 100% CH3CN, dried in a vacuum concentrator, and swollen and digested in 50 mM NH4HCO3 buffer (pH, 8.0) containing 12.5 ng trypsin/μL overnight at 37 °C. After digestion, the peptides were extracted three times with 5% (mL/mL) formic acid, 50% CH3CN, collected to new tubes, and dried in a vacuum concentrator. The peptide powder was dissolved in 0.1% formic acid and further analyzed by a μ-LC-MS/MS procedure.
2.7. μ-LC Mass Spectrometry Analysis and Protein Identification
Tryptic peptides from each of the gel slices were analyzed using an LTQ linear ion trap mass spectrometer (Thermo Finnigan, San Jose, CA, USA) equipped with a nanospray source (Thermo Finnigan) by slightly modifying the Hwang et al.’s method [14]. Briefly, samples in 12 μL of 0.1% formic acid were first injected into a peptide CapTrap cartridge for peptide concentration and desalting, and loaded into an in-house C18 microcolumn (5-μm bead size, 100-nm pore size, 100-μm inner diameter, 10-cm length, Column Engineering Inc., Ontario, Canada) with a microautosampler (EMMEN, The Netherlands) and separated by an Agilent 1100 high performance binary pump. Peptides were separated at a flow rate of 200 nL/min using a linear solvent gradient from 100% solvent A (5% (mL/mL) CH3CN, 0.4% (mL/mL) acetic acid, and 0.005% (mL/mL) heptafluorobutyric acid) to 80% solvent B (100% acetonitrile, 0.4% acetic acid, and 0.005% heptafluorobutyric acid) over 100 min. Positive ions were generated by electrospraying the eluent and directly introduced into an LTQ mass spectrometer. The mass spectrometer was operated in a data-dependent acquisition mode in the 300-1800 m/z range in which each full MS scan was followed by one MS/MS scan of the most intense ion with charge states +2 to +4 and fragmented by collision-induced dissociation (CID) using a normalized collision energy of 35%. The temperature of the heated capillary and electrospray voltage were 195 oC and 2.0 kV, respectively.
The peptide intensities were normalized against total intensities. Parent and fragment ions were selected with tolerances of ± 100 ppm and ± 1 Da, respectively. All acquired MS/MS data were queried against the NCBI rat protein database using the SEQUEST algorithm (Thermo Electron, San Jose, CA). The resulting SEQUEST output files were loaded for identification of peptides and proteins using PeptideProphet and ProteinProphet software [15, 16], and assembled a complete protein list from the rat urine fractions using the INTERACT software [17]. In addition, the peptide identification processes were further filtered using the size of the peptide greater than six amino acids and considering different modifications, such as, deamination, oxidation, and phosphorylation.
2.8. Western Blotting
For Western blotting, equal amounts of urinary proteins were loaded in each lane of 10% (mg/mL) SDS-PAGE gel and separated. After gel electrophoresis and protein transfer to PVDF membrane (Millipore, Billerica, MA, USA), the membranes were blocked with 3% (mg/mL) BSA, and probed with properly diluted (1:300-1:500) various monoclonal primary antibodies (Santa Cruz, Dallas, TX, USA) in 3% BSA with 0.1% (mL/mL) Tween-20 in PBS for 4 hr at RT, respectively. Following this, 1:10000 diluted goat anti-mouse secondary antibody conjugated with horse radish peroxidase (Sigma-Aldrich, St. Louis, MO, USA) in 0.1% Tween 20 in PBS was incubated with the membrane for 1 hr at RT. Immunoreactivity was detected using the LAS-3000 scanner (Fuji, Tokyo, Japan) after extensive washing with 0.1% Tween-20 in PBS and chemiluminescence staining (SuperSignal Picochemiluminescent Substrate, ThermoFisher, PA, USA). The intensities of the decorated bands were normalized against the total protein loaded in each lane.
