Abstract
Aging-related ED is predominantly attributed to neurovascular dysfunction mediated by NO suppression and increased oxidative stress in penis. The alterations of protein arginine methyltransferases 1 (PRMT1)/dimethylarginine dimethylaminohydrolase (DDAH)/asymmetrical dimethylarginine (ADMA)/NO synthase (NOS) pathway regulate NO production in the vascular endothelium. Epigallocatechin-3-gallate (EGCG) is one of the most abundant and antioxidative ingredients isolated from green tea. In the present study, 40 Sprague-Dawley rats were randomly distributed into four groups: one young rat group and three aged rat groups treated with daily gavage feedings of EGCG at doses of 0, 10 mg kg−1, and 100 mg kg−1 for 12 weeks, respectively. Erectile function was assessed by electrical stimulation of the cavernous nerves with intracavernous pressure (ICP) measurement. After euthanasia, penile tissue was investigated using Western blot and ELISA to assess the PRMT1/DDAH/ADMA/NOS metabolism pathway. Superoxide dismutase (SOD) and malondialdehyde (MDA) levels were detected by colorimetry. We also evaluated smooth muscle contents. The ratio of maximal ICP and mean systemic arterial pressure (MAP) was markedly higher in EGCG-treated aged rats than in untreated aged rats. We found that DDAH1 and DDAH2 were expressed in cavernosal tissue, and they were downregulated in corpora of aged rats. The administration of EGCG upregulated the expression and activity of DDAH. In contrast, EGCG treatment downregulated the expression of PRMT1 and ADMA content. Moreover, EGCG-treated rats showed an improvement in smooth muscle expression, the ratio of smooth muscle cell/collagen fibril, SOD activity, and MDA levels when compared with untreated aged rats.
Keywords: asymmetrical dimethylarginine, dimethylarginine dimethylaminohydrolase, epigallocatechin-3-gallate, erectile dysfunction, oxidative stress, protein arginine methyltransferases 1
INTRODUCTION
Erectile dysfunction (ED), defined as the inability to attain and/or maintain penile erection, is estimated to affect more than 150 million men worldwide, and this number is expected to double by 2025.1 Considered as the major public health problem that seriously affects the quality of life of patients and their partners, ED has become increasingly prevalent with age.2 The mechanism of aging-related ED has not yet been fully elucidated.
NO, which is synthesized from L-arginine by NO synthase (NOS), is well-known as the principal mediator of cavernous smooth relaxation and penile erection.3 Recent studies show that NO production may also be regulated by endogenous NOS inhibitors in vitro and in vivo, in particular, asymmetric dimethylarginine (ADMA).4,5 ADMA, a naturally occurring L-arginine analog synthesized endogenously during the methylation of protein arginine residues by protein arginine methyltransferases (PRMTs), is an endogenous inhibitor of all three types of NOS.6 ADMA exerts its biological effects by competing with L-arginine to bind to the active site of NOS, inhibiting NO synthesis and leading to NOS uncoupling.7 PRMT1 is the predominant member of the PRMT family that is involved in protein arginine methylation and it accounts for approximately 85% of arginine methylation reactions.8,9 The majority of ADMA (~80%) is intracellularly metabolized to citrulline and dimethylamine by dimethylarginine dimethylaminohydrolase (DDAH) and the rest is excreted in urine.10
In previous studies, increased levels of markers of oxidative stress were detected in both serum and cavernous tissues of patients with aging-related ED whereas markers of antioxidative status were decreased.11 Oxidative stress has been shown to increase PRMT1 activity and inactivate DDAH, which may be responsible for the increased ADMA concentration and subsequently decreased NO levels.4,12,13 Moreover, increased ADMA may then compete with arginine and “uncouple” NOS, contributing to oxidative stress that further inhibits NO genesis.7,12 Our previous study has demonstrated that the expression levels of DDAH and NOS were decreased in the cavernous tissues of aged rats whereas the ADMA concentration was increased.14
Green tea (Camellia sinensis), the second most consumed beverage worldwide, is chemically characterized by the presence of high amounts of polyphenolic compounds known as catechins, among which epigallocatechin-3-gallate (EGCG) is notable as the most abundant and active compound.15 EGCG has been reported to prevent oxidant injury and cell death via several mechanisms, such as scavenging oxygen radicals,16 protecting against oxidized LDL,17 and increasing the levels of antioxidant and antioxidant enzyme.18,19
In the present study, we examined the ability of EGCG to ameliorate erectile function in aged rats via the regulation of the PRMT1/DDAH/ADMA/NOS pathway by inhibiting oxidative stress.
MATERIALS AND METHODS
Animals
In this study, all procedures were approved by the Institutional Animal Care and Use Committee at Shandong University. Ten 12-week-old male Sprague-Dawley rats and 30 18-month-old male Sprague-Dawley rats were obtained from the Animal Breeding Center at Shandong University. Before the study, the rats were fed under the same standard laboratory conditions (temperature 22 ± 1°C, 12 h light: 12 h dark cycles) and had free access to food and water for 1 week. Ten 12-week-old male Sprague-Dawley rats, weighing 280–300 g, were classified as young group (Sham group). Thirty 18-month-old Sprague-Dawley rats, weighing 620–680 g, were randomly divided into three groups and fed with normal saline via oral gavage containing 0 (negative control group, NC group, n = 10), 10 mg kg−1 (EGCG-10 group, n = 10), or 100 mg kg−1 (EGCG-100 group, n = 10) epigallocatechin-3-gallate (EGCG, Sigma-Aldrich, St. Louis, MO, USA) daily for 12 weeks. The young rats received equivalent solutions of normal saline daily. Body weights were monitored regularly throughout the study and before euthanasia. All rats were maintained for 12 weeks, except for two rats in negative control group, one rat in EGCG-10 group and two rats in EGCG-100 group that died before the end of the study. No noticeable irritation was observed following administration of EGCG. After a 1-week washout period, we evaluated erectile function by cavernous nerve electrical stimulation. After functional testing, the rats were euthanized, and the penis was harvested for histochemical examination, Western blot, and enzyme-linked immunosorbent assay (ELISA) analyses.
