Abstract
The aim of the present work was to present the outcomes of the patients with Y-chromosome microdeletions treated by intracytoplasmic sperm injection (ICSI), either using fresh (TESE) or frozen-thawed (TESE-C) testicular sperm and ejaculated sperm (EJAC). The originality of this work resides in the comparisons between the different types of Y-microdeletions (AZFa, AZFb, and AZFc) and treatments, with detailed demographic, stimulation, embryological, clinical, and newborn (NB) outcomes. Of 125 patients with Y-microdeletions, 33 patients presented severe oligozoospermia (18 performed ICSI with ejaculated sperm) and 92 secretory azoospermia (65 went for TESE with 40 having successful sperm retrieval and performed ICSI). There were 51 TESE treatment cycles and 43 TESE-C treatment cycles, with a birth of 19 NB (2 in AZFa/TESE-C, 12 in AZFc/TESE, and 5 in AZFc/TESE-C). Of the 29 EJAC cycles, there was a birth of 8 NB (in AZFc). In TESE and EJAC cycles, there were no significant differences in embryological and clinical parameters. In TESE-C cycles, there was a significant lower oocyte maturity rate, embryo cleavage rate and mean number of embryos transferred in AZFb, and a higher mean number of oocytes and lower fertilization rate in AZFc. In conclusion, although patients with AZFc microdeletions presented a high testicular sperm recovery rate and acceptable clinical outcomes, cases with AZFa and AZFb microdeletions presented a poor prognosis. Due to the reported heredity of microdeletions, patients should be informed about the infertile consequences on NB and the possibility of using preimplantation genetic diagnosis for female sex selection.
Keywords: intracytoplasmic sperm injection, newborn outcomes, nonobstructive azoospermia, severe oligozoospermia, testicular sperm extraction, Y-chromosome microdeletions
INTRODUCTION
Infertility is a disease that attains about one in six couples at reproductive age.1 In approximately half of these cases, infertility is due to male factors. There are several causes of male infertility; however, the major causes are abnormal semen parameters and azoospermia.2 The incidence of chromosomal abnormalities in infertile men is as high as about 12.70%.3,4 Besides structural (11.70%) and numeric (0.94%) chromosomal abnormalities detected by karyotyping, microdeletions occurring on the long arm of the Y chromosome (Yq11) are also main causes of infertility (0.23%), compromising the correct progression of the spermatogenic process. Y-chromosome microdeletions are associated with severe oligozoospermia or nonobstructive azoospermia (secretory azoospermia), the latter presenting variable patterns of spermatogenesis failure.4,5
After Klinefelter syndrome, Y-chromosome microdeletions are the most frequent genetic causes of male infertility. The locus defined as Azoospermia Factor (AZF) in Yq11 contains the genes necessary for normal spermatogenesis, and deletions in this locus have been correlated with male infertility,6 being proposed as a tool for infertility diagnosis.7 This locus is divided into three major regions named AZFa, AZFb, and AZFc.5 Within the AZFc region is located the Deleted in Azoospermia (DAZ) gene family.8 Deletions of the DAZ1/DAZ2 gene doublet were found to be responsible for severe oligozoospermia, with possible evolution to secretory azoospermia with different testicular phenotypes,9 but later demonstrated that only DAZ1 was responsible for this phenotype.10
Distinct histopathology phenotypes were correlated with the site of the microdeletion, varying from complete absence of germ cells (Sertoli Cell-Only Syndrome, SCOS) in patients with AZFa microdeletions, maturation arrest at meiosis (MA) in cases with AZFb microdeletions, to hypospermatogenesis (HP) in patients with AZFc microdeletions.5,9,11,12,13,14,15,16 However, individual variation on the extent of microdeletions has led to the observation of incomplete microdeletions which enable the existence of spermatogenesis foci in SCOS and MA and thus successful testicular sperm retrieval.17,18,19,20 These situations may be referred as variants of classic deletions and were shown to correspond to specific subregions deletions on those classic AZF regions. Thus, the presence of these variants does not compromise the classic patterns that are related to full deletions of the AZFa or AZFb regions. The importance of careful evaluation of AZF microdeletions in male infertility before assisted reproduction is thus obvious.
We evaluated 128 patients with AZF microdeletions to determine whether there are any significant differences in outcomes between the different types of Y-microdeletions using fresh or frozen-thawed testicular sperm and ejaculated sperm. The results evidenced that although men with AZFc microdeletions have a high rate of sperm retrieval and acceptable clinical outcomes, in the presence of AZFa or AZFb microdeletions, the clinical outcomes are not satisfactory.
MATERIALS AND METHODS
Ethical considerations
Ethical guidelines were followed in the conduct of research, with written informed consent having been obtained before the beginning of the present work. This work did not involve human or animal experiments. An approval by an Ethics Committee and the provisions of the Declaration of Helsinki as revised in Tokyo 2004 on human experimentation does not apply to this kind of work. According to the National Law on Medically Assisted Procreation (Law 32/2006) and the National Council on Medically Assisted Procreation guidelines (CNPMA, 2015), blood samples, fertility treatments, and databases from patients with Y-chromosome microdeletions were analyzed without the need of further ethical obligations.
Patients
During the period from 1995 to 2014, we diagnosed 128 patients with Y-chromosome microdeletions. The mean male age of these men was 34.8 years (24–55) and the mean time of infertility was 4.8 (1–25) years.
Karyotype analysis
The karyotype was obtained using G-banding and included the analysis of at least 30 metaphases from peripheral blood lymphocytes, according to general protocols.21
Y-chromosome screening
Genomic DNA was obtained from peripheral blood lymphocytes using a modified salting out method (Citogene kit, Citomed, Lisbon, Portugal). Y-chromosome microdeletions in AZFa, AZFb, and AZFc regions were detected through routine molecular diagnosis of the Y chromosome following the EAA/EMQN Guidelines.19 Y-chromosome microdeletions in regions AZFa, AZFb, or AZFc were detected using sequence-tagged site (STS) and multiplex-PCR (Taq DNA polymerase from Thermo Scientific, Lithuania). The markers used were combined in four different multiplex-PCR, and the results were checked by capillary electrophoresis (QIAxcel, Qiagen, Hilden, Germany). For the optimal detection of AZF deletions, we followed the published indications for AZFa,11,12,13,15,19 AZFb, and AZFc.14,16,19,22 Besides the indicated STS markers, we also performed additional STS markers for spanning all AZF regions and SRY:11,12,13,14,15,16,20
For the AZFa region: sY84, sY86, sY82, sY83, sY1064, sY1065, sY1182, and sY88 and, additionally, USP9Y (DFFRY) and DDX3Y (DBY)
For the AZFb region: sY127, sY134, sY105, sY121, sY1224, sY143, sY1192, and sY153 and, additionally sY591, sY114, EIFIAY, sY135, and sY142
For the AZFc region: sY1197, sY1192, BPY2, sY152, sY254, sY 255, DAZ1, sY1291, CDY1, sY157, sY1201, and sY1206.
According to the actual nomenclature,19,22 Y-chromosome microdeletions were named as AZFa, AZFb (P5/proximal P1), AZFbc (P5/distal P1 or P4/distal P1), AZFc (b2/b4), and DAZ1, 2 (gr/gr).
