Abstract
Using conventional imaging modalities, it is difficult to detect recurrent lesions in prostate cancer patients who have undergone biochemical relapse, especially in patients with low prostate-specific antigen (PSA) levels. We retrospectively reviewed the files of fifty patients with histopathologically confirmed prostate cancer who underwent 99mTc-labeled prostate-specific membrane antigen (PSMA) single-photon emission computed tomography (SPECT)/computed tomography (CT), magnetic resonance imaging (MRI), and bone scan within a 30-day period. PSMA-SPECT/CT indicated metastatic lesions in 39 patients and had a higher detection rate (78.0%) than bone scan (34.0%) or MRI (40.0%). The diagnostic efficiency of PSMA-SPECT/CT imaging for bone and lymph node metastases (50.0% and 42.0%) was better than bone scan (34.0% and 0.0%) or MRI (24.0% and 20.0%). PSMA-SPECT/CT provided a higher detection rate at serum PSA levels of ≤1 ng ml−1, 1–4 ng ml−1, 4–10 ng ml−1, and >10 ng ml−1. No correlation was found between Gleason score, PSA level, and the tracer tumor/background ratio of metastatic lesions. With the aid of PSMA-SPECT/CT imaging, the therapeutic strategy was changed for 31 patients, and this may have enhanced their clinical outcome. In conclusion, PSMA-SPECT/CT imaging could detect more metastatic lesions and achieve a higher detection rate than conventional imaging modalities at different serum PSA levels in prostate cancer patients who had undergone biochemical relapse.
Keywords: 99mTc, CT, prostate cancer, PSMA, SPECT
INTRODUCTION
Prostate cancer (PCa) is the second most common cause of death in developed countries and is the most common solid cancer in men.1 The therapeutic treatment of PCa is principally influenced by the presence or absence of metastases. However, the detection of recurrent disease is currently a major challenge using conventional imaging modalities, especially at low prostate-specific antigen (PSA) levels. Studies employing computed tomography (CT), magnetic resonance imaging (MRI), and bone scan have shown disappointing sensitivity rates in the detection of small metastases, including lymph node metastases.2 A better diagnostic procedure is required to localize recurrences in PCa patients who have undergone biochemical relapse.
Prostate-specific membrane antigen (PSMA) has recently received increasing amounts of attention.3,4 PSMA is expressed in tissues such as the kidney, proximal small intestine, and salivary glands and is also overexpressed in PCa.5 As such, PSMA provides a promising target for PCa-specific imaging and therapy.
68Ga-PSMA-positron emission tomography (PET) imaging has been reported to improve the detection of metastatic disease, even at low serum PSA values.6,7 In this retrospective analysis, we compared the use of a novel single-photon emission CT (SPECT) imaging tracer, HYNIC-Glu-Urea-A (a 99mTc-labeled PSMA ligand), with conventional imaging modalities, such as bone scan and MRI, in the diagnosis of recurrence in PCa patients who had undergone biochemical recurrence.8
MATERIALS AND METHODS
Patients
For this retrospective analysis, we selected fifty consecutive patients who had undergone PSMA-SPECT/CT, a pelvic MRI, and a bone scan within a 30-day period. All patients had received a histopathological diagnosis of PCa. In all cases, progressive disease was suspected following conventional PCa treatment (hormone therapy, chemotherapy, radiation therapy, and/or surgery). The serum PSA level of all patients was >0.20 ng ml−1 when they underwent imaging. All patients had signed a written informed consent form allowing anonymized evaluation and publication of their data, and the Local Ethics Committee approved this retrospective analysis. Table 1 shows the clinical characteristics of all the patients.
Table 1.
The clinical characteristics of all the patients
Imaging
99mTc-labeled HYNIC-Glu-Urea-A was injected as an intravenous bolus, and whole-body SPECT and a noncontrast-enhanced (low-dose) CT scan were performed 2 h postinjection. Attenuation correction was performed using the low-dose nonenhanced CT data. No adverse effects were observed in any of the patients after tracer injection.