Measurement of erectile function
Erectile function was assessed by measuring the maximal intracavernous pressure (Max ICP) and the ratio of Max ICP/mean systemic arterial pressure (MAP). The rats were anesthetized with an intraperitoneal injection of 5% sodium pentobarbital. The lateral-prostatic space was dissected using a lower abdominal midline incision. The major pelvic ganglion and cavernous nerve were identified and isolated. The penile crus were exposed using a sagittal perineal incision. A 23-gauge butterfly needle connected to a polyethylene tube filled with 250 U ml−1 heparin-saline solutions was inserted in the cavernosum on the same side as the stimulated cavernous nerve. The systemic arterial blood pressure was monitored via a polyethylene tube placed into the left carotid artery. Electrostimulation (12 Hz; pulse width 5 ms; 5 V; duration of 60 s) was applied to the cavernous nerve with a stainless steel bipolar hook electrode. Both pressure lines (ICP and MAP) were measured continuously with a BL-420V pressure transducer system (Chengdu Implement Company, Chengdu, China).
ELISA for ADMA and cGMP
The concentration of total protein in the penile tissue was detected using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). The levels of the ADMA and cGMP in penile tissue were assessed using a commercial cGMP ELISA kit (R&D Systems, Inc., Minneapolis, MN, USA) and a commercial ADMA ELISA kit (Enzo Life Sciences, Lorrach, Germany), following the protocol provided by the manufacturer. Data are expressed as pmol mg pro−1 of wet weight tissue for cavernous tissue levels.
Masson's trichrome stain
Masson's trichrome stain was used to evaluate the smooth muscle cell/collagen fibril (SMC/CF) expression in cavernous tissue. Three-micrometer sections of formalin-fixed, paraffin-embedded (FFPE) tissues were deparaffinized in xylene (three washes for 3 min each) and hydrated in graded ethanol to distilled water. The slides were then stained with Masson's trichrome stain kit (Dako Sciences, Glostrup, Denmark), followed by dehydration in graded ethanol to xylene. The corpus cavernosum smooth muscle stained red, and the collagen fibril stained blue. The areas of smooth muscle and collagen were analyzed using the Image-Pro Plus 5.0 software package (Media Cybernetics, Inc., Bethesda, MD, USA).
Western blot assay for α-SMA, DDAH1, DDAH2, PRMT1, eNOS, p-eNOS, and nNOS
Rat penile tissues were homogenized in RIPA lysis buffer (Thermo Fisher Scientific, Waltham, MA, USA) on ice for 10 min and the supernatant was collected after centrifugation at 12 000 ×g for 15 min at 4°C. The protein concentration was assayed using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). The proteins were electrophoresed on 10% sodium dodecyl sulfate-polyacrylamide gels, transferred to a polyvinylidene fluoride membrane (Millipore Corp., Bedford, MA, USA) and blocked for 1 h of incubation at room temperature in TBS with 0.05% Tween 20 (TBST) containing 5% skim milk. The polyvinylidene fluoride membrane was then incubated overnight at 4°C with primary antibody against β-actin (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), α-SMA (1:1000; Abcam, Cambridge, UK), eNOS (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and phosphorylated eNOS (Ser1177, p-eNOS; 1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), nNOS (1:600, Santa Cruz Biotechnology, Santa Cruz, CA, USA), DDAH1 (1:1000; Abcam, Cambridge, UK), DDAH2 (1:500; Abcam, Cambridge, UK), or PRMT1 (1:500; Abcam, Cambridge, UK). After three washes in tris-buffered saline containing 0.1% Tween 20 (TBST) for 10 min each, the membrane was incubated for 1 h with the appropriate diluted horseradish peroxidase-conjugated secondary antibody. Subsequently, the membrane was washed 3 times again using TBST for 10 min each, and the protein bands were then detected using the enhanced chemiluminescence (ECL) system (Pierce Biotech Inc., Rockford, IL, USA). Signals were obtained in the linear range of detection and quantified by densitometry (Quantity One Analysis software; Bio-Rad, CA, USA) using a Fujifilm LAS-3000 imaging system (Fujifilm, Tokyo, Japan). Specifically, the integrated optical density (IOD) was measured after subtracting the background. IOD was also determined for β-actin to correct for any variations in total protein loading, and the amount of protein was represented as IOD/Std.
Measurement of DDAH activity
In the corpus cavernosum tissue homogenates, the DDAH activity was estimated using the colorimetric method and is expressed per gram of protein as previously described.20 This method is based on the L-citrulline production rate. To this end, corpus cavernosum tissue homogenate was mixed with sodium–phosphate buffer (pH 6.5), and 1 mmol l−1 ADMA (Sigma-Aldrich, St. Louis, MO, USA) was then added to each sample, which was then incubated at 37°C for 30 min. After the reaction had been stopped by the addition of 4% sulfosalicylic acid, the samples were centrifuged. Oxime (diacetic monoxime [0.08% w/v] in 5% acetic acid) mixed with antipyrine (antipyrine [0.5% w/v] in 50% sulfuric acid) was added to the samples during the next stage. Following 110 min of incubation at 60°C and 10 min of cooling on ice, the level of L-citrulline was measured using a spectrophotometric analysis at 466 nm. The obtained values were subtracted from the corresponding values in blind samples (without ADMA). Aliquots of L-citrulline were prepared to serve as standard samples, and the DDAH activity is presented as nmol L-citrulline mg pro−1 at 37°C.
Measurement of NOS activity
The concentration of total protein in the penile tissue was measured using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). The NOS activity was assessed using a Total Nitric Oxide Synthase assay kit (Nanjing Jiancheng Biotechnology Institute, Nanjing, China) following the protocol provided by the manufacturer. Data are expressed as U mg pro−1 of wet weight tissue for cavernous tissue levels.
Measurement of SOD activity and MDA levels
Approximately 20 mg of corpus cavernosum tissue was used for the experiment; the tissue was homogenized in 0.2 ml of normal saline. Subsequently, 0.05 ml of tissue homogenate was used for SOD determination and 0.1 ml of tissue homogenate was used for MDA determination. The total SOD activity was measured at 525 nm using spectrophotometric kits (Nanjing Jiancheng Biotechnology Institute, Nanjing, China). The MDA level was measured using spectrophotometric kits (Nanjing Jiancheng Biotechnology Institute, Nanjing, China) as described by Uchiyama and Mihara.21 The result is expressed as nanomoles per milligram protein.