Testicular biopsy procedure
The open testicular biopsy for sperm extraction was performed with spermatic cord block.23,24 For tissue preparation, small testicular seminiferous tubule fragments were fragmented and squeezed, and the resultant fluid fraction was treated according to a previous described protocol using erythrocyte-lysing buffer and enzymatic digestion with DNase and collagenase.25,26 The volume of each testicle was determined by two sequential methods, first using the Prader orchidometer (12 ellipsoid solid models of 1–25 ml) and then an orchidometer with ellipsoid rings of 1–30 ml. The testicular volumes were considered atrophic (A) when the volume was <6 ml, hypotrophyc (H) when between 6 and 15 ml, and normal (N) when >15 ml.27 The biopsy was multiple and extensive, with a preliminary microscopic check of the samples (2 mm) at the end of each biopsy, but the most conservative possible to keep the previous patient hormonal state. Retrieval was supported by an exhaustive search of sperm in the fragments obtained after enzymatic digestion. No postoperative complications were observed.
Testicular sperm cryopreservation
Cells were frozen with Sperm Freezing Medium or Cryosperm (Maløv, Denmark) in a 1:1 dilution. Samples were aspirated into labeled straws, sealed (L’Aigle, France), left over LN2 vapor, and finally immersed and stored in LN2.
Stimulation protocols
Women underwent controlled ovarian hyperstimulation with a GnRH short antagonist (cetrorelix; Merck Serono, Geneve, Switzerland; ganirelix; Organon, Oss, the Netherlands) protocol in the majority of the cases. For stimulation, rFSH was mainly used (Puregon; Organon; Gonal-F; Merck Serono, Germany). About 36 h before oocyte recovery, HCG was administered (Pregnyl; Organon). Estradiol serum levels were assayed at the day of HCG or 1 day before.28,29
Gamete and embryo handling
Gamete and embryo handling was performed with media from Medicult (Jyllinge, Denmark) or Vitrolife (Kungsbacka, Sweden). Microinjection was performed in an inverted microscope (Nikon DIAPHOT 200; Nikon, Tokyo, Japan), equipped with a thermal plate (37°C), Hoffman optics (Nikon) and Narishige micromanipulators (MO-188; Narishige, Tokyo, Japan), using micropipettes from Swemed (Goteborg, Sweden). ICSI was performed using the strong dislocation of the cytoplasm.30,31 Embryo grade was evaluated according to described methods.32,33 Embryo transfer was performed under ultrasonography, using a Sure View Wallace Embryo Replacement Catheter or Wallace malleable stylet (Smiths Medical Int., Kent, UK).
Luteal supplementation
All patients had luteal supplementation via intravaginal administration of 200 mg natural micronized progesterone, 8/8 h (600 mg per day) (Jaba, Besins Int., Montrouge, France) beginning on the day of oocyte retrieval. Implantation was confirmed by a rise in serum βHCG 12 days after embryo transfer. Clinical pregnancy was established by ultrasonography at 6 weeks of gestation. Progesterone was maintained until βHCG serum assay and, if positive, it was continued until 12 weeks of gestation.
Statistical analysis
Statistics was performed either using the software STATISTICA (Version 12.6, StatSoft, Dell Inc., Tulsa, OK, USA) or the PAST (Version 3.05c, Copyright Oyvind Hammer 1999-2015, Norway, based on Hammer et al., 2001), depending on the type of analysis and convenience (availability of tests and easiness of operation).34,35,36 For conducting parametric tests on continuous variables, normality and homogeneity of variances of datasets were checked by the Shapiro–Wilk W-test and by the Levene test, respectively. Whenever needed (for HCG and E2), data were log transformed.
One-way analysis of variance (ANOVA) was applied to compare the microdeletion groups (AZFa, AZFb, and AZFc/DAZ). Whenever ANOVA was significant, post-hoc analysis (paired comparisons) was performed with the Newman–Keuls test. As the “n” of some microdeletion groups is scarce, we conducted a parallel nonparametric approach, using the Kruskal–Wallis ANOVA, that whenever significant was followed by multiple pair comparisons using the Mann–Whitney U-test pairwise with sequential Bonferroni correction of the P values; as there was agreement between the parametric and nonparametric approaches, the significance levels are referred here only to the parametric approach. When at occasions there were only data for two microdeletion groups, comparisons between groups used the Student's t-test for independent samples, and, complementarily, the Mann–Whitney U-test; as above for ANOVA, facing agreement of both approaches, only the significance values displayed herein refer to the parametric testing. Regarding the analysis of proportions, groups were compared with the Z-test. Tests were always two-tailed, and differences were considered statistically significant when P < 0.05. Additional statistical analysis was carried out through the IBM SPSS Statistics 20 program for Windows (Armonk, IBM Corp, NY, USA), using the Chi-square tests, Fisher exact test, and the Independent Samples t-test for equality of means, two-sided.
RESULTS
During the period of study, 1387 Y-chromosome screenings were performed at FMUP and 128 AZF microdeletions were found: 17 in AZFa (1.2%), 26 in AZFb (1.9%), and 85 in AZFc (6.1%). On these 128 patients with AZF microdeletions, the rates of per type of microdeletion were: 13.3% in AZFa, 20.3% in AZFb, and 66.4% in AZFc. Of the 128 patients, 3 patients, despite having a diagnosis of an AZFc microdeletion, went only for consultations, and we were unable to perform karyotype analysis and determine if they had severe oligozoospermia or secretory azoospermia. Of these 125 cases, 33 (26.4%) patients presented severe oligozoospermia, of which 18 performed treatments with ejaculated sperm and 92 (73.6%) had secretory azoospermia, of which 65 performed TESE with 40 having a successful sperm retrieval. The clinical outcomes of these 58 patients were the objectives of the present study. For text convenience, cases with DAZ microdeletions were merged with those with AZFc microdeletions.
AZF microdeletions that involved the AZFa region presented five cases with microdeletions only in AZFa, four cases only in AZFab, and eight cases only in AZFabc. For the AZFb region, there was one case with microdeletions only in AZFb, one case only in AZFb + DAZ, two cases only in partial AZFb + DAZ, and 22 cases only in AZFbc. In the 125 patients (three patients with AZFc microdeletions did not perform karyotype analysis), there were 25 (20.0%) abnormal karyotypes: 8 in AZFabc (6.4%), 12 in AZFbc (9.6%), and 5 in AZFc (4.0%). The corresponding rates per type of microdeletion were: 100% in AZFabc as all eight patients had an abnormal karyotype, 54.5% of the 22 patients with AZFbc microdeletions, and 6.1% of the 82 patients with AZFc microdeletions.
Regarding male age, time of infertility, FSH, LH and testosterone serum levels, and total number of testicular fragments analyzed, comparisons between cases with and without sperm retrieval gave no significant differences except for FSH serum levels that were higher in cases without sperm retrieval in AZFc (Table 1).
Table 1.
