Abstract
Most widespread three-component penile prosthesis models are 700CX™ and Titan®. Our purpose is to assess patient and partner satisfaction after the first implant. This is a multicenter, retrospective, nonrandomized study in which all patients who met the inclusion criteria between 2009 and 2013 were included. In total, 248 patients agreed to participate. To evaluate patient satisfaction, a validated but modified 11-question questionnaire was completed (EDITS); and a nonvalidated two-item questionnaire was given to the partner. Statistical analysis used an ordinal logistic regression model. Two hundred and forty-eight patients (194 with 700CX™ vs 54 with Titan®) and 207 couples completed the questionnaire (165 with 700CX™ vs 42 with Titan®). Overall satisfaction was high. Both showed great reliability for sexual intercourse and high compliance with prior expectations. Most patients were able to manage the penile prosthesis correctly within 6 months. Postoperative penile shortening led to some dissatisfaction in 42% and 46% of cases (700CX™ /Titan®). Significant differences were found in three questions of patients’ questionnaire. There were more patients satisfied with the 700CX™ (P = 0.0001). No patient with Titan® implant took longer than 6 months to optimal management. Only 4% of patients with 700CX™ implant were dissatisfied with the deflation, in contrast to 24% with the Titan® (P = 0.0031). Of the two partners’ questions, one showed a statistically significant difference (P = 0.0026). It seems that group 700CX™ would recommend to re-implant the prosthesis with a greater tendency. The overall satisfaction was very high for both prostheses. The final aspect of the erected and flaccid penis was satisfactory, but both groups showed significant discontent with its final size. Partners’ overall satisfaction was high.
Keywords: erectile dysfunction, penile implantation, penile prosthesis
INTRODUCTION
Erectile dysfunction (ED) is a highly prevalent disorder. It is estimated that over 50% of men between the ages of 40 and 70 suffer from ED to some degree.1,2 Over a decade ago, the introduction of phosphodiesterase-5 inhibitors (PDE-5 inhibitors) aided and substantially improved ED treatment.3 Despite its effectiveness being high and estimated at around 60%-70%,4 other more invasive therapies may still be required, such as alprostadil treatment via intraurethral route or intracavernous injections, and vacuum devices. In fact, all the clinical practice guidelines recommend ED therapy to be gradual and progressive.5,6 When these treatment modalities are not sufficient or accepted by the patient, the only recourse is a penile prosthesis implantation (PP).
The idea of implanting a PP inside the corpora cavernous for treating ED is not new, and we would have to go back to the 1960s to find the first surgical operations that were performed.7 The first implants, which were semi-rigid or malleable, were almost replaced when the first inflatable penile prosthesis was developed in 1973 by Scott et al.8 Since then, significant advances and improvements have been produced both in design as well as in the materials used to manufacture the prosthesis. The surgical technique for PP implantation has also been subject to these advances. Today the most used prostheses are the three-component prostheses.
There are several commercial firms that manufacture and market the three-component PP. At present, the two most widespread models worldwide are 700CX™ (AMS; American Medical Systems Inc., Minneapolis, MN, USA) and Titan® (Coloplast Corp., Minneapolis, MN, USA). There have been numerous studies published until now which evaluated each type of prosthesis independently as regards the satisfaction of the patient, the partner and the surgeon. Similarly, studies have been conducted to assess both types of prostheses independently in very specific scenarios such as to evaluate new developments and technical advances of each of the prostheses, or to compare their effectiveness in special clinical situations such as for concomitant DE and Peyronie's disease treatment.3,7,9,10,11,12
To the best our knowledge, no studies have been published that compare patient and partner satisfaction after the implantation of each of the two types of PP. Therefore, we have developed this study, which assesses patient and partner satisfaction after the first implant of a 700CX™ prosthesis versus a Titan® prosthesis.