Statistical analysis
Data were expressed as mean ± standard deviation (mean ± s.d.). Statistical analysis was performed with one-way analysis of variance test with Bonferroni multiple comparison posttest. All statistical analyses were performed using the SPSS 16.0 statistical software (SPSS Inc., Chicago, IL, USA). The level of statistical significance was taken as P < 0.05.
RESULTS
Assessment of erectile responses
The ratios of Max ICP/MAP are shown in Figure 1. Compared to Sham group, the ratios of Max ICP/MAP were markedly lower in all groups of the aged rats (P < 0.01) whereas the aged rats treated with EGCG exhibited greater Max ICP/MAP ratios than untreated aged rats (P < 0.05).
Figure 1.
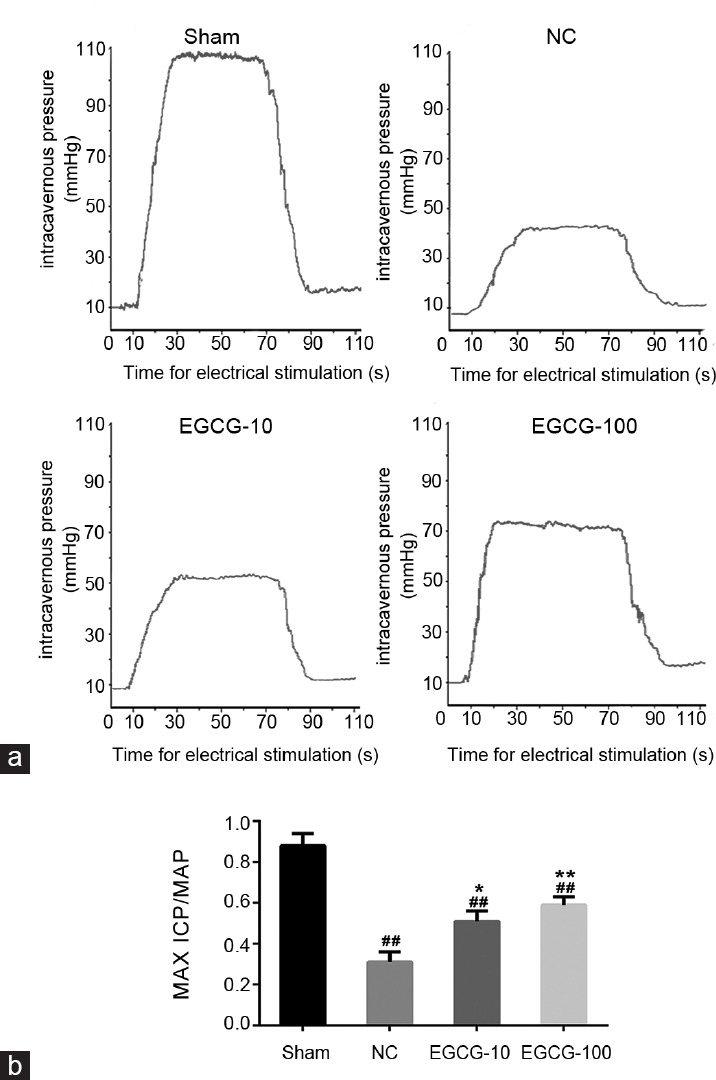
Erectile function evaluation. The ratio of maximal intracavernous pressure (Max ICP) and mean systemic arterial pressure (MAP) was shown to evaluate erectile function in each group. (a) Representative ICP in response to electrical stimulation of the cavernous nerve at 5 V in Sham group, NC group, EGCG-10 group, and EGCG-100 group. (b) Statistical chart of Max ICP/MAP ratio. Each bar depicted the mean values (± standard deviation) from n = 10 animals per group. *P < 0.05 versus NC group, **P < 0.01 versus NC group, ##P < 0.01 versus Sham group.
Effects of EGCG on ADMA and cGMP concentration
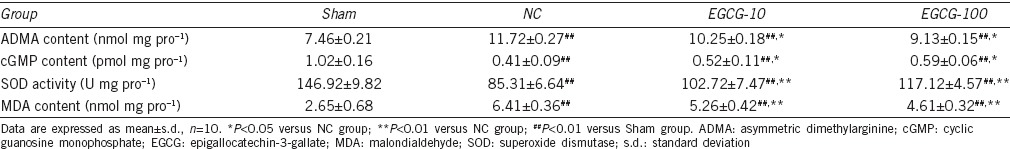
As demonstrated in Table 1, the concentrations of ADMA were higher in negative control group than EGCG-treated groups (P < 0.05) and Sham group (P < 0.01), and the ADMA concentrations remained higher in EGCG-treated groups than in Sham group (P < 0.01). The levels of cGMP were lower in negative control group than EGCG-treated groups (P < 0.05) and Sham group (P < 0.01), but the cGMP levels in EGCG-treated groups were still lower than those in Sham group (P < 0.01).
Table 1.
Comparisons of ADMA content, cGMP content, SOD activity, and MDA content in experimental animals after being treated for 12 weeks
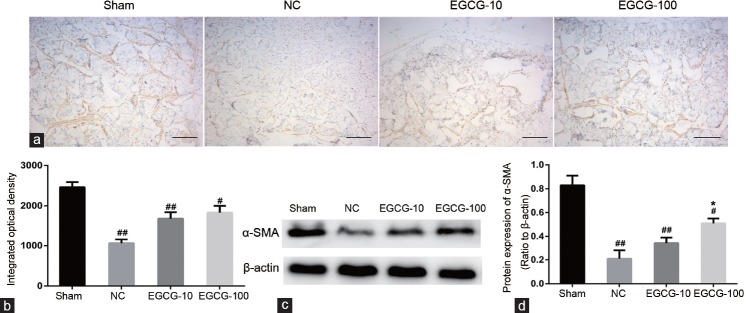
Effects of EGCG on cavernous smooth muscle and collagen content
In Figure 2, the ratio between the cavernous smooth muscle and collagen was reduced in negative control group compared with Sham group (P < 0.01). When compared with untreated aged rats, 100 mg kg−1 EGCG-treated aged rats had greater smooth muscle content (P < 0.05). Western blot and immunohistochemical staining of α-SMA content (Figure 3) also showed that the cavernous smooth muscle content was decreased in negative control group compared with Sham group (P < 0.01). In addition, the α-SMA content was lower in NC group than in EGCG-100 group (P < 0.05). There was no significant difference in α-SMA concentration between NC group and EGCG-10 group (P > 0.05).