Clinical data of patients with Y-chromosome microdeletions submitted to TESE
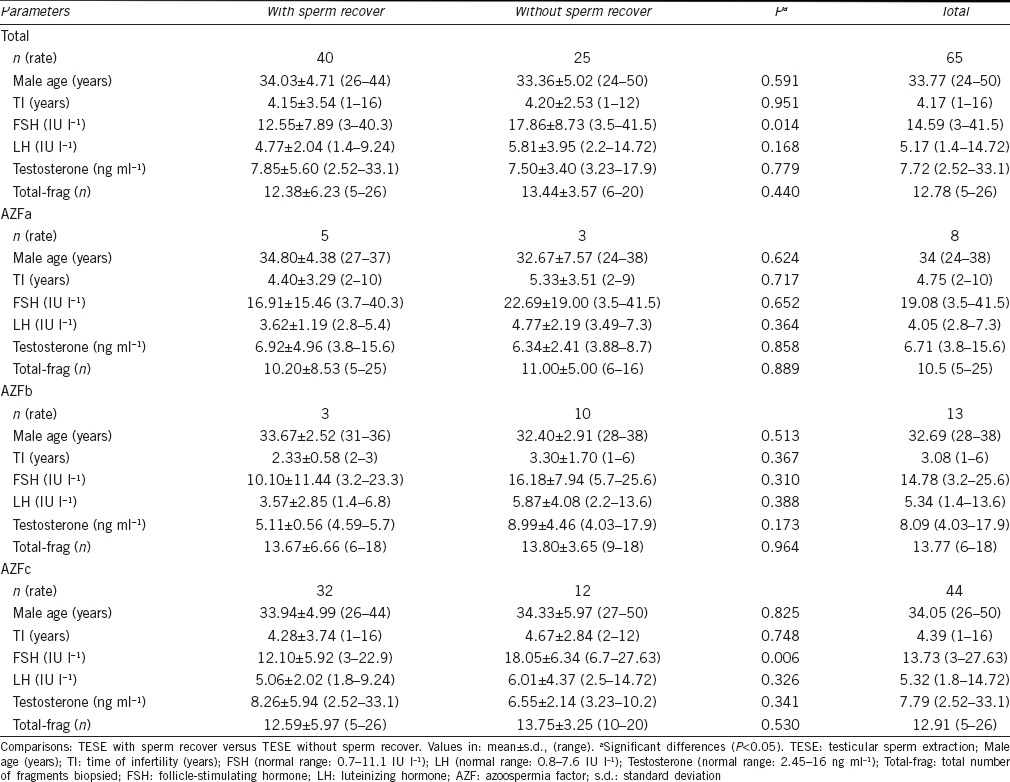
In 40 cases with sperm recovery, TESE was unilateral in 20 cases (50.0%) and bilateral in 20 patients (50.0%), with 14 (35.0%) patients in which only the right testicle was intervened and six patients (15.0%) where only the left testicle was analyzed. In 25 patients without sperm retrieval, both testicles were fully analyzed. Regarding the testicular volume (left/right testicle), of the 40 patients who had sperm retrieval at TESE, there were 25 cases N/N (62.5%), three cases N/H (7.5%), 11 cases H/H (27.5%), and one case H/A (2.5%). Of the 25 patients without sperm retrieval at TESE, there were 13 cases N/N (52%), 11 cases H/H (44%), and one case N/A (4%).
Patients with AZFa microdeletions
All patients with AZFa microdeletions presented secretory azoospermia. There were five cases with a microdeletion in AZFa: two performed no treatment, two were diagnosed as SCOS (one no treatment; one TESE without sperm recovered), and one as HP (one cycle TESE, without pregnancy, followed by four cycles with cryopreserved sperm, with a birth of 2 NB after a twin pregnancy). This patient has a normal karyotype and an isolated microdeletion of DDX3Y (DBY).
Four cases presented microdeletions in AZFab: 1 had SCOS (TESE negative), two cases had MA (one case with two TESE cycles and two cycles with cryopreserved testicular sperm, without pregnancy; and another case with one TESE cycle and two cycles with cryopreserved testicular sperm, without pregnancy), and one case had HP (two TESE-C cycles, without pregnancy). These three patients with sperm retrieved have normal karyotypes and an isolated microdeletion of DDX3Y (DBY) in AZFa associated with a P4/P4 deletion in AZFb (proximal border, between sY114/sY121 and sY1224/EIF1AY). Eight cases had microdeletions in AZFabc: 6 performed no treatment, 1 had SCOS (TESE negative), and 1 had MA (one cycle TESE, without fertilization). This patient has an abnormal karyotype, 46, X, del(Y)(q. 11.23) and presented isolated microdeletions of USP9Y (DFFRY) and DDX3Y (DBY) in AZFa, associated with a P4/distal P1 deletion (which means proximal border of AZFb and AZFc regions).
Regarding clinical treatments (Supplementary Tables 1 (37.8KB, pdf) and 2 (42.4KB, pdf) ), there were five cycles with fresh testicular sperm (four patients, with one having two cycles) and 10 cycles with cryopreserved sperm (two cycles: one patient from TESE-C; eight cycles: three patients from TESE), with 2 NB (with cryopreserved testicular sperm).
Embryological and clinical outcomes of patients with Y-chromosome microdeletions (TESE)
Newborn outcomes of patients with Y-chromosome microdeletions
Patients with AZFb microdeletions
Patients with AZFb microdeletions presented four cases with severe oligozoospermia and 22 cases with secretory azoospermia. There was only one case with a microdeletion in AZFb who presented secretory azoospermia and was diagnosed as SCOS (TESE negative). One case had AZFb + DAZ microdeletions and severe oligozoospermia (one cycle TESE, without pregnancy, followed by two cycles with cryopreserved sperm without pregnancy, and one cycle with ejaculated sperm without pregnancy). This patient had a normal karyotype and presented a P5/proximal P1 microdeletion.16 Two patients had partial AZFb + DAZ microdeletions and severe oligozoospermia: one case performed two cycles with ejaculated sperm, without fertilization, has a normal karyotype and a IR4/distal P2 microdeletion;16 and the other case performed one cycle TESE, with abortion of a singleton pregnancy, has a normal karyotype and a IR4/proximal P1 microdeletion.16 Of the 22 patients with AZFbc microdeletions, 1 had severe oligozoospermia (two cycles with ejaculated sperm, one cycle without pregnancy, and one cycle with abortion of a triplet pregnancy). This patient has a normal karyotype and a IR4/distal P1 microdeletion.14
Twenty-one cases presented secretory azoospermia. Of these, 10 performed no treatment, 9 were diagnosed as SCOS (one no treatment; eight TESE negative), 1 as MA (TESE negative), and 1 as HP (one cycle TESE, without pregnancy, followed by two cycles with cryopreserved testicular sperm also without pregnancy). This patient has a normal karyotype and a IR4/distal P1 microdeletion.14
Regarding clinical treatments (Supplementary Tables 1 (37.8KB, pdf) and 2 (42.4KB, pdf) ), there were three cycles with fresh testicular sperm (three patients), four cycles with cryopreserved sperm (two patients from TESE), and five cycles with ejaculated sperm (two patients; one patient from TESE), with no NB.