MATERIALS AND METHODS
After approval by the Institution's Ethics Committee, we conducted a multicenter, retrospective study from January 2009 to January 2013. The two centers included in the study belong to the Public Health System and are considered as National Reference Centers for PP implantation. One expert surgeon per center implanted all the prostheses in his center. Both surgeons performed the same standardized implant surgery technique as published previously: penoscrotal approach without dilation of the corpora cavernous,13 escrotal placement of the pump in front of the testicles, and retropubic insertion of the reservoir through the inguinal canal. Both centers shared the same strict protocol for clinical actions and guidelines of the PP before and after surgery, including the visits to the clinics and patient education for the management of the device. Whether the 700CX™ or the Titan® OTR prosthesis model was implanted depended on the public tender adjudication that takes place in each center. In this way, the PP implanted was the one available in each of the centers at the time of surgery. There was no possibility of choice either by the surgeon or by the patient. The inclusion criteria of patients are shown in Figure 1.
Figure 1.
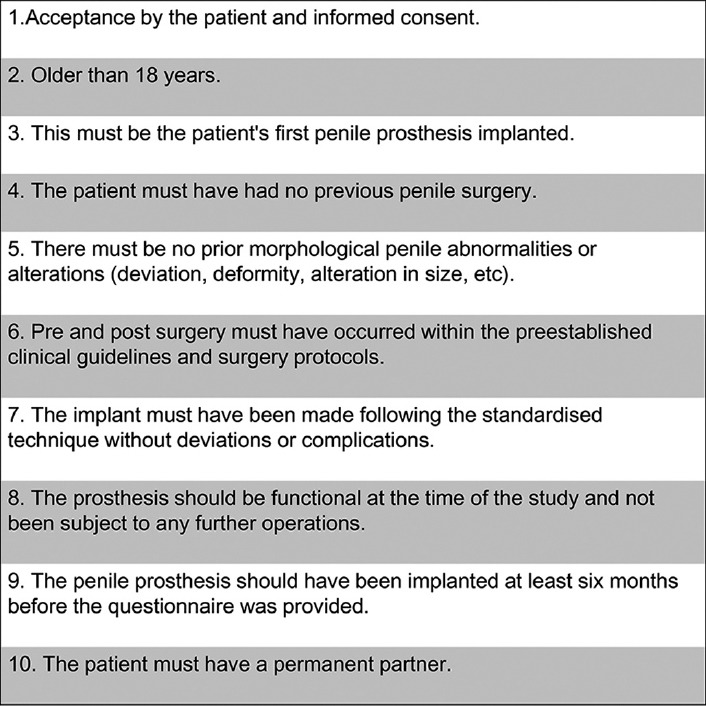
Inclusion criteria for the study.
In reviewing the medical records, 281 patients who fulfilled the above inclusion criteria were identified, of which 248 agreed to participate (88.3%), including 194 had been implanted a 700CX™ and 54 had a Titan® OTR. Two-hundred seven couples were included. A questionnaire was given to the patients and their partners, and this was returned anonymously.
All the data were collected anonymously and analyzed by the Statistical Service in a single center. The continuous variables were given as mean and standard deviation and the categorical variables are presented by the absolute and relative frequency. The association between the questionnaire responses, the dependent variables and the prosthesis used, were identified by an ordinal logistic regression model, based on the cumulative distribution of the categorical response probability.14 This model assumes a common slope associated with the predictor variable, i.e., it assumes that the odds ratio of the event Y ≤ j is independent of the category j. The assumption was checked, and if it defaulted then adjacent categories of the response variable were combined. The magnitude of the effect of the prosthesis type variable was evaluated through the odds ratio, together with the confidence interval at 95%, without and adjusted by the patient characteristics. The treatment and analysis of the data were performed using the SAS/STAT programme, version 9, SAS System for Windows (SAS Institute Inc., North Carolina, USA).