Figure 2.
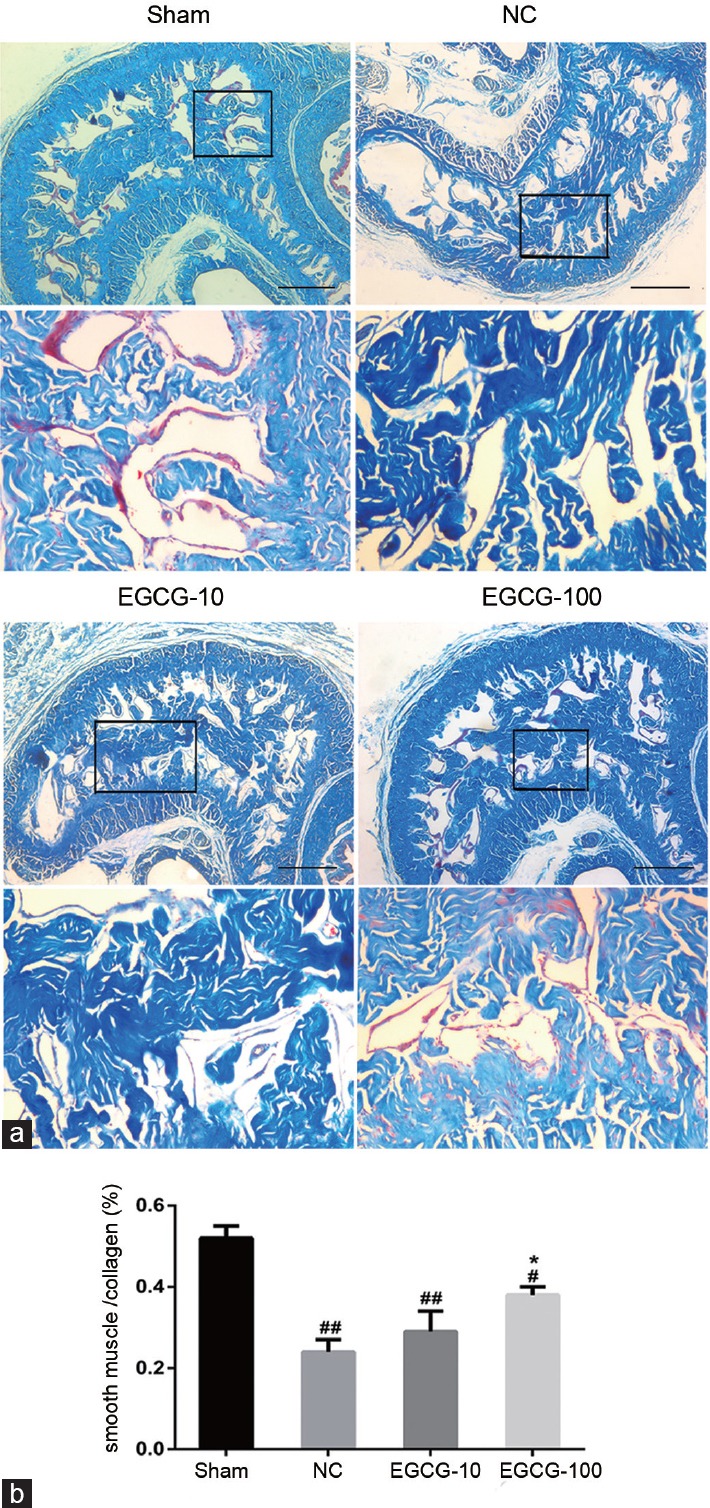
Masson's trichrome stain. Masson's trichrome stain was used to evaluate the cavernous tissue via smooth muscle cell (blue)/collagen fibril (red) (SMC/CF). (a) Masson's trichrome staining in corporal tissue of Sham group, NC group, EGCG-10 group, and EGCG-100 group. (b) Statistical chart of SMC/CF ratio. Each bar depicted the mean values (± standard deviation) from n = 10 animals per group. *P < 0.05 versus NC group. #P < 0.05 versus Sham group, ##P < 0.01 versus Sham group. Scale bar = 400 μm.
Figure 3.
Evaluation of α-SMA protein expression by immunohistochemistry and Western blot. (a) Immunohistochemical staining of α-SMA protein in Sham group, NC group, EGCG-10 group and EGCG-100 group. (b) Statistical chart of α-SMA integrated optical density among groups. (c) Western blot analysis of α-SMA protein in Sham group, NC group, EGCG-10 group, and EGCG-100 group. (d) Statistical chart of α-SMA relative to β-actin. Each bar depicted the mean values (± standard deviation) from n = 10 animals per group. *P < 0.05 versus NC group, #P < 0.05 versus Sham group, ##P < 0.01 versus Sham group. Scale bar = 200 μm.
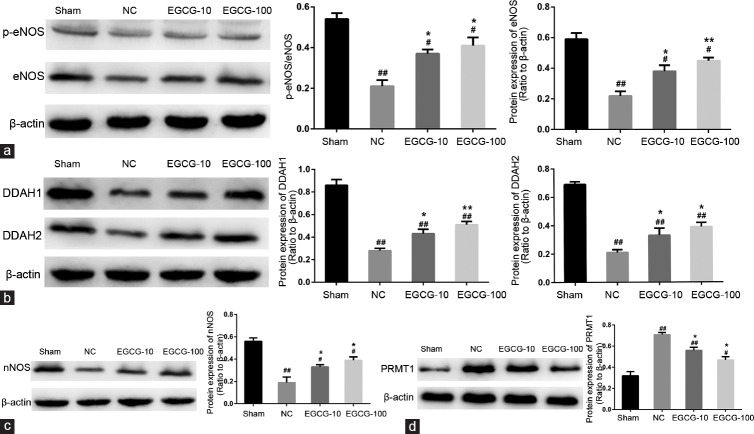
Western blot analysis of PRMT1, DDAH1, DDAH2, eNOS, p-eNOS, and nNOS expression
The protein expression levels of PRMT1, DDAH1, DDAH2, eNOS, p-eNOS, and nNOS in rat penile tissue detected by Western blotting are shown in Figure 4. Compared with young rats, the protein levels of PRMT1 were increased in untreated aged rats (P < 0.01). Both 10 mg kg−1 EGCG and 100 mg kg−1 EGCG treatment reduced the protein levels of PRMT1 in aged rats (P < 0.05). The protein levels of DDAH1, DDAH2, eNOS, p-eNOS, and nNOS in the penile tissue were lower in NC group than in Sham group (P < 0.01). However, both 10 mg kg−1 EGCG and 100 mg kg−1 EGCG treatment elevated the expression levels of DDAH1, DDAH2, p-eNOS, eNOS, and nNOS proteins (P < 0.05).