Patients with AZFc microdeletions
Patients with AZFc microdeletions presented 29 cases with severe oligozoospermia, 53 cases with secretory azoospermia, and three patients without this information. Of the cases with severe oligozoospermia, 12 had no treatment, 1 performed TESE (HP), and 16 patients had 24 ICSI cycles with ejaculated sperm (seven patients had eight more cycles). Of the cycles with ejaculated sperm, there were eight clinical pregnancies (four singletons, four twins), two abortions (twin pregnancy; singleton pregnancy), and 8 NB (three singletons; three twins: 5 NB as one case had a stillbirth).
Of these patients with severe oligozoospermia, one performed TESE (considered as HP) directly (one cycle with fresh testicular sperm and abortion of a singleton pregnancy, followed by one cycle with cryopreserved sperm without pregnancy) and another (considered as HP) performed two cycles with ejaculated sperm (no pregnancy), followed by two cycles with fresh testicular sperm (no pregnancy).
Of the cases with secretory azoospermia, there were 11 cases without treatment (two in HP cases), 17 cases with SCOS, 13 cases with MA, and 12 cases with HP.
Of the SCOS cases, sperm was recovered in seven patients. They went for 10 treatment cycles with fresh testicular sperm (two patients had three further cycles), with two clinical singleton pregnancies and 2 NB. There were three cycles with cryopreserved sperm (two patients from TESE cycles) without pregnancy.
Of the MA cases, sperm was recovered in 11 patients. Ten patients performed 17 treatment cycles with fresh testicular sperm (three patients had seven further cycles), with seven clinical singleton pregnancies, two abortions, and 5 NB. There were 12 cycles with cryopreserved sperm (one TESE-C cycle; 11 cycles from seven TESE patients), with one clinical singleton pregnancy that ended in abortion.
Sperm was recovered in all HP cases. Twelve patients had 16 treatment cycles with fresh testicular sperm (three patients had four further cycles), with six clinical pregnancies (four singletons; two twins), three abortions (singletons), and 5 NB. There were 14 treatment cycles with cryopreserved sperm (two patients from TESE-C with five cycles; nine cycles from five TESE patients), with four clinical pregnancies (three singletons; one twin) and 5 NB. In total, there were 32 (72.7%) cases of sperm retrieval after TESE in 44 patients.
Regarding clinical treatments (Supplementary Tables 1 (37.8KB, pdf) and 2 (42.4KB, pdf) ), there were 43 cycles with fresh testicular sperm (29 patients; 14 further cycles from eight patients), with 15 clinical pregnancies (13 singleton; 2 twins), 5 abortions (singleton pregnancies), and 12 NB (8 singletons, 2 twins); 29 cycles with cryopreserved sperm (three patients from TESE-C with 6 cycles; 14 patients from TESE cycles with 23 further cycles), with 5 clinical pregnancies (4 singleton; 1 twin), 1 abortion (singleton pregnancy), and 5 NB (3 singletons; 1 twin); and 24 cycles with ejaculated sperm (16 patients; 7 patients with 8 further cycles), with 8 clinical pregnancies (4 singletons; 4 twins), 2 abortions (1 singleton and 1 twin pregnancies), and 8 NB (3 singletons; 3 twins), which gives in total 25 NB (12 with fresh testicular sperm; 5 with cryopreserved testicular sperm; 8 with ejaculated sperm).
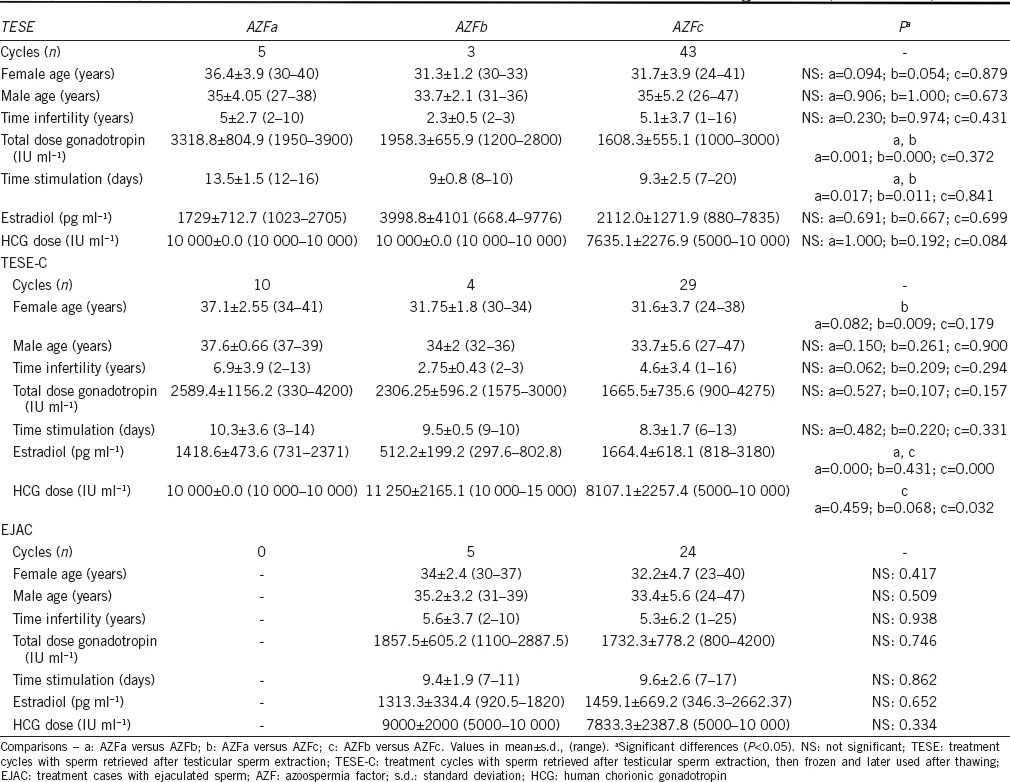
In relation to stimulation characteristics of TESE treatment cycles (Table 2), in AZFa, there was a significant higher total gonadotropin dose used and time of stimulation. For TESE-C treatment cycles, in AZFa, the mean female age was significant higher to AZFc and, in AZFb, the estradiol levels were significant lower and the HCG dose was significant higher to AZFc. For EJAC treatment cycles, there were no significant differences regarding all parameters analyzed.
Table 2.
Demographic and stimulation characteristics of patients with Y-chromosome microdeletions
In total, there were 123 treatment cycles, 112 embryo transfers, 31 CP (22 singleton; 8 twin; 1 triplet), and 27 NB (2 in AZFa; 25 in AZFc; 14 singleton; 7 twin; 12 males; 15 females). Treatments included 51 (41.5%) cycles with fresh testicular sperm, 43 (35.0%) cycles with frozen-thawed testicular sperm, and 29 (23.6%) cycles with ejaculated sperm (Supplementary Table 1 (37.8KB, pdf) ). In cycles with fresh testicular sperm, there were no significant differences between AZFa, AZFb, and AZFc microdeletions for all embryological and clinical parameters (Supplementary Table 1a (37.8KB, pdf) ). In AZFc, the mean gestation age was 38.2 years, with 1 case of very-preterm delivery and three cases of low birth weight (Supplementary Table 2 (42.4KB, pdf) ). In cycles with cryopreserved testicular sperm, comparisons showed that in AZFc, the mean number of cumulus-oocyte complexes retrieved was significant higher and that the fertilization rate was significant lower whereas in AZFb, the maturation rate and the embryo cleavage rate were significantly lower (Supplementary Table 1b (38.5KB, pdf) ). In AZFa, the 2 NB had a normal gestation age (37 weeks) and weight (2500 g; 3000 g). In AZFc, the mean gestation age was 36.5 years, with 1 case of preterm delivery and two cases of low birth weight (Supplementary Table 2 (42.4KB, pdf) ). In cycles with ejaculated sperm, there were no significant differences between AZFa, AZFb, and AZFc microdeletions for all embryological and clinical parameters (Supplementary Table 1c (37.4KB, pdf) ). In AZFc, the mean gestation age was 38.8 years, with 1 case of preterm delivery and three cases of low birth weight (Supplementary Table 2 (42.4KB, pdf) ).