Consistent results whether the lesions identified by PSMA SPECT/CT were metastatic or not were obtained by discussion of the three experienced nuclear medicine experts with their experience in image analysis. Any nonphysiological focal areas of increased uptake that were higher than the background level were interpreted as suspicious of prostate cancer, lymph node metastasis, bone metastasis, or soft-tissue metastasis. The tumor/background (T/B) ratio was calculated from regions of interest that were manually drawn over the sites of increased uptake. For background correction, a region of interest was drawn over the gluteus muscle. In cases of multiple metastases, only the five lesions with the highest intensity were included for further analysis.
Statistical analysis
For statistical analysis, dedicated statistical software was used (SPSS 15.0; SPSS Inc., Chicago, Illinois, USA). Correlations between the Gleason score, PSA levels, and T/B ratio were assessed using the Spearman's rank correlation coefficient. All results are expressed as the mean ± standard deviation. P < 0.05 was considered statistically significant.
RESULTS
Presence of PSMA-positive lesions
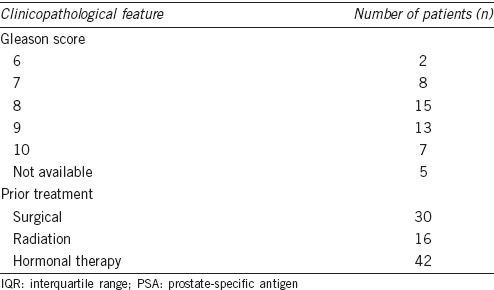
No adverse or clinically detectable pharmacological effects were observed in any of the patients after injection of the PSMA-SPECT/CT tracers. PSMA-SPECT/CT indicated metastatic lesions in 39 patients and had a higher detection rate (78.0%); there were 46 lymph node metastases, 95 bone metastases, and six soft-tissue metastases (two pulmonary and four hepatic) detected (Figure 1a). The median age was 71 years (interquartile range: 67-75). The median PSA value was 7.61 ng ml-1 (interquartile range: 1.25-51).
Figure 1.
Comparison of different imaging modalities for metastatic lesions in PCa patients. (a) Prostate-specific membrane antigen (PSMA) single-photon emission computed tomography (SPECT)/computed tomography (CT) imaging provided a higher detection ratio than bone scan or magnetic resonance imaging (MRI). (b) The tumor/background (T/B) ratio was higher for lymph node metastases than soft-tissue or bone metastases.
The T/B ratio was highest in lymph node metastases (37.42 ± 6.65). Soft tissue (26.33 ± 9.07) and bone metastases (21.40 ± 4.34) had lower T/B ratios although no significant difference in the T/B ratio was found between lymph node, soft tissue, and bone metastases (Figure 1b). There were no correlations between the Gleason score, PSA level, and T/B ratio of the metastatic lesions.
Comparison with different imaging modalities
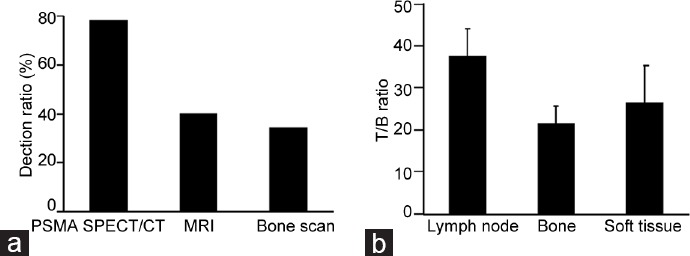
With the help of bone scan, metastatic bone lesions were found in 15 patients, suspicious bone lesions in two patients, and normal results in 33 patients. PSMA/SPECT imaging confirmed bone metastases in 15 patients. In patients with suspicious bone lesions, one patient was demonstrated by PSMA SPECT/CT. In addition, 23 patients who had normal bone scans were shown to have multiple metastatic bone or lymph node metastases using PSMA-SPECT/CT imaging. In general, bone lesions (n = 35) were observed in 17 patients by bone scan (detection ratio: 34.0%) (Figure 1a).