Figure 4.
Evaluation of PRMT1, DDAH1, DDAH2, eNOS, p-eNOS, and nNOS protein expression by Western blot in Sham group, NC group, EGCG-10 group and EGCG-100 group. (a) Representative Western blot analysis of eNOS and p-eNOS in corpus cavernosum among groups. β-actin was used as a loading control. (b) Representative Western blot analysis of DDAH1 and DDAH2 in corpus cavernosum among groups. β-actin was used as a loading control. (c) Representative Western blot analysis of nNOS in corpus cavernosum among groups. β-actin was used as a loading control. (d) Representative Western blot analysis of PRMT1 in corpus cavernosum among groups. β-actin was used as a loading control. Each bar depicted the mean values (± standard deviation) from n = 10 animals per group. *P < 0.05 versus NC group, **P < 0.01 versus NC group, #P < 0.05 versus Sham group, ##P < 0.01 versus Sham group.
Effects of EGCG on DDAH and NOS activities
The DDAH and NOS activities in the corpus cavernosum are shown in Figure 5. The DDAH and NOS activities were lower in untreated aged rats than in young rats (P < 0.01). Both 10 mg kg−1 EGCG and 100 mg kg−1 EGCG-treated aged rats exhibited higher DDAH and NOS activities than were seen in untreated aged rats (P < 0.05).
Figure 5.
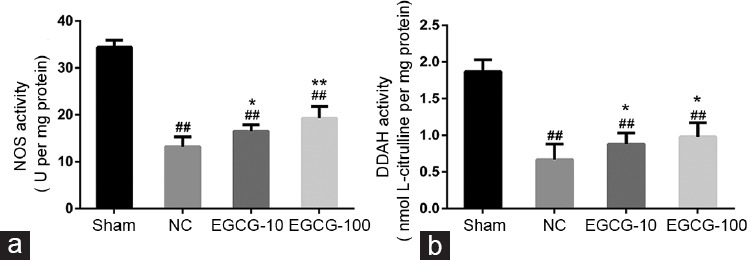
Evaluation of NOS activity and DDAH activity in corpus cavernosum from Sham group, NC group, EGCG-10 group and EGCG-100 group. (a) NOS activity in corpus cavernosum among groups. (b) DDAH activity in corpus cavernosum among groups. Each bar depicted the mean values (± standard deviation) from n = 10 animals per group. *P < 0.05 versus NC group, **P < 0.01 versus NC group, ##P < 0.01 versus Sham group.
SOD activity and MDA levels in the corpus cavernosum
The SOD activity and MDA levels in the corpus cavernosum are shown in Table 1. Decreased penile SOD activity and elevated levels of penile MDA were found in the corpus cavernosum of untreated aged rats compared with young rats (P < 0.01). Both 10 mg kg−1 EGCG and 100 mg kg−1 EGCG-treated aged rats exhibited increased penile SOD activity, as well as reduced levels of MDA activity compared with untreated aged rats (P < 0.01).
DISCUSSION
The present study found the following: (1) EGCG treatment can effectively ameliorate erectile function in aged rats by inhibiting oxidative stress to regulate the PRMT1/DDAH/ADMA/NOS metabolic pathway (Figure 6). (2) EGCG treatment can markedly improve the structural impairment in the corpus cavernosum of aged rats.
Figure 6.
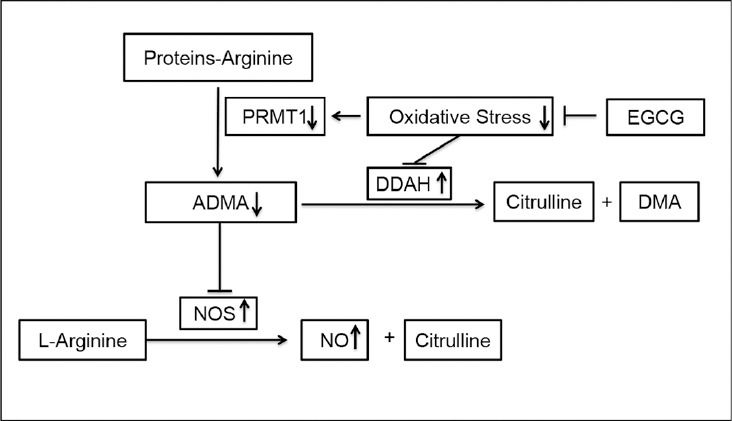
Evaluation of EGCG on PRMT1/DDAH/ADMA/NOS pathway in corpus cavernosum of the aged rats. PRMT1: protein arginine methyltransferases 1; ADMA: asymmetric dimethylaminohydrolase; DDAH: dimethylarginine dimethylaminohydrolase; NOS: nitric oxide synthase; NO: nitric oxide; DMA: dimethylamine.
Oxidative damage to the vasculature due to an increased production of reactive oxygen species (ROS) plays an important role in the natural aging process.22,23 As shown in previous studies,24 oxidative stress induces vascular endothelial cell and smooth muscle cell damage in the corpus cavernosum, which are the main pathophysiological mechanisms of aging-related ED. In this study, the administration of EGCG increased the amount of smooth muscle and the smooth muscle/collagen ratio.