2.9. Statistical Analysis
Statistical analyses were performed using SPSS software (SPSS Korea Plus, Seoul, Korea). The mean ± SEM was calculated for each group and compared by paired Student’s t-tests. P<0.05 was considered statistically significant.
3. RESULTS and DISCUSSIONS
Isolation of biomarkers for non-invasive diagnosis of osteoporosis is very demanding in the prevention and monitoring of the disease. The orthogonal rat model for osteoporosis and the urine-based proteomic approach using 2-D gel analysis and mass spectrometry could allow us to reduce the environmental and biological variations, and provide us a premier platform for isolating potential osteoporosis-associated biomarkers, which can be used for developing non-invasive diagnosis.
We designed the experiment to enhance the possibility of the selection of the osteoporosis-associated biomarkers by comparing the urinary proteins from sham operated and ovariectomized 8 month old rats after 4 month of the operation, and at the same time to compare urinary proteins from 2 month old rats (Young group) with sham and OVX to select and eliminate the simply age-related up- or down-regulated proteins. The comparison between Young control and Sham for the spot intensities on 2-D gel and spectral counts from MS analysis could provide us information which proteins are changed as rats are aged. And the information on the age-related proteins helped us focus on the other osteoporosis related protein markers from the lists of proteins obtained from Sham and OVX groups by 2-D gel and MS/MS analysis.
3.1. Serum Estrogen Level and Bone Density
The induction of osteoporosis to rats was assessed four months after ovariectomy by analyzing the serum estrogen level (pg/mL) and BMD. The lower serum estrogen level in the ovariectomized group (44±2.14, n=10), which is similar to the before puberty group (45.6±2.07, n=3), when compared with the Sham (99.4±7.5, n=10) or 12 week-old young adult group (103±6.13, n=3) (Fig. 1A), suggests that the operation of ovariectomy was successful. The BMD (g/mL) of the femurs from the ovariectomized rats (0.18±0.016, n=10) were significantly lower than those of the sham operated rats (0.26±0.022, n=10) (Fig. 1B). As the reduction of bone mineral density of femur measured by DEXA is considered as the standard method and a good hallmark for the progression of osteoporosis [6], we could proceed for the osteoporosis associated biomarker development in urine.
Fig. (1).
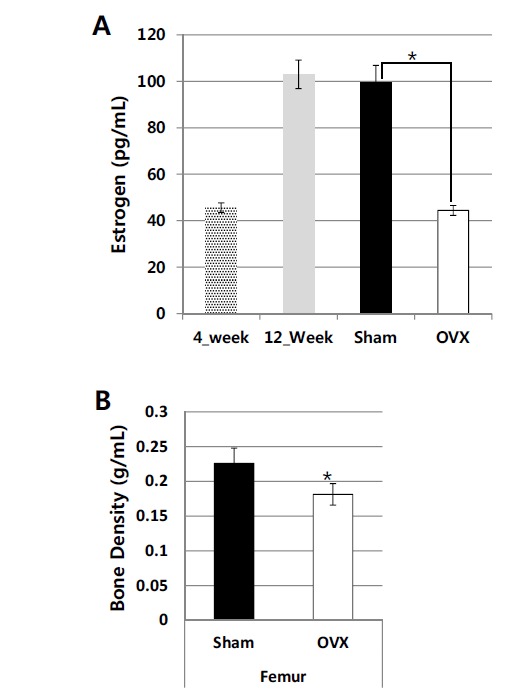
Serum estradiol levels (A) and BMD (B) were measured from 4-week-old (n=3), 12-week-old (n=8), sham (12-month-old, n=10), and OVX (12-month-old, n=10) rats. Blood and bone samples were taken 4 months post-ovariectomy. The statistical differences between sham and OVX samples are designated with * (p<0.05). The serum estrogen level and BMD were determined by ELISA kit and DEXA, respectively.