A validated questionnaire was used to evaluate patient satisfaction. This was the Erectile Dysfunction Inventory of Treatment Satisfaction (EDITS), which was modified for this study and included 11 questions.15 A nonvalidated two-question questionnaire was used to evaluate the partner's satisfaction. Table 1 shows both questionnaires.
Table 1.
Patient and partner satisfaction questionnaires

RESULTS
Patient characteristics
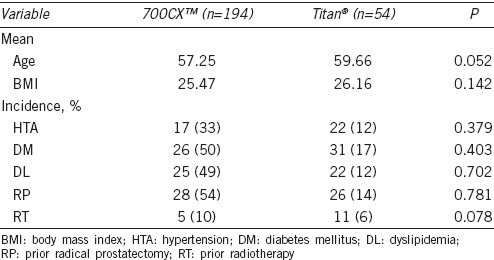
A total of 248 patients met the necessary requirements and agreed to participate in this study. One hundred and ninety-four of them were implanted a 700CX™ and 54 were implanted a Titan®. The mean age of the 700CX™ group was 57.25 years whereas the Titan® group was 59.66. A total of 207 partners agreed, and 41 refused to complete the specific questionnaire (165 with 700CX™ and 42 with Titan®).
The variables analyzed in each group included age, body mass index (BMI), and the presence or absence of comorbidities or relevant medical history such as hypertension (HTA), diabetes mellitus (DM), dyslipidemia (DL), prior radical prostatectomy (RP), and prior radiotherapy (RT). No statistically significant differences were found between the two groups for any of these variables, so we can conclude that they were homogeneous. The characteristics of the groups are summarized in Table 2.
Table 2.
Demographic variables
Patient satisfaction
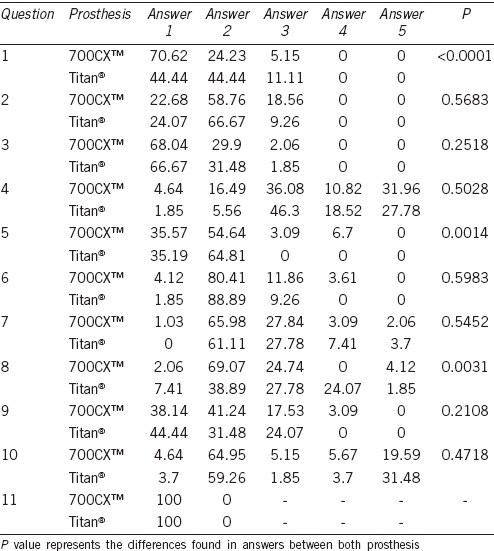
The overall responses to the patient satisfaction test (modified EDITS) are shown in Table 3. The three aspects that showed statistically significant differences were overall satisfaction with sexual intercourse, time to the optimal management of the prosthesis and ease of deflation. Detailed analysis was as follows.
Table 3.
Answer given to patient satisfaction test (percentage in each answer)
Question number 1
We assessed the overall satisfaction with the sexual intercourse. Although with both PPs the patients were satisfied, it seems that there were more patients satisfied with the 700CX™ than with Titan®. It is noteworthy that no patient was dissatisfied or very dissatisfied after the PP implantation (P = 0.0014).
Question number 5
We evaluated the time that passed until the patient had the optimal management of the prosthesis. The vast majority of patients managed the PP in <6 months. However, it seems that while no patient with the Titan® implant took longer than this time, 10% of patients with the 700CX™ implant went over this length of time (P = 0.0014).
Question number 8
We assessed the ease of deflating the prosthesis. While only 4% of patients with the 700CX™ implant were dissatisfied with the deflation of the prosthesis, up to 24% of the patients with the Titan® implant were dissatisfied (P = 0.0031).