Embryological and clinical outcomes of patients with Y-chromosome microdeletions (TESE-C)
Embryological and clinical outcomes of patients with Y-chromosome microdeletions: Ejaculated sperm
DISCUSSION
This study represents the third higher series of patients (n = 128) presenting Y-microdeletions 105,37 143,15 185,38 and 110.39 Fifty-eight patients performed ICSI, either using fresh or frozen-thawed testicular sperm retrieved by TESE (n = 40), and ejaculated sperm (n = 18). The originality of the present results resided in the comparisons performed between the different types of Y-microdeletions and treatments with detailed demographic, stimulation, embryological, clinical, and NB outcomes.
Regarding AZFa microdeletions
All 17 patients with AZFa microdeletions presented secretory azoospermia. Of these, 8 remained without testicular diagnosis (two in AZFa, six in AZFabc) as they refused treatment and one case had SCOS (diagnostic biopsy) but did not perform clinical treatment. Of the eight patients that performed TESE, five had retrieved sperm. There were 3 (37.5%) cases with SCOS (no sperm retrieved), 3 (37.5%) cases with MA (with sperm retrieved), and 2 (25%) cases with HP (with sperm retrieved).
The AZFa region was deleted at distinct subregions, 5 at AZFa, 4 at AZFb, and 8 at AZFabc. In AZFa, there were two cases without treatment, two cases with SCOS (no sperm retrieved), and one case with HP (1 tween pregnancy with 2 NB after 4 further cycles of ICSI with cryopreserved testicular sperm). In AZFab, there was one case with SCOS (no sperm retrieved), two cases with MA (no pregnancy), and one case with HP (no pregnancy). In AZFabc, there were six cases without treatment, one case with SCOS (no sperm retrieved) and one case with MA (no fertilization). Thus, in patients with AZFa microdeletions, sperm could be retrieved only in MA and HP cases, but only in those with HP a term pregnancy could be achieved.
All eight cases with AZFabc microdeletions had abnormal karyotypes. This included six cases without treatment, the case with SCOS and the case with MA. Considering our 125 patients with karyotypes, this gives a rate of 6.4%. Previous studies had found abnormal karyotypes at a rate of 1%–3% in AZFa and of 11%–22% in AZFabc.37,38,40,41 In our study, there was a lower rate in AZFabc. In addition, the absence of abnormal karyotypes in AZFa had already been noticed.37,40
Patients with AZFa microdeletions do not present sperm in their seminiferous tubules. Our results are in accordance with these findings, as sperm could be retrieved only in the presence of an isolated DDX3Y (DBY) microdeletion (the only case with NB; one case AZFa) and in cases with an isolated DDX3Y (DBY) microdeletion associated with a P4/P4 microdeletion (all without pregnancy; three cases AZFab). The case with an AZFabc microdeletion and retrieved sperm has an abnormal karyotype, 46, X, del(Y)(q. 11.23) and presented isolated microdeletions of USP9Y (DFFRY) and DDX3Y (DBY) in AZFa, associated with a P4/distal P1 deletion. The rare immature testicular sperm found could not fertilize the oocyte. Although several studies indicated that deletions of the AZFa genes USPP9Y (DFFRY) and DDX3Y (DBY) cause SCOS with total absence of sperm in the testis,5,13,42,43 other reports referred the retrieval of testicular sperm in patients with isolated deletions in one of these gene regions,13,44,45 and even when both genes were deleted.11,46
Regarding AZFb microdeletions
Of the 26 patients with AZFb microdeletions, 4 (15.4%) presented severe oligozoospermia and 22 (84.6%) secretory azoospermia. Of the cases with severe oligozoospermia, two patients performed ICSI cycles with ejaculated sperm and two performed TESE (diagnosed as HP at treatment). In one case with ejaculated sperm, there was a singleton pregnancy that ended in abortion, and in one case with HP there was a triplet pregnancy that ended in abortion. Of the secretory azoospermia cases, 11 did not performed treatment, of which 10 had no testicular phenotype and one had SCOS. Of the 11 cases that performed treatment, there were 9 (81.8%) cases with SCOS (no sperm retrieved), 1 (9.1%) case with MA (no sperm retrieved), and 1 (9.1%) case with HP (no pregnancy).
The AZFb region was deleted at distinct subregions, 1 at AZFb (secretory azoospermia), 1 at AZFb + DAZ (severe oligozoospermia), 2 at partial AZFb + DAZ (severe oligozoospermia), and 22 at AZFbc (1 with oligozoospermia, 21 with secretory azoospermia). In AZFb, there was one case of SCOS (no sperm retrieved). In AZFb + DAZ, there was one case with severe oligozoospermia but that performed TESE (no pregnancy). In partial AZFb + DAZ, there was one case with ICSI with ejaculated sperm (no pregnancy), and one case that performed TESE (abortion of a singleton pregnancy). In AZFbc, there was one case treated with ejaculated sperm (abortion of a triplet pregnancy), eight cases with SCOS (no sperm retrieved), one case with MA (no sperm retrieved), and one case with HP (no pregnancy). Thus, in patients with AZFb microdeletions, sperm could be retrieved only in cases with ejaculated sperm and HP, although no term pregnancy could be achieved.
Abnormal karyotypes were observed only in AZFbc (12 cases). These included six cases without treatment (from 10 cases), five cases with SCOS (from 10 cases), and one case MA. Considering our 125 patients with karyotypes, this gives a rate of 9.6%, which is similar to that previous reported of 9%–50% in AZFbc.37,38,40,41
Patients with AZFb microdeletions have no sperm in the seminiferous tubules. Our results are in accordance with these findings as sperm could be retrieved only in the presence of a P5/proximal P1 microdeletion (AZFb + DAZ), with no pregnancy,16 a IR4/distal P2 microdeletion (partial AZFb + DAZ) with ejaculated sperm and no fertilization,16 a IR4/proximal P1 microdeletion (partial AZFb + DAZ) with a singleton abortion,16 and IR4/distal P1 microdeletions (two cases AZFbc), with ejaculated sperm (triplet abortion) or with testicular sperm (no pregnancy).14 Although microdeletions in AZFb are considered to be associated with MA and absence of sperm retrieval,5,40,47,48 there are reported cases showing that there is the possibility of sperm retrieval in AZFb,14,16,41,49 AZFbc,16,39 and partial AZFb,38,50 due to different localization and extension of the deletion.