3.2. Proteomic Discovery of Osteoporosis Associated Biomarkers in Rat Urine
3.2.1. Selection of Rat Urinary Biomarkers on 2-D Gels
Having experienced that the extraneous protein contamination to the urine sample via fecal contamination and any possibility of proteolytic cleavage of the urinary protein could affect the urinary protein profiles, we took extra cares in urine sampling by adding 2 ml of 2x concentrated protease inhibitors cocktail to the collecting bottles in the metabolic cages and in person monitoring. An average of 4 mL of urine was collected from each rat. Equal volume of urine was taken from each sample to make pooled urine for each group of 10 rats. Treatment of 2 mM EDTA to urine to remove divalent cation by dialysis could improve the resolution of 2D gels without sacrificing the representation of the total proteins. Our sample preparation method, which minimizes sample handling, efficiently represents proteins from a sample. Expectedly, the 2-D gel profiles of the rat urine proteome were much more comparable within and between sample runs (Fig. 2) as reported previously [8, 12]. During the period of inducing osteoporosis for 4 month, urine samples were taken and pooled at 2 month, and the protein profiles were compared on 2-D gels. Several candidate osteoporosis associated proteins were selected including SOD and A1AT. Final 2-D analysis on Sham and OVX urine was performed at 4 month. This time, the significantly and repeatedly changed urine protein spots in 2 month and 4 month of inducing osteoporosis in rats were isolated from the 2D gels and identified by MS analysis. The differential proteins in OVX urine were identified as up-regulated alpha-1-anti-trypsin, and down-regulated superoxide dismutase (Fig. 2 and Table 1).
Fig. (2).
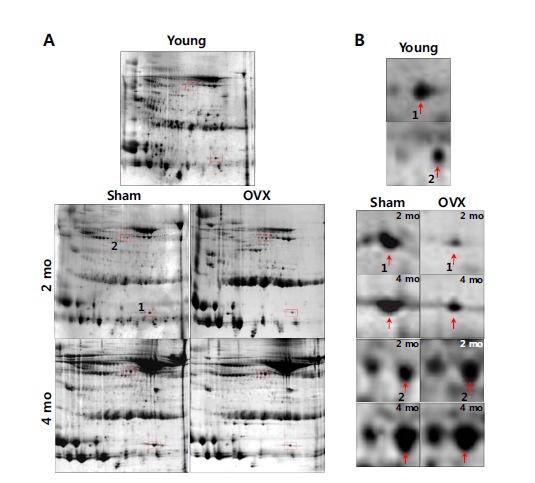
Selection of osteoporosis-associated urinary proteins on 2-D gel. A) Urine from sham (n=10) and OVX (n=10) rats was collected for 2 months (2 mo) and 4 months (4 mo) post-operation, and processed to prepare proteins for 2-D gel analysis (pH 4-7). A urine control from 3-month-old (Young, n=8) rats was included to exclude age-related differential proteins, and select osteoporosis-associated proteins from sham and OVX (boxed in broken line) rats. B) The enlarged panels of the differential proteins from 2-D gels are indicated by arrows.
Table 1.
Osteoporosis-associated differential urinary proteins selected from 2-D gel analysis and identified by peptide mass fingerprinting.
3.2.2. Osteoporosis Associated Urinary Proteins Selected by Quantitative MS/MS Analysis
Due to the low reproducibility and biased representation of protein profiles on 2-D gels, we tried another option for seeking more protein markers and verifying the results from 2-D gel analysis, urine proteins from Young, Sham and OVX rats were digested with trypsin and analysed by LC-MS/MS [14]. One hundred and one differential proteins (>2-folds) were selected from rat urinary proteins prepared from Young, sham and OVX groups by comparing the MS/MS spectrum counts. From comparison between the normalized spectrum counts from Young and sham samples, we firstly defined aging related proteins if the ratios of Young/sham are >1.5 or <0.67, respectively, and deleted them from the list of the differential proteins. Then, the proteins with the ratio of sham/OVX greater than 2 or lower than 0.5 were selected as down- or up-regulated osteoporosis associated proteins, respectively. We selected 19 up-regulated and 11 down-regulated osteoporosis associated proteins (Table 2) from OVX urine. As one third of the selected 30 osteoporosis-associated proteins are known to be correlated with bone metabolism (designated as “up or down” in Table 2), the result suggests that our selection procedure using the orthogonal rat model fairly represents human osteoporosis-associated biomarkers in urine. Interestingly, the 2 differential proteins, SOD and A1AT, selected from 2-DE analysis, are all listed in Table 2, of which lists were selected by quantitative MS/MS analysis.