The current study also identified the SOD activity and MDA levels as markers of oxidative stress in the corpus cavernosum. In our study, the SOD activity was decreased and the levels of MDA were increased in aged rats compared with young rats. In addition, this abnormality in aged rats was attenuated by EGCG treatment, which suggests that EGCG treatment restored SOD activity and reduced MDA production via inhibiting oxidative stress in the corpus cavernosum. Recent studies show that tea catechins are powerful hydrogen-donating antioxidants and free radical scavengers in a number of in vitro systems25 and in vivo models.26 Our data were also consistent with other studies. Nanjo et al. have reported that EGCG not only induces the activity of SOD but also augments SOD levels.27 Mostafa et al. demonstrated that streptozotocin-induced diabetic rats on green tea show decrease in cavernous MDA concentrations compared with untreated diabetic rats.28
Thus, we speculate that EGCG treatment ameliorates erectile function in aged rats via NO release. To address this issue, the cGMP levels and NOS protein expression in the corpus cavernosum were measured. The results from our experiments showed that p-eNOS expression, eNOS expression, nNOS expression, NOS activity, and cGMP levels in the corpus cavernosum were decreased in aged rats, which were corroborated our pervious observation.14 Moreover, EGCG supplement significantly increased p-eNOS expression, eNOS expression, nNOS expression, NOS activity, and cGMP levels in aged rats in our study. Accordingly, these results support our hypothesis that EGCG can improve impaired erectile function via the NO/cGMP pathway in aged rats. Similar results were also found in other studies. Persson et al. reported that tea flavanols increase NO production in the endothelial cells.29 Babu and Liu showed that catechins regulate vascular tone by activating eNOS where a catechin-associated improvement of vascular function and anti-atherosclerosis effect could delay or prevent the onset of aging-related ED.30 Mostafa et al. suggested that EGCG markedly increased the cavernous eNOS and cGMP levels in diabetic rats.28
According to a literature review of NOS, there are two important mechanisms for impaired NO synthesis in endothelial dysfunction. One of them is the decrease of eNOS and nNOS expression, and the other one is the accumulation of endogenous NOS inhibitors in the corpus cavernosum such as ADMA. In addition, several studies have indicated an association between ROS, ADMA concentration, and endothelial dysfunction.7,10,12,31 ADMA may increase ROS production in a concentration-dependent manner.12 Moreover, ROS can stimulate ADMA production and/or inhibit ADMA degradation, which may further increase ADMA production in a positive feedback manner.10,12 Thus, we speculate that EGCG treatment improves erectile dysfunction in aged rats via the PRMT1/DDAH/ADMA/NOS metabolic pathway. To confirm this hypothesis, we measured the protein concentration of PRMT1, DDAH1, and DDAH2 by Western blotting and assessed the DDAH activity and ADMA concentration in the corpus cavernosum. The results from our experiments showed that the DDAH1 expression, DDAH2 expression, and the DDAH activity in the corpus cavernosum were decreased in untreated aged rats compared with young rats. In addition, the PRMT1 expression and ADMA concentration in the corpus cavernosum were increased in untreated aged rats. We also revealed that EGCG treatment increased the DDAH1 expression, DDAH2 expression, and DDAH activity in corpus cavernosum. PRMT1 expression and ADMA concentration in the corpus cavernosum were significantly decreased in EGCG-treated aged rats. In support of our observation, Park et al. found that decreased cavernosal DDAH activity results in cavernosal ADMA accumulation, which leads to reduced cavernosal NOS activity and impairment of erectile function in a rat model of atherosclerosis. Moreover, they suggested that the decreased DDAH activity was associated with the NOS activity showing a functional correlate of erectile function.20 Imamura et al. previously described that impaired DDAH activity due to decreased DDAH1 protein expression results in the accumulation of ADMA in rabbit corpus cavernosum and at least partly lead to the impaired NO production after the administration of cigarette smoke extract.32 Masuda et al. suggested that the impaired NO-mediated cavernosal relaxations after cavernosal ischemia caused by partial vessel occlusion in rabbits are closely related to the decreased NOS activity and the increased accumulation of ADMA.33
In current experiment, we first revealed that the protein expression of PRMT1 was increased the cavernosal tissues of aged rats and was decreased in the EGCG treatment group. As a similar study showed that PRMT1 expression was higher in the hearts of aged rats than in those of young rats,34 oxidative stress had been shown to increase the activity and protein levels of PRMT1 leading to increased ADMA concentrations.35,36 In addition, Jiang et al. reported that the protective effect of a potent lipophilic antioxidant, probucol, on endothelium is related to reduction in the ADMA concentration via the inhibition of PRMT1 expression and enhancement of DDAH activity.37 In addition, we indicate that both DDAH activity and expression of DDAH in the corpus cavernosum significantly decrease at the protein level in aged rats and increase in the EGCG treatment group. Recent studies have identified DDAH as an extremely oxidant-sensitive enzyme, and its sulfhydryl group predisposes DDAH to easy oxidation after which it loses its activity.38,39 In support of our observation, Tang et al. have described that EGCG protects endothelial dysfunction induced by native LDL in vivo or by ox-LDL in endothelial cells, and the protective effect of EGCG on the endothelium is related to a decrease in the ADMA level due to an increase in DDAH activity.40 In summary, the increased PRMT1 concentration and diminished activity and protein concentration of cavernosal DDAH might be one of the potential causes of impaired erectile function in aged rats. Although we have identified changes in the DDAH activity and protein concentrations of PRMT1, DDAH1, and DDAH2 in different groups, we have not elucidated the individual functions in corpus cavernosum. Further studies are needed to determine the potential contribution of cavernosal PRMT1, ADMA, and DDAH to erectile function using isoform-specific inhibitor or transgenic animals. Another limitation of the study is that a positive control group, such as a phosphodiesterase type 5 inhibitors treatment group, was not used in the study. Thus, EGCG will be combined with other medicines in future studies.
In this study, we found that daily gavage feedings of a lower (10 mg kg−1) or higher dose (100 mg kg−1) of EGCG improves erectile function in aged rats by exerting antioxidative effects in the corpus cavernosum. However, cavernous smooth muscle content did not markedly differ between negative control and lower dose EGCG groups. Moreover, a higher dose of EGCG treatment showed more ameliorated levels of erectile function compared with a lower dose treatment according to the study. This difference indicated that EGCG improved ED in aged rats with in a dose-dependent manner.