Using MRI scans, metastatic lesions were found in twenty patients and normal results in thirty patients. PSMA-SPECT/CT imaging confirmed metastases in all twenty patients and showed additional metastatic lesions in 19 patients. In general, metastatic lesions (n = 35) were observed in twenty patients by MRI imaging (detection ratio: 40.0%) (Figure 1a).
PSMA-SPECT/CT imaging could find 95 bone metastases in 25 patients (50.0%). Bone scan could find 35 bone metastases in 17 patients (34.0%). MRI could find twenty bone metastases in 12 patients (24.0%). The diagnostic efficiency on bone metastases with PSMA-SPECT/CT imaging was better than bone scan and MRI (Figure 2a).
Figure 2.
The diagnostic efficiency of PSMA-SPECT/CT imaging for different metastatic lesions in PCa patients. The efficiency of PSMA-SPECT/CT imaging in (a) diagnosing bone, (b) lymph node, and (c) soft-tissue metastases was greater than bone scan or MRI. The diagnostic efficiency on (d) pelvic lymph node metastases and (e) pelvic bone metastases with PSMA SPECT/CT imaging was better than bone scan and MRI.
PSMA-SPECT/CT was more efficient at diagnosing lymph node and soft-tissue metastases. Using PSMA-SPECT/CT, 46 metastatic lymph node lesions were detected in 21/50 (42.0%) of patients; MRI indicated 15 metastatic lesions in 10/50 (20.0%) of patients (Figure 2b). In addition, PSMA-SPECT/CT imaging identified six soft-tissue metastases in 6/50 (12.0%) of patients, but MRI and bone scan found no soft-tissue metastases (Figure 2c).
The detection efficiency by MRI, bone scan, and PSMA-SPECT/CT imaging for pelvic metastases was also compared. For detection of lymph nodes’ metastases, using PSMA-SPECT/CT, thirty metastatic lymph node lesions were detected in 18/50 (36.0%) of patients. MRI indicated 15 metastatic lesions in 10/50 (20.0%) of patients (Figure 2d). For detection of bone metastases, PSMA-SPECT/CT imaging found fifty bone metastases in 20/50 (40.0%) of patients. Bone scan could find 15 bone metastases in 10/50 (20.0%) of patients. MRI could find twenty bone metastases in 12/50 (24.0%) of patients (Figure 2e). The diagnostic efficiency on pelvic lymph node and bone metastases with PSMA SPECT/CT imaging was better than bone scan and MRI.
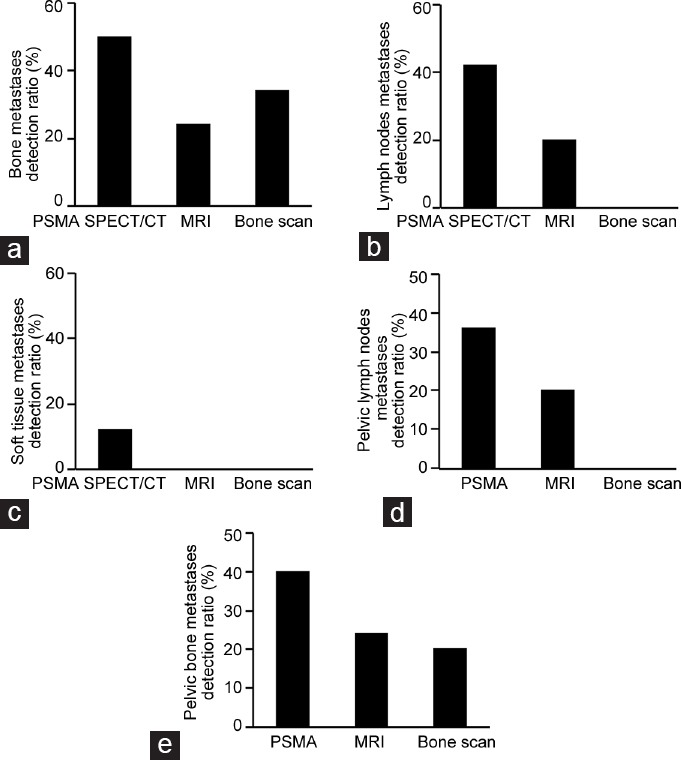
In our retrospective analysis, we found that PSMA-SPECT/CT detected five lesions in 3/10 (30.0%) of patients with ≤1 ng ml−1 PSA; in patients with 1–4 ng ml−1 PSA, twenty lesions were identified in 8/10 (80.0%) of patients; in patients with 4–10 ng ml−1 PSA, twenty lesions were identified in 5/5 (100%) of patients; and in patients with >10 ng ml−1 PSA, 102 lesions were identified in 23/23 (100%) of patients (Figure 3). Using the same thresholds, MRI detected one lesion in 1/10 (10.0%) of patients