Table 2.
Osteoporosis-associated differential urinary proteins analyzed by a quantitative MS/MS.
| Accession # | Protein Name | Normalized Spectrum Count |
Plasma Level in
Osteoporosis |
References | ||
|---|---|---|---|---|---|---|
| Young | Sham | OVX | ||||
| P17475 | A1AT | 0 | 0 | 16 | Down | [22] |
| Q63213 | Alpha-2u globulin | 17 | 10 | 43 | NRa | |
| Q7TPI9 | Antithrombin-III | 0 | 0 | 8 | NR | |
| P04639 | Apolipoprotein A-I | 7 | 10 | 27 | NR | |
| M0R8A9 | Apolipoprotein A-IV | 0 | 0 | 27 | NR | |
| P26644 | Beta-2-glycoprotein 1 | 8 | 10 | 26 | NR | |
| Q8K3U6 | Coagulation factor VII | 0 | 0 | 32 | NR | |
| P01026 | Complement C3 | 0 | 0 | 11 | NR | |
| Q8VHC1 | Cystatin-M | 0 | 0 | 10 | NR | |
| P14740 | Dipeptidyl peptidase 4 | 27 | 24 | 58 | Up | [23] |
| Q62894 | Extracellular matrix protein 1 | 0 | 0 | 12 | Down | [24] |
| Q7TQ70 | Fibrinogen alpha chain | 0 | 0 | 14 | Up | [25] |
| P07314 | Gamma-glutamyltranspeptidase | 17 | 12 | 44 | Up | [26] |
| P20059 | Hemopexin | 0 | 0 | 9 | NR | |
| P35572 | Insulin-like growth factor-binding protein | 0 | 0 | 8 | Down | [27] |
| P28826 | Meprin A beta-subunit | 7 | 10 | 52 | NR | |
| P12346 | Serotransferrin | 7 | 9 | 50 | Up | [28] |
| P07724 | Serum albumin | 214 | 287 | 718 | No association | [29] |
| P02767 | Transthyretin | 0 | 0 | 8 | Up | [30] |
| P23593 | Apolipoprotein D | 16 | 25 | 8 | NR | |
| O08779 | CD44 antigen | 29 | 11 | 0 | NR | |
| Q9Z0M0 | Complement decay-accelerating factor | 11 | 17 | 0 | NR | |
| Q08420 | Extracellular superoxide dismutase [Cu-Zn] | 11 | 15 | 0 | Down | [31] |
| Q6AXM6 | Intercellular adhesion molecule 2 | 12 | 9 | 0 | NR | |
| Q63691 | Monocyte differentiation antigen CD14 | 8 | 11 | 0 | NR | |
| P08721 | Osteopontin | 12 | 10 | 0 | Down | [32] |
| Q56A20 | Phosphoinositide-3-kinase-interacting protein | 21 | 23 | 0 | NR | |
| P07632 | Superoxide dismutase [Cu-Zn] | 19 | 19 | 9 | Down | [17] |
| P34901 | Syndecan-4 | 29 | 22 | 0 | NR | |
| D3ZAE6 | Vasorin | 44 | 41 | 13 | NR | |
a NR, not reported
A study analyzing the relationship between the activities of anti-oxidative enzymes, SOD, glutathione peroxidase and catalase in the plasma, and osteoclastic activity demonstrated that reactive oxygen species quenching suppresses bone turnover to restore bone strength and microarchitecture [18-20]. These results are in line with our present finding that SOD level in urine is decreased in OVX rats than in age-matched sham individuals.