Regarding AZFc microdeletions
Of the 82 patients with AZFc microdeletions (three patients without diagnosis of severe oligozoospermia or secretory azoospermia), 29 (35.4%) had severe oligozoospermia and 53 (64.6%) secretory azoospermia. Of the cases with severe oligozoospermia, 12 did not perform treatment and had no testicular phenotype diagnosed, 16 had ICSI treatment cycles with ejaculated sperm (8 NB, 2 abortions), and 1 had HP and performed TESE (1 abortion). Of the cases with secretory azoospermia, 9 did not performed treatment and had no testicular phenotype diagnosed. Of the 44 cases with diagnosis, 17 (38.6%) had SCOS (10 with no sperm retrieved; 7 with sperm retrieved and 2 NB), 13 (29.5%) had MA (2 without sperm retrieved; 11 with sperm retrieved and 5 NB), and 14 (31.8%) had HP (10 NB). Our present results are thus in accordance with previous studies, which demonstrated that AZFc microdeletions do not prevent the possibility of sperm retrieval at TESE and that the phenotype may vary from SCOS to severe oligozoospermia.5,9,40,51,52
Abnormal karyotypes were found in five cases. These included two cases treated with ejaculated sperm (one case with 2 NB), one case with SCOS (no pregnancy), one case with MA (no pregnancy), and one case with HP (abortion of a singleton pregnancy). Considering our 125 patients with karyotypes, this gives a rate of 4%, which is similar to that previous reported of 5%–25% in AZFc.37,38,40,41
In relation to patient hormonal characteristics, comparisons between cases with and without sperm recovery by each type of microdeletion revealed no significant differences regarding age and hormone levels, with exception of higher FSH levels in patients without sperm recovered in AZFc. Previous studies, although with comparisons to controls, also showed no significant differences for these parameters.39,47,52,53,54,55
In relation to stimulation parameters, in TESE cycles, there were no significant differences regarding ages, time of infertility, estradiol levels, and HCG dose, with the total dose of gonadotropins and time of stimulation being significant higher in AZFa. In TESE-C cycles, the female age was significant higher in AZFa than in AZFc cases, the estradiol levels lower in AZFb, and the HCG dose lower in AZFc than in AZFb. For EJAC cycles, no significant differences were found. In the literature, we could not find data for comparisons to our present results.
In relation to clinical outcomes, several studies evaluated the impact of ICSI using sperm from patients with Y-chromosome microdeletions. In almost all of these previous studies, authors compared controls (patients without microdeletions) against patients with AZFc microdeletions. In those studies, authors did not find significant differences for all embryological, clinical and NB outcomes, with the exception of a significant lower FR.40,41,54,56
We did not compare our results to such type of controls but between the different types of microdeletions (AZFa, AZFb, and AZFc) and treatments (TESE, TESE-C, and EJAC) as this comparison has not been performed before. In relation to embryological and clinical outcomes, in TESE cycles, there were no significant differences for all parameters evaluated, including the mean number of oocytes recovered, and the rates of oocyte maturation, fertilization, embryo cleavage, high embryo grade, blastocyst, clinical and ongoing pregnancies, implantation, live-birth delivery, and NB. In relation to NB outcomes, there was one case of very-preterm delivery and three cases of low birth weight (AZFc). For TESE-C cycles, AZFc cases showed a significant higher mean number of oocytes retrieved, the maturation rate was lower in AZFb, the fertilization rate was lower in AZFc, and the embryo cleavage rate was lower in AZFb. There was one case of preterm birth delivery and two cases of low birth weight (AZFc). For EJAC cycles, there were no significant differences between groups, with one case of preterm delivery and three cases of low birth weight (AZFc). These satisfactory results were only for AZFc, as in AZFa there was only 1 CP (twin NB) and in AZFb there was only 2 CP that ended in abortion.
CONCLUSIONS
Comparisons between the different types of microdeletions (AZFa, AZFb, and AZFc) and treatments (TESE, TESE-C, and EJAC) revealed that with the exception of AZFc, cases with AZFa and AZFb microdeletions presented a poor clinical prognosis. Due to the reported heredity of microdeletions and the association with abnormal karyotypes, patients should be informed about the infertile consequences on the NB and the possibility of using preimplantation genetic diagnosis for female sex selection.
AUTHOR CONTRIBUTIONS
CG performed database construction, data processing, statistical analysis, critical discussion, and text draft; MC carried out IVF embryology and acquired data; ER performed statistical teaching and supervision; SF performed Y chromosome genetic analysis and critically reviewed the manuscript; JS supervised the IVF laboratory and acquired data; Luís Ferráz: male patient evaluation and testicular biopsy; CO evaluated female patients, carried out controlled ovarian hyperstimulation, embryo transfer and patient follow-up; AB recruited the patients, supervised the IVF laboratory, and critically reviewed the manuscript; MS carried out the study conception and design, data analysis, critical discussion, and manuscript writing.
COMPETING INTERESTS
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
This work was financed by the Institutions of the authors and in part by Multidisciplinary Unit for Biomedical Research-UMIB, ICBAS-UP (National Funds through FCT-Foundation for Science and Technology, under the Pest-OE/SAU/UI0215/2014). We also would like to acknowledge:
For oocyte retrieval to Jorge Beires, MD, PhD, Gynecologist, Dept. of Gynecology and Obstetrics, Director of the Unit of Gynecology and Reproductive Medicine, Hospital of S. John, E.P.E, Porto, Portugal) and José Manuel Teixeira da Silva, MD, Ginecologist
For anesthesiology to José Correia, MD, Anesthetist (Department of Anesthesiology, Hospital of S. John, E.P.E, Porto, Portugal)
For complementary Female patient evaluation, controlled ovarian hyperstimulation, embryo transfer, patient follow to José Teixeira da Silva, MD, Gynecologist, Subspecialization in Reproductive Medicine (CGRABarros)
For complementary IVF embryology and data acquisition to Paulo Viana BSc, Clinical Embryologist-ESHRE (GRAABarros)
For spermiology laboratorial work to Cláudia Osório, BSc, Ana Gonçalves, BSc, and Nuno Barros BSc (CGR-ABarros)
For cytogenetics support to Sofia Dória, PhD, Carolina Almeida, PhD, Ana Grangeia, PhD and Maria João Pinho, PhD (Department of Genetics, FMUP)
For Critical Review of the manuscript to Maria de Lourdes Pereira, PhD, Full Professor (Department of Biology, CICECO, University of Aveiro, Portugal).