with ≤1 ng ml−1 PSA; in patients with 1–4 ng ml−1 PSA, nine lesions were identified in 5/10 (50.0%) of patients; in patients with 4–10 ng ml−1 PSA, three metastatic lesions were found in 2/5 (40.0%) of patients; and in patients with >10 ng ml−1 PSA, 22 lesions were identified in 12/23 (52.0%) of patients (Figure 3). Using the same thresholds, bone scan identified one metastatic bone lesion in 1/10 (10.0%) of patients with ≤1 ng ml−1 PSA; in patients with 1–4 ng ml−1 PSA, two metastatic bone lesions were identified in 2/10 (20.0%) of patients; in patients with 4–10 ng ml−1 PSA, three metastatic bone lesions were found in 2/5 (40.0%) of patients; and in patients with >10 ng ml−1 PSA, 27 lesions were identified in 10/23 (43.5%) of patients and two suspicious lesions were found in 2/23 (8.7%) of patients (Figure 3).
Figure 3.
The diagnostic efficiency of PSMA-SPECT/CT imaging at a range of serum PSA levels in PCa patients. PSMA-SPECT/CT provided a higher detection rate at serum prostate-specific antigen levels of (a) ≤1 ng ml−1, (b) 1–4 ng ml−1, (c) 4–10 ng ml−1, and (d) >10 ng ml−1.
Clinical consequences
Following the identification of additional localized metastatic lesions using PSMA-SPECT/CT imaging, 15 patients all received androgen deprivation therapy (ADT) and PSA control were not satisfactory. After selective radiotherapy, PSA levels of these patients decreased, indicating that the PSMA-positive lesions were PCa metastases (Figure 4). Following PSMA-SPECT/CT imaging, the therapeutic strategy of ten patients was changed from a standard pelvic lymphadenectomy to an extended pelvic lymphadenectomy and the PSMA-positive lesions were also proven to be PCa by histology (Figure 5). In addition, six patients changed the therapeutic strategies after PSMA SPECT/CT showing multiple bone metastasis and received ADT or chemotherapy with docetaxel (Figure 6). In summary, PSMA-SPECT/CT imaging provided valuable additional evidence to support further treatment and may have improved the therapeutic strategy of 31 patients (Supplementary Information (1.2MB, pdf) ).
Figure 4.
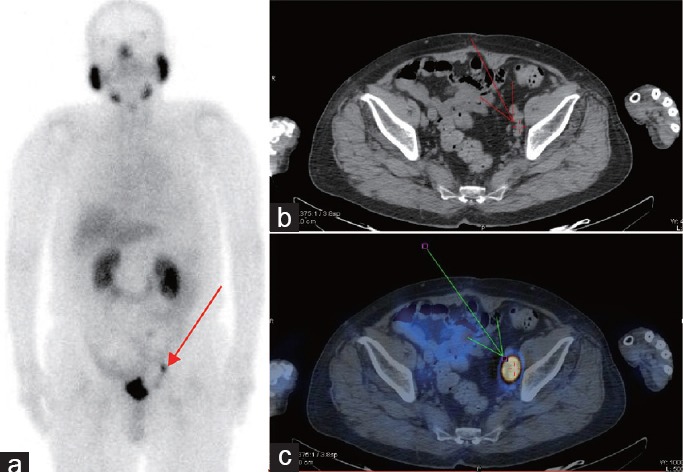
Example results for a patient who received selected radiation therapy. The patient was 70-year-old male with a biopsy-proven prostate cancer Gleason score of 9. After radical prostatectomy (RP), PSA level increased to 4 ng ml−1 and he became refractory to androgen deprivation therapy (ADT). PSMA-SPECT/CT imaging indicated lymph node metastases near the left iliac blood vessels ([a] Whole-body planar images; [b] CT plain scan and [c] fused PSMA-SPECT/CT images.). MRI was unable to confirm the metastatic lymph node. Following PSMA-SPECT/CT imaging, the patient received selected radiation therapy and his PSA level decreased to 1.20 ng ml−1.