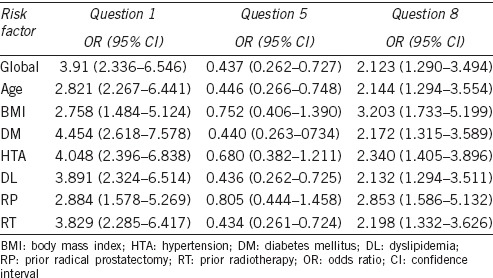
For each of these three issues, an adjusted test for the various analyzed risk factors was used, to find out if any of them was interfering in the results. The results are shown in Table 4. For question 11 no statistical analysis was performed because all the prostheses were functioning at the time of completing the questionnaire, which was one of the inclusion criteria.
Table 4.
OR for risk factors in questions where significant statistically differences were found between both prosthesis
Partner satisfaction
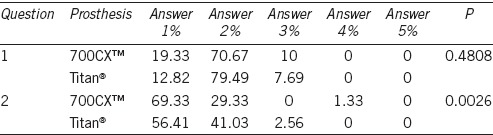
A total of 207 partners agreed to participate in the study and completed the questionnaire (83.5%). The results are summarized in Table 5. Of the two questions contained therein, one showed a statistically significant difference between the two prostheses (P = 0.0026). It seems that although both groups would “strongly” recommend to their partners to re-implant the PP, it seems there is a greater tendency that group 700CX™ would recommend it more than group Titan® with 69% versus 56%, respectively.
Table 5.
Answer given to partner satisfaction test (percentage in each answer)
DISCUSSION
This article, to our knowledge, is the first to compare patient and partner satisfaction for the two types of prosthesis most used in our sector: 700CX™ versus Titan®. This study provides information as to the satisfaction of both the patient and his partner for the penile prosthesis in an overall manner, as well as for each of them separately.
Technically both penile prostheses are very similar in their design. The 700CX™ cylinders are manufactured with Dacron-Lycra fabric between two layers of silicone and coated on both sides with a Parylene layer, which improves the lubrication and mechanical reliability.16 The Titan® cylinders are made from Bioflex, a polyurethane-like material, coated with a hydrophilic compound. Both polyurethane and Bioflex seem to be very resistant to abrasion, and large series published with the Mentor Alpha-1 (also polyurethane) show very few mechanical cylinder failures.17 In our study, we only included patients who had the implant functioning at the time of analysis and who had not required any intervention because of malfunction. Therefore, we cannot evaluate technical differences.
The 700CX™ reservoir is spherical, with different capacities depending on the length of the cylinders that are implanted. The Titan® reservoir, however, has a more cylindrical shape. The latest pump developed by AMS, called Momentary Squeeze (MS) showed an 86% patient satisfaction, and virtually eliminated the cases of auto-inflation.7 It consists of a bulb that must be squeezed to inflate the cylinders, with a small button located just above it, which when pressed for a few seconds completely deflates the prosthesis. The Titan® mechanism is similar, except that the button is bigger and it has to be pressed on both sides at the same time. Questionnaire question number 7, which referred to the ease of inflating the prosthesis, showed no statistically significant differences between them. However, question number 8 which assessed the ease of deflation did show a difference, a 2.12 OR was obtained for 700CX™ against Titan® (95% CI: 1.290–3.494; Table 4). At first, we thought that this result was probably explained by the subjects’ characteristics, such as, for example, the more obese individuals who could have more problems when it came to accessing the Titan® deflation button. After statistical adjustment for various possible confounding factors including the body mass index, significant differences continued to be found in both groups for ease of deflation.
Both prostheses consist of three-components, including a reservoir that stores the serum that fills the cylinders when the penis is flaccid. This gives a more natural appearance to the flaccid penis, which is an improvement with respect to the one- or two-component prostheses.18 For question number 9, regarding the appearance of the flaccid penis, the overall satisfaction was high, and no significant differences were found. When asked about the appearance of the erect penis (question 10) no differences were found. With regard to penile length (question 4), postoperative penile shortening is one of the most common causes of dissatisfaction in the literature and in our series.19 This fact has been tried to be resolved with the new techniques for dilation and measurement of the corpora cavernosa with little success.20 In this study, 25% of patients with a 700CX™ and 34% of those with a Titan® reported being dissatisfied or very dissatisfied with the appearance of the erect penis; as well as the 42% and 46% had some degree of dissatisfaction with the 700CX™ and Titan® respectively.