Supplementary information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.WHO. WHO Laboratory Manual fo the Examination and Processing of Human Semen. 5th ed. Cambridge, UK: University Press; 2010. p. 272. [Google Scholar]
- 2.Sen S, Pasi AR, Dada R, Shamsi MB, Modi D. Y chromosome microdeletions in infertile men: prevalence, phenotypes and screening markers for the Indian population. J Assist Reprod Genet. 2013;30:413–22. doi: 10.1007/s10815-013-9933-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagvenkar P, Desai K, Hinduja I, Zaveri K. Chromosomal studies in infertile men with oligozoospermia and non-obstructive azoospermia. Indian J Med Res. 2005;122:34–42. [PubMed] [Google Scholar]
- 4.Mierla D, Jardan D, Stoian V. Chromosomal abnormality in men with impaired spermatogenesis. Int J Fertil Steril. 2014;8:35–42. [PMC free article] [PubMed] [Google Scholar]
- 5.Vogt PH, Edelmann A, Kirsch S, Henegariu O, Hirschmann P, et al. Human Y chromosome azoospermia factors (AZF) mapped to different subregions in Yq11. Hum Mol Genet. 1996;5:933–43. doi: 10.1093/hmg/5.7.933. [DOI] [PubMed] [Google Scholar]
- 6.Tiepolo L, Zuffardi O. Localization of factors controlling spermatogenesis in the nonfluorescent portion of the human Y chromosome long arm. Hum Genet. 1976;34:119–24. doi: 10.1007/BF00278879. [DOI] [PubMed] [Google Scholar]
- 7.Ma K, Inglis JD, Sharkey A, Bickmore WA, Hill RE, et al. A Y chromosome gene family with RNA-binding protein homology: candidates for the azoospermia factor AZF controlling human spermatogenesis. Cell. 1993;75:1287–95. doi: 10.1016/0092-8674(93)90616-x. [DOI] [PubMed] [Google Scholar]
- 8.Saxena R, de Vries JW, Repping S, Alagappan RK, Skaletsky H, et al. Four DAZ genes in two clusters found in the AZFc region of the human Y chromosome. Genomics. 2000;67:256–67. doi: 10.1006/geno.2000.6260. [DOI] [PubMed] [Google Scholar]
- 9.Fernandes S, Huellen K, Goncalves J, Dukal H, Zeisler J, et al. High frequency of DAZ1/DAZ2 gene deletions in patients with severe oligozoospermia. Mol Hum Reprod. 2002;8:286–98. doi: 10.1093/molehr/8.3.286. [DOI] [PubMed] [Google Scholar]
- 10.Fernandes AT, Fernandes S, Gonçalves R, Sá R, Costa P, et al. DAZ gene copies: evidence of Y chromosome evolution. Mol Hum Reprod. 2006;12:519–23. doi: 10.1093/molehr/gal051. [DOI] [PubMed] [Google Scholar]
- 11.Foresta C, Ferlin A, Moro E. Deletion and expression analysis of AZFa genes on the human Y chromosome revealed a major role for DBY in male infertility. Hum Mol Genet. 2000;9:1161–9. doi: 10.1093/hmg/9.8.1161. [DOI] [PubMed] [Google Scholar]
- 12.Sun C, Skaletsky H, Rozen S, Gromoll J, Nieschlag E, et al. Deletion of azoospermia factor a (AZFa) region of Y chromosome caused by recombination between HERV15 proviruses. Hum Mol Genet. 2000;9:2291–6. doi: 10.1093/oxfordjournals.hmg.a018920. [DOI] [PubMed] [Google Scholar]
- 13.Kamp C, Huellen K, Fernandes S, Sousa M, Schlegel PN, et al. High deletion frequency of the complete AZFa sequence in men with Sertoli-cell-only syndrome. Mol Hum Reprod. 2001;7:987–94. doi: 10.1093/molehr/7.10.987. [DOI] [PubMed] [Google Scholar]
- 14.Costa P, Gonçalves R, Ferrás C, Fernandes S, Fernandes AT, et al. Identification of new breakpoints in AZFb and AZFc. Mol Hum Reprod. 2088;14:251–8. doi: 10.1093/molehr/gan014. [DOI] [PubMed] [Google Scholar]
- 15.Kleiman SE, Almog R, Yogev L, Hauser R, Lehavi O, et al. Screening for partial AZFa microdeletions in the Y chromosome of infertile men: is it of clinical relevance? Fertil Steril. 2012;98:43–7. doi: 10.1016/j.fertnstert.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 16.Soares AR, Costa P, Silva J, Sousa M, Barros A, et al. AZFb microdeletions and oligozoospermia-which mechanisms? Fertil Steril. 2012;97:858–63. doi: 10.1016/j.fertnstert.2012.01.099. [DOI] [PubMed] [Google Scholar]
- 17.Foresta C, Moro E, Ferlin A. Prognostic value of Y deletion analysis. The role of current methods. Hum Reprod. 2001;16:1543–7. doi: 10.1093/humrep/16.8.1543. [DOI] [PubMed] [Google Scholar]
- 18.Sadeghi-Nejad H, Farrokhi F. Genetics of azoospermia: current knowledge, clinical implications, and future directions. Part II. Y chromosome microdeletions. Urol J. 2007;4:192–206. [PubMed] [Google Scholar]
- 19.Krausz C, Hoefsloot L, Simoni M, Tüttelmann F. EAA/EMQN best practice guidelines for molecular diagnosis of Y-chromosomal microdeletions state-of-art 2013. Andrology. 2014;2:5–19. doi: 10.1111/j.2047-2927.2013.00173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suganthi R, Vijesh VV, Vandana N, Banazir JA. Y chromosomal microdeletion screening in the workup of male infertility and its current status in India. Int J Fertil Steril. 2014;7:253–66. [PMC free article] [PubMed] [Google Scholar]
- 21.Rooney DE, Czepulkowski BH. Human Chromosome Preparation. In: Rickwood B, editor. Essential Techniques. UK: John Wiley & Sons Ltd; 1997. p. 154. [Google Scholar]
- 22.Simoni M, Bakker E, Krausz C. EAA/EMQN best practice guidelines for molecular diagnosis of Y-chromosomal microdeletions. State of the art 2004. Int J Androl. 2004;27:240–9. doi: 10.1111/j.1365-2605.2004.00495.x. [DOI] [PubMed] [Google Scholar]
- 23.Li PS, Li S, Schlegel PN, Goldstein M. External spermatic sheath injection for vasal nerve block. Urology. 1992;39:173–6. doi: 10.1016/0090-4295(92)90278-5. [DOI] [PubMed] [Google Scholar]
- 24.Gorgy A, Meniru GI, Naumann N, Beski S, Bates S, et al. The efficacy of local anesthesia for percutaneous epididymal sperm aspiration and testicular sperm aspiration. Hum Reprod. 1998;13:646–50. doi: 10.1093/humrep/13.3.646. [DOI] [PubMed] [Google Scholar]
- 25.Crabbé E, Verheyen G, Silber S, Tournaye H, Van de Velde H, et al. Enzymatic digestion of testicular tissue may rescue the intracytoplasmic sperm injection cycle in some patients with non-obstructive azoospermia. Hum Reprod. 1998;13:2791–6. doi: 10.1093/humrep/13.10.2791. [DOI] [PubMed] [Google Scholar]
- 26.Sousa M, Cremades N, Silva J, Oliveira C, Ferraz L, et al. Predictive value of testicular histology in secretory azoospermic subgroups and clinical outcome after microinjection of fresh and frozen-thawed sperm and spermatids. Hum Reprod. 2002;17:1800–10. doi: 10.1093/humrep/17.7.1800. [DOI] [PubMed] [Google Scholar]