Figure 5.
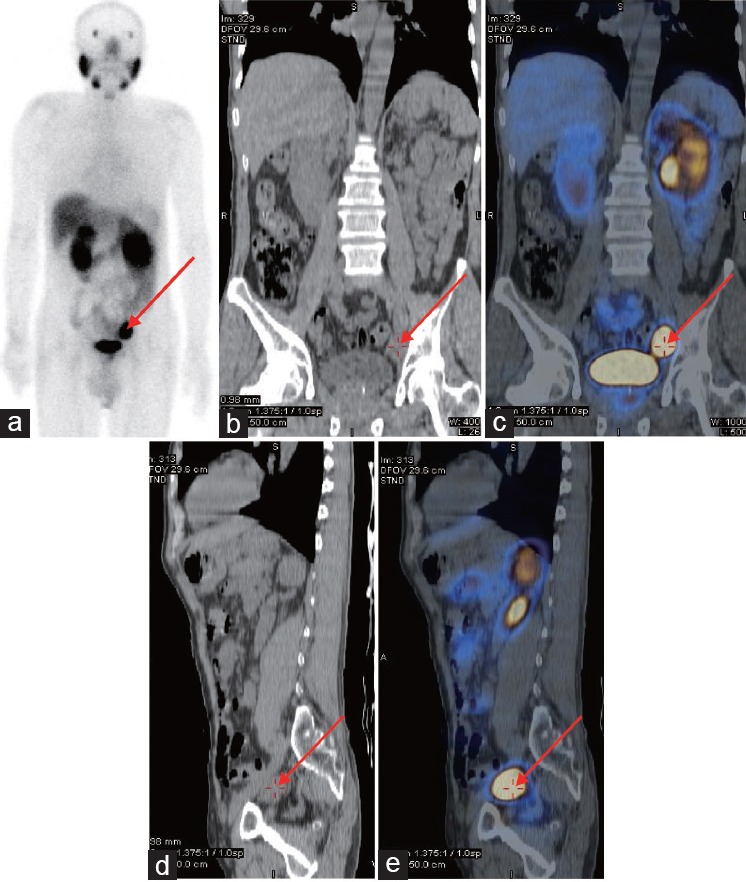
Example results for a patient who received RP and extended pelvic lymphadenectomy. The patient was 62-year-old male with a biopsy-proven prostate cancer Gleason score of 8 and PSA level of 46 ng ml−1. PSMA-SPECT/CT indicated lymph node metastases near the left iliac blood vessels ([a] Whole-body planar images; [b] coronal plane of CT plain scan; [c] coronal plane of fused PSMA-SPECT/CT images; [d] vertical plane of CT plain scan; [e] vertical plane of fused PSMA-SPECT/CT images.). Following PSMA-SPECT/CT imaging, the patient underwent RP and extended pelvic lymphadenectomy. The PSMA-positive lesions near the left iliac blood vessels were proven to be prostate cancer using histology. After surgery, the patient received ADT and his PSA level decreased to 0.10 ng ml−1. Arrows indicate metastatic lymph node lesions detected using PSMA-SPECT/CT.
Figure 6.