According to question number 6, more than 90% of patients had sexual intercourse at least once a week, with no difference between these two, and this is perhaps somewhat higher than that published in literature.21 In this era of oral therapy for erectile dysfunction, the three-component penile prosthesis is the treatment that produces the highest patient satisfaction rate.19,22 In this work which assesses patient satisfaction with the treatment they have received, questions 2 and 3 show that the confidence to have sexual intercourse (question number 3) was 98% with no difference between the two prostheses, and the fulfillment of the pretreatment expectations (question number 2) was about 90% for Titan® and over 80% for 700CX™. However, for question 1, which asked about the overall satisfaction with sexual intercourse, statistically significant differences were found. The OR for 700CX™ versus Titan® was 3.91 (95% CI: 2.336–6.546; Table 4), and the risk subgroup analysis did not provide any additional information. From a detailed analysis of the responses given in Table 3, we can see that the fundamental difference is that while 70% of patients with 700CX™ report being very satisfied, only 44% of those with Titan® give this answer. Subsequently, the differences decrease, as none refer to being somewhat or very dissatisfied. We are unable to reach more conclusions based on the results of this study, given its limitations. However, it may be a factor to consider in the design of future studies.
The postoperative period after a PP implant is very important, especially in terms of patient education for the management of the device. This is what provides the couple with the necessary autonomy to enjoy sexual intercourse, with the psychological advantages that entail and therefore overall satisfaction with the procedure.20 We consider the optimal management of the device when the patient can use it for sexual intercourse with no help or with the help of his partner. In our study, 100% of patients with the Titan® and 90% of patients with the 700CX™ had learnt to manage the device within 6 months or less. However, the differences observed were statistically significant and also it is seen that in the subgroup analysis, BMI, hypertension (HTN) and prior radical prostatectomy (RP) showed statistically significant OR (Table 4). In the case of BMI, it seems logical that the more obese the patient is, the more difficult it will be to manage the pump, due to the increase of prepubic fat and a lesser visual range of the penis and scrotum. However, we did not find justification as to why prior radical prostatectomy (RP) or hypertension (HTN) can act as confounding factors. Moreover, since both groups were found to be homogeneous for the different risk factors from the beginning, it cannot be explained that these differences can be justified by them.
In the case of the partner satisfaction questionnaire, as shown in Table 5, in question number 1 over 90% considered that the sexual intercourse was good or very good, with no significant differences between the two prostheses, whereas for question number 2, 98% would recommend to their partners to have a PP implant again. For this second question significant differences were found, with an OR = 2.58 for 700CX™ versus Titan® (P = 0.0026).
It should be mentioned that this paper has limitations in its design. On one hand, it is retrospective, with the usual limitations that this design entails especially when we want to assess satisfaction, for the passage of time can change or blur our patient's point of view. Another detail to keep in mind is that we must consider there are two centers and the objective is to assess the satisfaction of both the patient and the partner after the first PP implant. For this reason, we made a large sample selection, and excluded patients who had not followed our established clinical guidelines or the standardized surgical protocol. Similarly, cases that began with a pathological or previously operated penis were deliberately excluded, because satisfaction could be affected by the outcome of the latter pathology.12 Hence, these should be considered as the best results that can be expected after the PP implant. Moreover, the cases were not randomized. This is because the penile prostheses that were available in the centers involved in the study were allocated by public tender or public auction. This meant that the prosthesis implanted had to be the one available in the center at that time. Although this is a clear limitation, we believe that the final sample is large enough and representative of the patients who received a penile prosthesis implant for the first time. It is important to point out that both groups were found to be homogeneous as regards analyzed risk factors and the series obtained was sufficiently wide. In a similar way, a strict exclusion criterion was applied when the patients did not follow the clinical guidelines or the standardized surgical protocol. Besides, we think that the fact that neither the patient, nor the surgeon can choose the device to implant is a clear strength of this study. Therefore, we believe that this work provides valid and interesting information in this field.