With an anti-inflammatory property, the plasma concentration of A1AT, a serine protease inhibitor is known to be correlated to bone density [21-22]. In this study, however, A1AT in OVX urine is up-regulated. Similar kind of inverse relationship between urine and plasma level of adiponectin has been reported in kidney disease [23]. The relationships of urine and plasma A1AT level need to be confirmed.
The biomarkers already known to be associated with osteoporosis in Table 2 with references [17, 22, 24-33] are all blood borne. However, in this study, we report urine origin biomarkers, which are repeatedly found by both 2-D gel and LC-MS/MS methods.
3.3. Validation of the Potential Osteoporosis Biomarkers in Urine by Western Blotting
As SOD and A1AT are overlapped urinary biomarker for osteoporosis by proteomic analysis in 2 D gel and MS/MS analyses, we tried to validate the results by Western blotting. A comparable amount of urine proteins from the pooled Young, sham and OVX samples were run together with the protein from a human cell line as a positive control on 1-D gel (Fig. 3). The Western blotting result shows that the SOD level in the pooled urine from sham operated rats (12 month old) was decreased compared to that of 3 month-old Young rats. Likewise, decreased SOD level in the pooled urine of OVX rats was observed compared to the pooled urine of Sham rats (Fig. 3. left panel). Similar result was shown from the individual urine of Sham and OVX rats (Fig. 3, right panel). As expected from the results of 2-D gel analyses, anti-SOD antibody showed decreased band intensity of 18 kDa protein in OVX urine compared to Young and sham. Contrarily, anti-A1AT antibody decorated increased band intensity of the 53 kDa protein in OVX urine, while Young and sham group showed lower intensities. These results consistently suggest that SOD and A1AT can be used as urinary biomarker for osteoporosis diagnosis.
Fig. (3).
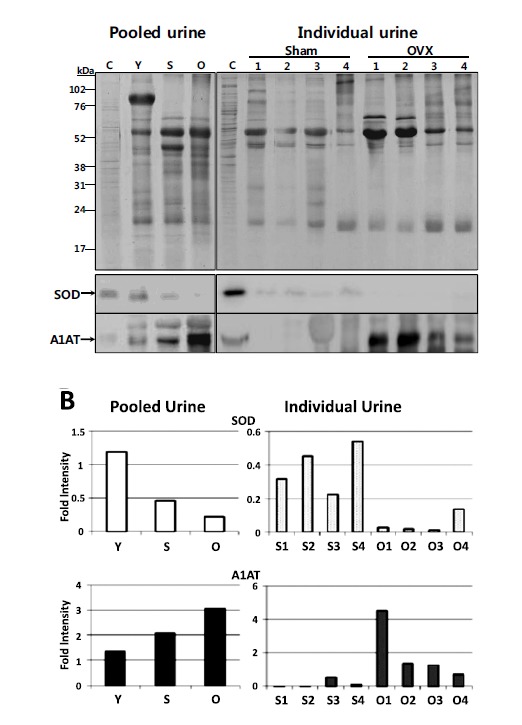
Western blot analysis of SOD and A1AT expression in urine. A) Equal amounts of proteins from 3-month-old (Y), sham (S), and OVX (O) rats, either pooled or individual (1 to 4), were separated on 12% SDS-PAGE gels and stained with Coomassie Brilliant Blue R-250 (upper panel). The major proteins in the urine samples are transferrin (78 kDa, *), albumin (66 kDa, **), immunoglobulin, heavy chain (53 kDa, ***), and immunoglobulin, light chain (23 kDa, ****), respectively. For Western blotting (lower panel), the separated proteins were transferred to PVDF membrane and immunoblotted by anti-SOD and anti-A1AT antibodies, respectively (designated by arrows). Protein from HepG2 cell (C) was used as a positive control. The individual samples of Sham and OVX were chosen if there were enough amounts of urine left for Western blotting analysis. B) Densitometry analysis data of the SOD and A1AT bands on the Western blots were normalized by the densities of the controls from HepG2 cell.