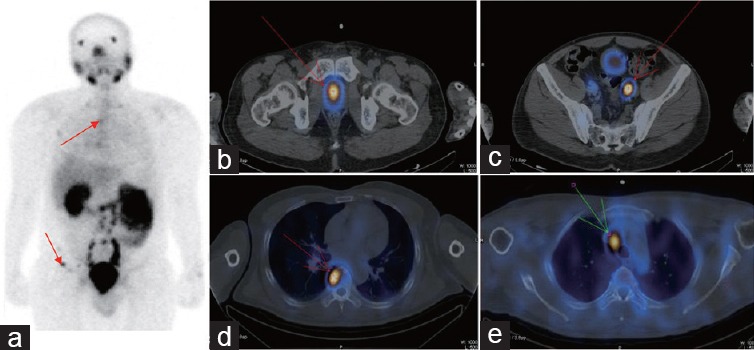
Example results for a patient who received chemotherapy with docetaxel. The patient was 50-year-old male with a biopsy-proven prostate cancer Gleason score of 10 and a PSA level of 110 ng ml−1. MRI showed prostate cancer accompanied by enlarged pelvic lymph nodes and bone scan showed no metastatic bone lesions. The patient was ready to undergo salvage RP, but the therapeutic strategy was changed after PSMA-SPECT/CT imaging showed multiple metastatic lesions (a) in the pelvis, (b) prostate cancer, (c) near to the iliac blood vessels and at (a and d) vertebrae and (e) mediastinal sites. The patient received chemotherapy with docetaxel and his PSA level decreased to 3 ng ml−1. The metastatic lesions are indicated by arrows.
DISCUSSION
PCa is one of the most common cancers, and understanding the exact diagnosis and the location of recurrence is essential for its management.9 However, it can be difficult to conduct accurate staging and detect early recurrence, especially at low PSA levels, using conventional imaging modalities.2 99mTc-methylene diphosphonate bone scan is the primary imaging procedure for diagnosing bone metastasis. However, bone scan has a low specificity, and many benign bone lesions can show increased radiotracer uptake, leading to false-positive results.10 MRI is also commonly used for PCa diagnosis. However, for patients with low PSA levels, the MRI detection rate is low, and it can be difficult to detect metastatic lesions that overlap muscles using this modality.
PSMA is a transmembrane cell surface protein that is expressed in all stages of PCa. The expression of PSMA increases with tumor aggressiveness, metastatic disease, and disease recurrence. As such, PSMA is an excellent target for PCa imaging.11 68Ga-PSMA PET/CT imaging is useful in diagnosing and staging prostate cancer. However, PET is not always available in China, especially in the remote areas. Furthermore, PET is also more expensive than SPECT/CT imaging. All these factors indicate that PSMA-based SPECT imaging could play an important role in PCa imaging, especially in institutions and areas where PET is not available.
Some studies have shown that a 111In-labeled anti-PSMA nanobody designed for targeted SPECT/CT imaging also exhibits good tumor targeting with low uptake in nontarget tissues, allowing excellent SPECT/CT imaging of PCa.12,13 In addition, 99mTc-MIP-1404- and 99mTc-MIP-1405-labeled PSMA-SPECT/CT can identify the majority of metastatic bone lesions and rapidly detected soft tissue PCa lesions, including subcentimeter lymph nodes metastases.14,15 However, to date, few studies have investigated the efficacy of SPECT/CT imaging using 99mTc-HYNIC-Glu-Urea-A in the diagnosis of PCa patients who have undergone biochemical recurrence.
In this retrospective analysis, we investigated the efficacy of 99mTc-HYNIC-Glu-Urea-A SPECT/CT imaging for the detection of metastatic PCa lesions. We found that PSMA-SPECT/CT was useful for evaluating metastatic lesions in PCa patients. High radiotracer uptake was observed at the sites of bone, lymph node, and soft-tissue metastates. A high T/B uptake ratio is advantageous in the evaluation of suspected lesions, and, consistent with a previous study,16 we found no correlation between the Gleason score, PSA level, and the T/B ratio.