CONCLUSIONS
We found that EGCG ameliorates erectile function in aged rats by inhibiting oxidative stress to regulate the PRMT1/DDAH/ADMA/NOS metabolic pathway. However, the molecular mechanism underlying the protective effects of EGCG requires further investigation.
AUTHOR CONTRIBUTIONS
DC, BL, and DQS performed the molecular genetic studies, participated in Western blot analysis and drafted the manuscript. DC and KQZ conducted the immunoassays and ELISA analysis. DQS and HZ participated in the design of the study and performed the statistical analyses. QF conceived of the study, participated in its design, helped draft the manuscript and have given final approval of the version to be published. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no conflict of interest.
REFERENCES
- 1.McVary KT. Clinical practice. Erectile dysfunction. N Engl J Med. 2007;357:2472–81. doi: 10.1056/NEJMcp067261. [DOI] [PubMed] [Google Scholar]
- 2.Lewis RW, Fugl-Meyer KS, Corona G, Hayes RD, Laumann EO, et al. Definitions/epidemiology/risk factors for sexual dysfunction. J Sex Med. 2010;7:1598–607. doi: 10.1111/j.1743-6109.2010.01778.x. [DOI] [PubMed] [Google Scholar]
- 3.Kandeel FR, Koussa VK, Swerdloff RS. Male sexual function and its disorders: physiology, pathophysiology, clinical investigation, and treatment. Endocr Rev. 2001;22:342–88. doi: 10.1210/edrv.22.3.0430. [DOI] [PubMed] [Google Scholar]
- 4.Lin KY, Ito A, Asagami T, Tsao PS, Adimoolam S, et al. Impaired nitric oxide synthase pathway in diabetes mellitus: role of asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase. Circulation. 2002;106:987–92. doi: 10.1161/01.cir.0000027109.14149.67. [DOI] [PubMed] [Google Scholar]
- 5.Leiper JM, Santa Maria J, Chubb A, MacAllister RJ, Charles IG, et al. Identification of two human dimethylarginine dimethylaminohydrolases with distinct tissue distributions and homology with microbial arginine deiminases. Biochem J. 1999;343(Pt 1):209–14. [PMC free article] [PubMed] [Google Scholar]
- 6.Beltowski J, Kedra A. Asymmetric dimethylarginine (ADMA) as a target for pharmacotherapy. Pharmacol Rep. 2006;58:159–78. [PubMed] [Google Scholar]
- 7.Antoniades C, Shirodaria C, Leeson P, Antonopoulos A, Warrick N, et al. Association of plasma asymmetrical dimethylarginine (ADMA) with elevated vascular superoxide production and endothelial nitric oxide synthase uncoupling: implications for endothelial function in human atherosclerosis. Eur Heart J. 2009;30:1142–50. doi: 10.1093/eurheartj/ehp061. [DOI] [PubMed] [Google Scholar]
- 8.Pawlak MR, Scherer CA, Chen J, Roshon MJ, Ruley HE. Arginine N-methyltransferase 1 is required for early postimplantation mouse development, but cells deficient in the enzyme are viable. Mol Cell Biol. 2000;20:4859–69. doi: 10.1128/mcb.20.13.4859-4869.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang J, Frankel A, Cook RJ, Kim S, Paik WK, et al. PRMT1 is the predominant type I protein arginine methyltransferase in mammalian cells. J Biol Chem. 2000;275:7723–30. doi: 10.1074/jbc.275.11.7723. [DOI] [PubMed] [Google Scholar]
- 10.Teerlink T, Luo Z, Palm F, Wilcox CS. Cellular ADMA: regulation and action. Pharmacol Res. 2009;60:448–60. doi: 10.1016/j.phrs.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aversa A, Bruzziches R, Francomano D, Natali M, Gareri P, et al. Endothelial dysfunction and erectile dysfunction in the aging man. Int J Urol. 2010;17:38–47. doi: 10.1111/j.1442-2042.2009.02426.x. [DOI] [PubMed] [Google Scholar]
- 12.Sydow K, Munzel T. ADMA and oxidative stress. Atheroscler Suppl. 2003;4:41–51. doi: 10.1016/s1567-5688(03)00033-3. [DOI] [PubMed] [Google Scholar]
- 13.Boger RH, Sydow K, Borlak J, Thum T, Lenzen H, et al. LDL cholesterol upregulates synthesis of asymmetrical dimethylarginine in human endothelial cells: involvement of S-adenosylmethionine-dependent methyltransferases. Circ Res. 2000;87:99–105. doi: 10.1161/01.res.87.2.99. [DOI] [PubMed] [Google Scholar]
- 14.Wang JH, Chen D, Zhang KQ, Zhang H, Fu Q. Effect of DDAH/ADMA/NOS regulation pathway on cavernae corporum cavernosorum rat penis of different age. Andrologia. 2015;48:262–7. doi: 10.1111/and.12441. [DOI] [PubMed] [Google Scholar]
- 15.Kim HS, Quon MJ, Kim JA. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014;2:187–95. doi: 10.1016/j.redox.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srividhya R, Jyothilakshmi V, Arulmathi K, Senthilkumaran V, Kalaiselvi P. Attenuation of senescence-induced oxidative exacerbations in aged rat brain by (-)-epigallocatechin-3-gallate. Int J Dev Neurosci. 2008;26:217–23. doi: 10.1016/j.ijdevneu.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Ou HC, Song TY, Yeh YC, Huang CY, Yang SF, et al. EGCG protects against oxidized LDL-induced endothelial dysfunction by inhibiting LOX-1-mediated signaling. J Appl Physiol (1985) 2010;108:1745–56. doi: 10.1152/japplphysiol.00879.2009. [DOI] [PubMed] [Google Scholar]
- 18.Na HK, Kim EH, Jung JH, Lee HH, Hyun JW, et al. (-)-Epigallocatechin gallate induces Nrf2-mediated antioxidant enzyme expression via activation of PI3K and ERK in human mammary epithelial cells. Arch Biochem Biophys. 2008;476:171–7. doi: 10.1016/j.abb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Ohno S, Yu H, Dickinson D, Chu TC, Ogbureke K, et al. Epigallocatechin-3-gallate modulates antioxidant and DNA repair-related proteins in exocrine glands of a primary Sjogren's syndrome mouse model prior to disease onset. Autoimmunity. 2012;45:540–6. doi: 10.3109/08916934.2012.710860. [DOI] [PubMed] [Google Scholar]