The comparative proteomic analysis of the ovariectomized rat model provides a panel of significantly up- or down-regulated urinary proteins that are associated with osteoporosis. The expression patterns of single or multiple proteins in urine of osteoporosis patients can give us insights to diagnose the disease in non-invasive ways. Especially, SOD and A1AT proteins, which were found to be significantly altered in osteoporotic urine by 2D gel and MS/MS analyses, and also validated by Western blotting, can be promising biomarkers for developing non-invasive diagnostic assays.
CONCLUSION
In this study, we present urinary protein biomarkers, which can be used for the diagnosis and monitoring of osteoporosis. The comparative proteomic analysis of the OVX rat model provides a panel of significantly upregulated or downregulated urinary proteins that are associated with osteoporosis. Especially, SOD and A1AT proteins, which are found to be significantly altered in osteoporotic urine by 2D gel and MS/MS analyses and validated by Western blotting, appear to be promising biomarkers for the development of non-invasive diagnostic assays. These rat urinary proteins can be good candidates for evaluation in human osteoporosis urine samples to test the possibilities for their use in the diagnosis of the disease.
ACKNOWLEDGEMENTS
This research was supported by the Kyungpook National University Research Fund, 2011.
LIST OF ABBREVIATIONS
- 2-DE
2-Dimensional Gel Electrophoresis
- A1AT
Alpha-1-Antitrypsin
- BMD
Bone Mineral Density
- BSA
Bovine Serum Albumin
- EDTA
Ethylene Diamine Tetra Acetic Acid
- IEF
Isoelectric Focusing
- MALDI
Matrix Assisted Laser Desorption Ionization
- MS
Mass Spectrometry
- MS/MS
Tandem Mass Spectrometry
- OVX
Ovariectomized
- SDS-PAGE
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis
- SOD
Superoxide Dismutase
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.WHO Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ. Tech. Rep. Ser. 1994;843:1–129. [PubMed] [Google Scholar]
- 2.Boyle W.J., Simonet W.S., Lacey D.L. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 3.Ho T.Y., Santora K., Chen J.C., Frankshun A.L., Bagnell C.A. Effects of relaxin and estrogens on bone remodeling markers, receptor activator of NF-kB ligand (RANKL) and osteoprotegerin (OPG), in rat adjuvant-induced arthritis. Bone. 2011;48:1346–1353. doi: 10.1016/j.bone.2011.03.684. [DOI] [PubMed] [Google Scholar]
- 4.Tella S.H., Gallagher J.C. Biological agents in management of osteoporosis. Eur. J. Clin. Pharmacol. 2014;70:1291–1301. doi: 10.1007/s00228-014-1735-5. [DOI] [PubMed] [Google Scholar]
- 5.Garnero P. New developments in biological markers of bone metabolism in osteoporosis. Bone. 2014;66:46–55. doi: 10.1016/j.bone.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 6.Vasikaran S., Eastell R., Bruyere O., Foldes A.J., Garnero P., Griesmacher A., McClung M., Morris H.A., Silverman S., Trenti T., Wahl D.A., Cooper C., Kanis J.A. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: A need for international reference standards. Osteoporos. Int. 2011;22:391–420. doi: 10.1007/s00198-010-1501-1. [DOI] [PubMed] [Google Scholar]
- 7.Zou L., Sun W. Human urine proteome: A powerful source for clinical research. Adv. Exp. Med. Biol. 2015;845:31–42. doi: 10.1007/978-94-017-9523-4_4. [DOI] [PubMed] [Google Scholar]