Lymph node, bone, and soft-tissue metastases commonly occur in PCa, and these metastases are adverse prognostic factors.17,18 Using PSMA-SPECT/CT imaging, we observed high levels of radiotracer uptake at the site of metastatic lesions, and, regardless of the lesion size, the detection rate was higher than either MRI or bone scan. In addition, PSMA-SPECT/CT helped confirm the diagnosis of PCa in patients with suspicious metastatic lesions and helped improve therapeutic schedules. In this retrospective analysis, PSMA-SPECT/CT might affect the clinical outcomes of 31 patients in a positive way.
Since whole-body MRI is time-consuming and expensive, pelvic MRI and bone scan are used to diagnose PCa by most urologists in China. In this retrospective analysis, we found that the diagnostic efficiency on pelvic lymph node and bone metastases with PSMA SPECT/CT imaging was better than bone scan and MRI. In addition, PSMA SPECT/CT imaging was cheaper and provided more information – also information that the regular urologist was more able to understand by himself (instead of interpreting imaging of whole body MRI), which helped guide clinical diagnosis.
This retrospective analysis has certain limitations, such as its small patient population. Another limitation is that the bone and lymph node lesions identified using PSMA-SPECT/CT were not pathologically confirmed. As such, it is possible that there were some false-positive lesions identified. However, 15 patients who were treated with selective radiation following PSMA-SPECT/CT imaging, subsequently, showed decreased PSA levels, indicating that the PSMA-positive lesions were most probably PCa. In addition, in ten patients, the PSMA-positive lesions were histologically shown to be PCa. Further analyses are required to confirm our findings and to verify the sensitivity and specificity of PSMA-SPECT/CT imaging in the detection of metastatic lesions in PCa patients.
CONCLUSIONS
At a range of serum PSA levels, PSMA-SPECT/CT imaging identified more metastatic lesions and provided a higher detection rate than conventional imaging modalities. This helped guide clinical diagnosis and treatment, which indicates the value of PSMA-SPECT/CT imaging in the evaluation of metastasis in PCa patients who have undergone biochemical relapse, even in patients with low PSA levels.
AUTHOR CONTRIBUTIONS
HCS and YZ designed the retrospective analysis, collected, analyzed, and interpreted the clinical data, and wrote and revised the manuscript. GWL, SLH, and XPX helped collect the clinical data. BD revised the manuscript. DWY supervised the project and revised the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This work was supported by the Shanghai Rising Star Program (No. 16QA1401100) and the National Natural Science Foundation of China (Grant No. 81602222, 81272837 and 81472377). All these study sponsors have no roles in the study design, in the collection, analysis, and interpretation of data.
Supplementary information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Brawley OW. Prostate cancer epidemiology in the United States. World J Urol. 2012;30:195–200. doi: 10.1007/s00345-012-0824-2. [DOI] [PubMed] [Google Scholar]
- 2.Kosuri S, Akhtar NH, Smith M, Osborne JR, Tagawa ST. Review of salvage therapy for biochemically recurrent prostate cancer: the role of imaging and rationale for systemic salvage targeted anti-prostate-specific membrane antigen radioimmunotherapy. Adv Urol 2012. 2012 doi: 10.1155/2012/921674. 921674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eder M, Schäfer M, Bauder-Wüst U, Hull WE, Wängler C, et al. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem. 2012;23:688–97. doi: 10.1021/bc200279b. [DOI] [PubMed] [Google Scholar]
- 4.Maurer T, Eiber M, Schwaiger M, Gschwend JE. Current use of PSMA-PET in prostate cancer management. Nat Rev Urol. 2016;13:226–35. doi: 10.1038/nrurol.2016.26. [DOI] [PubMed] [Google Scholar]