- 20.Park K, Lee DG, Kim SW, Paick JS. Dimethylarginine dimethylaminohydrolase in rat penile tissue: reduced enzyme activity is responsible for erectile dysfunction in a rat model of atherosclerosis. Int J Impot Res. 2009;21:228–34. doi: 10.1038/ijir.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–8. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 22.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915–22. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrara N, Abete P, Ambrosio G, Landino P, Caccese P, et al. Protective role of chronic ubiquinone administration on acute cardiac oxidative stress. J Pharmacol Exp Ther. 1995;274:858–65. [PubMed] [Google Scholar]
- 24.Yu W, Wan Z, Qiu XF, Chen Y, Dai YT. Resveratrol, an activator of SIRT1, restores erectile function in streptozotocin-induced diabetic rats. Asian J Androl. 2013;15:646–51. doi: 10.1038/aja.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rice-Evans C. Implications of the mechanisms of action of tea polyphenols as antioxidants in vitro for chemoprevention in humans. Proc Soc Exp Biol Med. 1999;220:262–6. doi: 10.1046/j.1525-1373.1999.d01-45.x. [DOI] [PubMed] [Google Scholar]
- 26.Levites Y, Weinreb O, Maor G, Youdim MB, Mandel S. Green tea polyphenol (-)-epigallocatechin-3-gallate prevents N-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine-induced dopaminergic neurodegeneration. J Neurochem. 2001;78:1073–82. doi: 10.1046/j.1471-4159.2001.00490.x. [DOI] [PubMed] [Google Scholar]
- 27.Nanjo F, Honda M, Okushio K, Matsumoto N, Ishigaki F, et al. Effects of dietary tea catechins on alpha-tocopherol levels, lipid peroxidation, and erythrocyte deformability in rats fed on high palm oil and perilla oil diets. Biol Pharm Bull. 1993;16:1156–9. doi: 10.1248/bpb.16.1156. [DOI] [PubMed] [Google Scholar]
- 28.Mostafa T, Sabry D, Abdelaal AM, Mostafa I, Taymour M. Cavernous antioxidant effect of green tea, epigallocatechin-3-gallate with/without sildenafil citrate intake in aged diabetic rats. Andrologia. 2013;45:272–7. doi: 10.1111/and.12005. [DOI] [PubMed] [Google Scholar]
- 29.Persson IA, Josefsson M, Persson K, Andersson RG. Tea flavanols inhibit angiotensin-converting enzyme activity and increase nitric oxide production in human endothelial cells. J Pharm Pharmacol. 2006;58:1139–44. doi: 10.1211/jpp.58.8.0016. [DOI] [PubMed] [Google Scholar]
- 30.Babu PV, Liu D. Green tea catechins and cardiovascular health: an update. Curr Med Chem. 2008;15:1840–50. doi: 10.2174/092986708785132979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooke JP. Does ADMA cause endothelial dysfunction? Arterioscler Thromb Vasc Biol. 2000;20:2032–7. doi: 10.1161/01.atv.20.9.2032. [DOI] [PubMed] [Google Scholar]
- 32.Imamura M, Waseda Y, Marinova GV, Ishibashi T, Obayashi S, et al. Alterations of NOS, arginase, and DDAH protein expression in rabbit cavernous tissue after administration of cigarette smoke extract. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2081–9. doi: 10.1152/ajpregu.00406.2007. [DOI] [PubMed] [Google Scholar]
- 33.Masuda H, Tsujii T, Okuno T, Kihara K, Goto M, et al. Accumulated endogenous NOS inhibitors, decreased NOS activity, and impaired cavernosal relaxation with ischemia. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1730–8. doi: 10.1152/ajpregu.00277.2001. [DOI] [PubMed] [Google Scholar]
- 34.Hong E, Lim Y, Lee E, Oh M, Kwon D. Tissue-specific and age-dependent expression of protein arginine methyltransferases (PRMTs) in male rat tissues. Biogerontology. 2012;13:329–36. doi: 10.1007/s10522-012-9379-2. [DOI] [PubMed] [Google Scholar]
- 35.Kaida Y, Ueda S, Yamagishi S, Nakayama Y, Ando R, et al. Proteinuria elevates asymmetric dimethylarginine levels via protein arginine methyltransferase-1 overexpression in a rat model of nephrotic syndrome. Life Sci. 2012;91:301–5. doi: 10.1016/j.lfs.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y, Xu X, Sheng M, Zhang X, Gu Q, et al. PRMT-1 and DDAHs-induced ADMA upregulation is involved in ROS- and RAS-mediated diabetic retinopathy. Exp Eye Res. 2009;89:1028–34. doi: 10.1016/j.exer.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Jiang JL, Zhang XH, Li NS, Rang WQ, Feng Y, et al. Probucol decreases asymmetrical dimethylarginine level by alternation of protein arginine methyltransferase I and dimethylarginine dimethylaminohydrolase activity. Cardiovasc Drugs Ther. 2006;20:281–94. doi: 10.1007/s10557-006-9065-1. [DOI] [PubMed] [Google Scholar]
- 38.Boger RH, Maas R, Schulze F, Schwedhelm E. Asymmetric dimethylarginine (ADMA) as a prospective marker of cardiovascular disease and mortality - An update on patient populations with a wide range of cardiovascular risk. Pharmacol Res. 2009;60:481–7. doi: 10.1016/j.phrs.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 39.Palm F, Onozato ML, Luo Z, Wilcox CS. Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. Am J Physiol Heart Circ Physiol. 2007;293:H3227–45. doi: 10.1152/ajpheart.00998.2007. [DOI] [PubMed] [Google Scholar]
- 40.Tang WJ, Hu CP, Chen MF, Deng PY, Li YJ. Epigallocatechin gallate preserves endothelial function by reducing the endogenous nitric oxide synthase inhibitor level. Can J Physiol Pharmacol. 2006;84:163–71. doi: 10.1139/y05-156. [DOI] [PubMed] [Google Scholar]