- 8.Oh J., Pyo J.H., Jo E.H., Hwang S.I., Kang S.C., Jung J.H., Park E.K., Kim S.Y., Choi J.Y., Lim J. Establishment of a near-standard two-dimensional human urine proteomic map. Proteomics. 2004;4:3485–3497. doi: 10.1002/pmic.200401018. [DOI] [PubMed] [Google Scholar]
- 9.Thongboonkerd V., Mungdee S., Chiangjong W. Should urine pH be adjusted prior to gel-based proteome analysis? J. Proteome Res. 2009;8:3206–3211. doi: 10.1021/pr900127x. [DOI] [PubMed] [Google Scholar]
- 10.Zerefos P.G., Vougas K., Dimitraki P., Kossida S., Petrolekas A., Stravodimos K., Giannopoulos A., Fountoulakis M., Vlahou A. Characterization of the human urine proteome by preparative electrophoresis in combination with 2-DE. Proteomics. 2006;6:4346–4355. doi: 10.1002/pmic.200500671. [DOI] [PubMed] [Google Scholar]
- 11.Ho Y.J., Wang C.F., Hsu W.Y., Tseng T., Hsu C.C., Kao M.D., Tsai Y.F. Psychoimmunological effects of dioscorea in ovariectomized rats: Role of anxiety level. Ann. Gen. Psychiatry. 2007;6:21. doi: 10.1186/1744-859X-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J.W., Lee J.Y., Lim J. Improvement of resolution in 2-D gel analysis by simple pre-treatment of human urine with EDTA. Open J. Clin. Diagn. 2012;2:40–43. [Google Scholar]
- 13.Soskic V., Godovac-Zimmermann J. Improvement of an in-gel tryptic digestion method for matrix-assisted laser desorption/ionization-time of flight mass spectrometry peptide mapping by use of volatile solubilizing agents. Proteomics. 2001;1:1364–1367. doi: 10.1002/1615-9861(200111)1:11<1364::AID-PROT1364>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 14.Hwang S.I., Lundgren D.H., Mayya V., Rezaul K., Cowan A.E., Eng J.K., Han D.K. Systematic characterization of nuclear proteome during apoptosis: A quantitative proteomic study by differential extraction and stable isotope labeling. Mol. Cell. Proteome. 2006;5:1131–1145. doi: 10.1074/mcp.M500162-MCP200. [DOI] [PubMed] [Google Scholar]
- 15.Keller A., Nesvizhskii A.I., Kolker E., Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 16.Nesvizhskii A.I., Keller A., Kolker E., Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 17.Han D.K., Eng J., Zhou H., Aebersold R. Quantitative profiling of differentiation-induced microsomal proteins using isotope-coded affinity tags and mass spectrometry. Nat. Biotechnol. 2001;19:946–951. doi: 10.1038/nbt1001-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoo Y.M., Han T.Y., Kim H.S. Melatonin suppresses autophagy induced by Clinostat in preosteoblast MC3T3-E1 cells. Int. J. Mol. Sci. 2016;17:526. doi: 10.3390/ijms17040526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ardawi M.S., Badawoud M.H., Hassan S.M., Rouzi A.A., Ardawi J.M., AlNosani N.M., Qari M.H., Mousa S.A. Lycopene treatment against loss of bone mass, microarchitecture and strength in relation to regulatory mechanisms in a postmenopausal osteoporosis model. Bone. 2016;83:127–140. doi: 10.1016/j.bone.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 20.Bhardwaj P., Rai D.V., Garg M.L. Zinc as a nutritional approach to bone loss prevention in an ovariectomized rat model. Menopause. 2013;20:1184–1193. doi: 10.1097/GME.0b013e31828a7f4e. [DOI] [PubMed] [Google Scholar]
- 21.Gordon S.M., McKenzie B., Kemeh G., Sampson M., Perl S., Young N.S., Fessler M.B., Remaley A.T. Rosuvastatin alters the proteome of high density lipoproteins: Generation of alpha-1-antitrypsin enriched particles with anti-inflammatory properties. Mol. Cell. Prote. 2015;14:3247–3257. doi: 10.1074/mcp.M115.054031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao J.J., Gregoire B.R., Sun L., Song S. Alpha-1 antitrypsin reduces ovariectomy-induced bone loss in mice. Ann. N. Y. Acad. Sci. 2011;1240:E31–E35. doi: 10.1111/j.1749-6632.2011.06370.x. [DOI] [PubMed] [Google Scholar]