CONCLUSIONS
Given the data in this study, it can be said that in the best possible scenario for a PP implant, the overall satisfaction is very high for both types of prosthesis. And while both groups found the final appearance of the penis, when it erects and especially when it flaccids, to be satisfactory, both showed significant discontent with its final size. The vast majority of the patients could manage the prosthesis within 6 months or less, and the degree of satisfaction of the partners is high. However, the optimal management of the 700CX™ took longer than with the Titan®, and the dissatisfaction with the deflation was higher with the Titan®.
AUTHOR CONTRIBUTIONS
JRO and CRC conceived and designed the study. JRO put in contact the centres. JRO, CRC, BGG, JSG and JMP collected the data. JRO, CRC and BGG collaborated in writing the paper. ERC and ARA revised the paper.
COMPETING INTERESTS
All the authors declare no competing interests.
REFERENCES
- 1.Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts male aging study. J Urol. 1994;1:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 2.Martin-Morales A, Sanchez-Cruz JJ, Saenz de Tejada I, Rodriguez-Vela L, Jimenez-Cruz JF, et al. Prevalence and independent risk factors for erectile dysfunction in Spain: results of the Epidemiologia de la Disfuncion Erectil Masculina Study. J Urol. 2001;166:569–74. doi: 10.1016/s0022-5347(05)65986-1. [DOI] [PubMed] [Google Scholar]
- 3.Ohl DA, Brock G, Ralph D, Bogache W, Jones LR, et al. Prospective evaluation of patient satisfaction, and surgeon and patient trainer assessment of the Coloplast Titan one touch release three-piece inflatable penile prosthesis. J Sex Med. 2012;9:2467–74. doi: 10.1111/j.1743-6109.2012.02819.x. [DOI] [PubMed] [Google Scholar]
- 4.Morales A, Gingell C, Collins M, Wicker PA, Osterloh IH. Clinical safety of oral sildenafil citrate (VIAGRA) in the treatment of erectile dysfunction. Int J Impot Res. 1998;10:69–73. doi: 10.1038/sj.ijir.3900354. [DOI] [PubMed] [Google Scholar]
- 5.Hatzimouratidis K, Eardley I, Giuliano F, Hatzichristou D, Moncada I, et al. Guidelines on Male Sexual Dysfunction: Erectile Dysfunction and Premature Ejaculation. 2014. Available from: http://www.uroweb.org/gls/pdf/14%20 Male%20Sexual%20Dysfunction_LR.pdf . [DOI] [PubMed]
- 6.Montague DK, Jarow JP, Broderick GA, Dmochowski RR, Heaton JP, et al. AUA Guidelines: Management of Erectile Dysfunction. 2011. Available from: http://www.auanet.org .