- 5.Afshar-Oromieh A, Malcher A, Eder M, Eisenhut M, Linhart HG, et al. PET imaging with a (Ga-68) gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging. 2013;40:486–95. doi: 10.1007/s00259-012-2298-2. [DOI] [PubMed] [Google Scholar]
- 6.Afshar-Oromieh A, Haberkorn U, Schlemmer HP, Fenchel M, Eder M, et al. Comparison of PET/CT and PET/MRI hybrid systems using a 68Ga-labelled PSMA ligand for the diagnosis of recurrent prostate cancer: initial experience. Eur J Nucl Med Mol Imaging. 2014;41:887–97. doi: 10.1007/s00259-013-2660-z. [DOI] [PubMed] [Google Scholar]
- 7.Bluemel C, Krebs M, Polat B, Linke F, Eiber M, et al. 68Ga-PSMA-PET/CT in patients with biochemical prostate cancer recurrence and negative 18F-choline-PET/CT. Clin Nucl Med. 2016;41:515–21. doi: 10.1097/RLU.0000000000001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu XP, Zhang JP, He SM, Luo JM, Bao X, et al. The imaging study of a small-molecular inhibitor targeting prostate specific membrane antigen. Oncoradiology. 2015;3:173–8. [In Chinese] [Google Scholar]
- 9.Carroll PR, Parsons JK, Andriole G, Bahnson RR, Castle EP, et al. NCCN guidelines insights: prostate cancer early detection, version 2.2016. J Natl Compr Canc Netw. 2016;14:509–19. doi: 10.6004/jnccn.2016.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kabasakal L, Demirci E, Ocak M, Akyel R, Nematyazar J, et al. Evaluation of PSMA PET/CT imaging using a 68Ga-HBED-CC ligand in patients with prostate cancer and the value of early pelvic imaging. Nucl Med Commun. 2015;36:582–7. doi: 10.1097/MNM.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 11.Perner S, Hofer MD, Kim R, Shah RB, Li H, et al. Prostate-specific membrane antigen expression as a predictor of prostate cancer progression. Hum Pathol. 2007;38:696–701. doi: 10.1016/j.humpath.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Chatalic KL, Veldhoven-Zweistra J, Bolkestein M, Hoeben S, Koning GA, et al. A novel 111In-labeled anti–prostate-specific membrane antigen nanobody for targeted SPECT/CT imaging of prostate cancer. EJNMMI Res. 2015;56:1094–9. doi: 10.2967/jnumed.115.156729. [DOI] [PubMed] [Google Scholar]
- 13.Schottelius M, Wirtz M, Eiber M, Maurer T, Wester HJ. [111In] PSMA-I&T: expanding the spectrum of PSMA-I and T applications towards SPECT and radioguided surgery. EJNMMI Res. 2015;5:68. doi: 10.1186/s13550-015-0147-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vallabhajosula S, Nikolopoulou A, Babich JW, Osborne JR, Tagawa ST, et al. 99mTc-labeled small-molecule inhibitors of prostate-specific membrane antigen: pharmacokinetics and biodistribution studies in healthy subjects and patients with metastatic prostate cancer. J Nucl Med. 2014;55:1791–8. doi: 10.2967/jnumed.114.140426. [DOI] [PubMed] [Google Scholar]
- 15.Hillier SM, Maresca KP, Lu G, Merkin RD, Marquis JC, et al. 99mTc-labeled small-molecule inhibitors of prostate-specific membrane antigen for molecular imaging of prostate cancer. J Nucl Med. 2013;54:1369–76. doi: 10.2967/jnumed.112.116624. [DOI] [PubMed] [Google Scholar]
- 16.Dietlein M, Kobe C, Kuhnert G, Stockter S, Fischer T, et al. Comparison of [18F] DCFPyL and [68Ga] GaPSMA- HBED-CC for PSMA-PET imaging in patients with relapsed prostate cancer. Mol Imaging Biol. 2015;17:575–84. doi: 10.1007/s11307-015-0866-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gakis G, Boorjian SA, Briganti A, Joniau S, Karazanashvili G, et al. The role of radical prostatectomy and lymph node dissection in lymph node positive prostate cancer: a systematic review of the literature. Eur Urol. 2014;66:191–9. doi: 10.1016/j.eururo.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 18.Heck MM, Souvatzoglou M, Retz M, Nawroth R, Kübler H, et al. Prospective comparison of computed tomography, diffusion-weighted magnetic resonance imaging and [11C] choline positron emission tomography/computed tomography for preoperative lymph node staging in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2014;41:694–701. doi: 10.1007/s00259-013-2634-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