- 23.Zheng T., Yang L., Liu Y., Liu H., Yu J., Zhang X., Qin S. Plasma DPP4 activities are associated with osteoporosis in postmenopausal women with normal glucose tolerance. J. Clin. Endocrinol. Metab. 2015;100:3862–3870. doi: 10.1210/jc.2015-2233. [DOI] [PubMed] [Google Scholar]
- 24.Bonnet N., Standley K.N., Bianchi E.N., Stadelmann V., Foti M., Conway S.J., Ferrari S.L. The matricellular protein periostin is required for sost inhibition and the anabolic response to mechanical loading and physical activity. J. Biol. Chem. 2009;284:35939–38950. doi: 10.1074/jbc.M109.060335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J.T., Kotani K. Inverse correlation between fibrinogen and bone mineral density in women: Preliminary findings. J. Formos. Med. Assoc. 2016;115:54–56. doi: 10.1016/j.jfma.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 26.Kim B.J., Baek S., Ahn S.H., Kim S.H., Jo M.W., Bae S.J., Kim H.K., Park G.M., Kim Y.H., Lee S.H., Kim G.S., Choe J., Koh J.M. A higher serum gamma-glutamyl transferase level could be associated with an increased risk of incident osteoporotic fractures in Korean men aged 50 years or older. Endocr. J. 2014;61:257–263. doi: 10.1507/endocrj.ej13-0463. [DOI] [PubMed] [Google Scholar]
- 27.Koutroubakis I.E., Zavos C., Damilakis J., Papadakis G., Neratzoulakis J., Karkavitsas N., Kouroumalis E.A. Role of ghrelin and insulin-like growth factor binding protein-3 in the development of osteoporosis in inflammatory bowel disease. J. Clin. Gastroenterol. 2011;45:e60–e65. doi: 10.1097/MCG.0b013e3181f42543. [DOI] [PubMed] [Google Scholar]
- 28.Yang Q., Jian J., Abramson S.B., Huang X. Inhibitory effects of iron on bone morphogenetic protein 2-induced osteoblastogenesis. J. Bone Miner. Res. 2011;26:1188–1196. doi: 10.1002/jbmr.337. [DOI] [PubMed] [Google Scholar]
- 29.Afshinnia F., Wong K.K., Sundaram B., Ackermann R.J., Pennathur S. Hypoalbuminemia and osteoporosis: Reappraisal of a controversy. J. Clin. Endocrinol. Metab. 2016;101:167–175. doi: 10.1210/jc.2015-3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rusińska A.1., Świątkowska M., Koziołkiewicz W., Skurzyński S., Golec J., Chlebna-Sokół D. Proteomic analysis of plasma profiles in children with recurrent bone fractures. Acta Biochim. Pol. 2011;58:553–561. [PubMed] [Google Scholar]
- 31.Wang Y., Zhao L., Xu J., Nie Y., Guo Y., Tong Y., Qin L., Zhang Q. Curculigoside isolated from Curculigo orchioides prevents hydrogen peroxide-induced dysfunction and oxidative damage in calvarial osteoblasts. Acta Biochim. Biophys. Sin. (Shanghai) 2012;44:431–441. doi: 10.1093/abbs/gms014. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka S., Narusawa K., Onishi H., Miura M., Hijioka A., Kanazawa Y., Nishida S., Ikeda S., Nakamura T. Lower osteocalcin and osteopontin contents of the femoral head in hip fracture patients than osteoarthritis patients. Osteoporos. Int. 2011;22:587–597. doi: 10.1007/s00198-010-1328-9. [DOI] [PubMed] [Google Scholar]
- 33.Yaturu S., Reddy R.D., Rains J., Jain S.K. Plasma and urine levels of resistin and adiponectin in chronic kidney disease. Cytokine. 2007;37:1–5. doi: 10.1016/j.cyto.2007.02.003. [DOI] [PubMed] [Google Scholar]