- 7.Knoll LD, Henry G, Culkin D, Ohl DA, Otheguy J, et al. Physician and patient satisfaction with the new AMS 700 momentary squeeze inflatable penile prosthesis. J Sex Med. 2009;6:1773–8. doi: 10.1111/j.1743-6109.2009.01251.x. [DOI] [PubMed] [Google Scholar]
- 8.Scott FB, Bradley WE, Timm GW. Management of erectile impotence. Use of implantable prosthesis. Urology. 1973;2:80–2. doi: 10.1016/0090-4295(73)90224-0. [DOI] [PubMed] [Google Scholar]
- 9.Henry GD, Jennermann C, Eid JF. Evaluation of satisfaction and axial rigidity with titan XL cylinders. Adv Urol 2012. 2012:1–6. doi: 10.1155/2012/896070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung E, Solomon M, Deyoung L, Brock GB. Comparison between AMS 700™ CX and Coloplast™ titan inflatable penile prosthesis for Peyronie's disease treatment and remodeling: clinical outcomes and patient satisfaction. J Sex Med. 2012;10:2855–60. doi: 10.1111/jsm.12009. [DOI] [PubMed] [Google Scholar]
- 11.Kramer AC, Schweber A. Patient expectations prior to Coloplast Titan penile prosthesis implant predicts postoperative satisfaction. J Sex Med. 2010;7:2261–6. doi: 10.1111/j.1743-6109.2010.01799.x. [DOI] [PubMed] [Google Scholar]
- 12.Usta MF, Bivalacqua TJ, Sanabria J, Koksal IT, Moparty K, et al. Patient and partner satisfaction and long-term results after surgical treatment for Peyronie's disease. Urology. 2003;62:105–19. doi: 10.1016/s0090-4295(03)00244-9. [DOI] [PubMed] [Google Scholar]
- 13.Moncada I, Martínez-Salamanca JI, Jara J, Cabello R, Moralejo M, et al. Inflatable penile prosthesis implantation without corporeal dilation: a cavernous tissue sparing technique. J Urol. 2010;183:1123–6. doi: 10.1016/j.juro.2009.11.048. [DOI] [PubMed] [Google Scholar]
- 14.Agresti A. Categorical Data Analysis. 2nd ed. Miami: Wiley; 2002. p. 86. [Google Scholar]
- 15.Althof SE, Corty EW, Levine SB, Levine F, Burnett AL, et al. EDITS: development of questionnaires for evaluating satisfaction with treatments for erectile dysfunction. Urology. 1999;53:793–9. doi: 10.1016/s0090-4295(98)00582-2. [DOI] [PubMed] [Google Scholar]
- 16.Salem EA, Wilson SK, Neeb A, Delk JR, Cleves MA. Mechanical reliability of AMS 700 CX improved by parylene coating. J Sex Med. 2009;6:2615–20. doi: 10.1111/j.1743-6109.2009.01382.x. [DOI] [PubMed] [Google Scholar]
- 17.Wilson SK, Cleves MA, Delk JR., 2nd Comparison of mechanical reliability of original and enhanced Mentor Alpha 1 penile prosthesis. J Urol. 1999;162:715–8. doi: 10.1097/00005392-199909010-00022. [DOI] [PubMed] [Google Scholar]
- 18.Wilson SK, Cleves M, Delk JR. Long term results with Hydrofelx and Dynaflex penile prostheses: device survival comparision to multicomponent inflatables. J Urol. 1996;155:1621–3. [PubMed] [Google Scholar]
- 19.Cruz N. Tratado de Andrología y Medicina Sexual. Madrid: Editorial Médica Panamericana; 2011. pp. 156–74. [Google Scholar]
- 20.Henry G, Houghton L, Culkin D, Otheguy J, Shabsigh R, et al. Comparison of a new length measurement technique for inflatable penile prosthesis implantation to standard techniques: outcomes and patient satisfaction. J Sex Med. 2011;8:2640–6. doi: 10.1111/j.1743-6109.2011.02340.x. [DOI] [PubMed] [Google Scholar]
- 21.Henry GD, Brinkman MJ, Mead SF, Delk JR, 2nd, Cleves MA, et al. A survey of patients with inflatable penile prostheses: assessment of timing and frequency of intercourse and analysis of implant durability. J Sex Med. 2012;9:1715–21. doi: 10.1111/j.1743-6109.2012.02729.x. [DOI] [PubMed] [Google Scholar]
- 22.Bernal RM, Henry GD. Contemporary patient satisfaction rates for three-piece inflatable penile prostheses. Adv Urol 2012. 2012 doi: 10.1155/2012/707321. 707321. [DOI] [PMC free article] [PubMed] [Google Scholar]