- 27.Bujan L, Mieusset R, Mansat A, Moatti JP, Mondinat C, et al. Testicular size in infertile men: relationship to semen characteristics and hormonal blood levels. Br J Urol. 1989;64:632–7. doi: 10.1111/j.1464-410x.1989.tb05325.x. [DOI] [PubMed] [Google Scholar]
- 28.Huirne JA, Homburg R, Lambalk CB. Are GnRH antagonists comparable to agonists for use in IVF? Hum Reprod. 2007;22:2805–13. doi: 10.1093/humrep/dem270. [DOI] [PubMed] [Google Scholar]
- 29.Pinto F, Oliveira C, Cardoso MF, Teixeira da Silva J, Silva J, et al. Impact of GnRH ovarian stimulation protocols on intracytoplasmic sperm injection outcomes. Reprod Biol Endocrinol. 2009;7:1–10. doi: 10.1186/1477-7827-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tesarik J, Sousa M, Testart J. Human oocyte activation after intracytoplasmic sperm injection. Hum Reprod. 1994;9:511–8. doi: 10.1093/oxfordjournals.humrep.a138537. [DOI] [PubMed] [Google Scholar]
- 31.Tesarik J, Sousa M. Key elements of a highly efficient intracytoplasmic sperm injection technique: Ca2+ fluxes and oocyte cytoplasmic dislocation. Fertil Steril. 1995;64:770–6. doi: 10.1016/s0015-0282(16)57853-4. [DOI] [PubMed] [Google Scholar]
- 32.Vandervorst M, Liebaers I, Sermon K, Staessen C, De Vos A, et al. Successful preimplantation genetic diagnosis is related to the number of available cumulus-oocyte complexes. Hum Reprod. 1998;13:3169–76. doi: 10.1093/humrep/13.11.3169. [DOI] [PubMed] [Google Scholar]
- 33.Gardner DK, Lane M, Stevens J, Schlenker T, Scoolcraft WM. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155–8. doi: 10.1016/s0015-0282(00)00518-5. [DOI] [PubMed] [Google Scholar]
- 34.Hammer O, Harper DA, Ryan PD. PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron. 2001;4:1–9. [Google Scholar]
- 35.Zar JH. Biostatistical Analysis. 5th ed. USA: Pearson; 2009. p. 960. [Google Scholar]
- 36.StatSoft, Inc. Electronic Statistics Textbook. Tulsa, OK: StatSoft; 2013. [Last accessed on 2015 Aug 14]. Available from: http://www.statsoft.com/textbook/ [Google Scholar]
- 37.Kumtepe Y, Beyazyurek C, Cinar C, Ozbey I, Ozkan S, et al. A genetic survey of 1935 Turkish men with severe male factor infertility. Reprod Biomed Online. 2009;18:465–74. doi: 10.1016/s1472-6483(10)60121-6. [DOI] [PubMed] [Google Scholar]
- 38.Totonchi M, Meybodi AM, Boroujeni PB, Gilani MS, Almadani N, et al. Clinical data for 185 infertile Iranian men with Y-chromosome microdeletion. J Assist Reprod Genet. 2012;29:847–53. doi: 10.1007/s10815-012-9798-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi DK, Gong IH, Hwang JH, Oh JJ, Hong JY. Detection of Y chromosome microdeletion is valuable in the treatment of patients with nonobstructive azoospermia and oligoasthenoteratozoospermia: sperm retrieval rate and birth rate. Korean J Urol. 2013;54:111–6. doi: 10.4111/kju.2013.54.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patrat C, Bienvenu T, Janny L, Faure AK, Fauque P, et al. Clinical data and parenthood of 63 infertile and Y-microdeleted men. Fertil Steril. 2010;93:822–32. doi: 10.1016/j.fertnstert.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 41.Kim MJ, Choi HW, Park SY, Song IO, Seo JT, et al. Molecular and cytogenetic studies of 101 infertile men with microdeletions of Y chromosome in 1,306 infertile Korean men. J Assist Reprod Genet. 2012;29:539–46. doi: 10.1007/s10815-012-9748-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krausz C, Degl’Innocenti S, Nuti F, Morelli A, Felici F, et al. Natural transmission of USP9Y gene mutations: a new perspective on the role of AZFa genes in male fertility. Hum Mol Genet. 2006;15:2673–81. doi: 10.1093/hmg/ddl198. [DOI] [PubMed] [Google Scholar]
- 43.Hotaling J, Carrell DT. Clinical genetic testing for male factor infertility: current applications and future directions. Andrology. 2014;2:339–50. doi: 10.1111/j.2047-2927.2014.00200.x. [DOI] [PubMed] [Google Scholar]
- 44.Sargent CA, Boucher CA, Kirsch S, Brown G, Weiss B, et al. The critical region of overlap defining the AZFa male infertility interval of proximal Yq contains three transcribed sequences. J Med Genet. 1999;36:670–7. [PMC free article] [PubMed] [Google Scholar]
- 45.Luddi A, Margollicci M, Gambera L, Serafini F, Cioni M, et al. Spermatogenesis in a man with complete deletion of USP9Y. N Engl J Med. 2009;360:881–5. doi: 10.1056/NEJMoa0806218. [DOI] [PubMed] [Google Scholar]
- 46.Kleiman SE, Maymon BB, Yogev L, Paz G, Yavetz H. The prognostic role of the extent of Y microdeletion on spermatogenesis and maturity of Sertoli cells. Hum Reprod. 2001;16:399–402. doi: 10.1093/humrep/16.3.399. [DOI] [PubMed] [Google Scholar]
- 47.Martinez MC, Bernabe MJ, Gomez E, Ballesteros A, Landeras J, et al. Screening for AZF deletion in a large series of severely impaired spermatogenesis patients. J Androl. 2000;21:651–5. [PubMed] [Google Scholar]
- 48.Stouffs K, Lissens W, Tournaye H, van Steirteghem A, Liebaers I. The choice and outcome of the fertility treatment of 38 couples in whom the male partner has a Yq microdeletion. Hum Reprod. 2005;20:1887–96. doi: 10.1093/humrep/deh847. [DOI] [PubMed] [Google Scholar]
- 49.Ferrás C, Fernandes S, Marques CJ, Carvalho F, Alves C, et al. AZF and DAZ gene copy-specific deletion analysis in maturation arrest and Sertoli cell-only syndrome. Mol Hum Reprod. 2004;10:755–61. doi: 10.1093/molehr/gah104. [DOI] [PubMed] [Google Scholar]
- 50.Choi JM, Chung P, Veeck L, Mielnik A, Palermo GD, et al. AZF microdeletions of the Y chromosome and in vitro fertilization outcome. Fertil Steril. 2004;81:337–41. doi: 10.1016/j.fertnstert.2003.06.030. [DOI] [PubMed] [Google Scholar]
- 51.Calogero AE, Garofalo MR, Barone N, Palma AD, Vicari E, et al. Spontaneous regression over time of the germinal epithelium in a Y chromosome-microdeleted patient: case report. Hum Reprod. 2001;16:1845–8. doi: 10.1093/humrep/16.9.1845. [DOI] [PubMed] [Google Scholar]
- 52.Oates RD, Silber S, Brown LG, Page DC. Clinical characterization of 42 oligospermic or azoospermic men with microdeletion of the AZFc region of the Y chromosome, and of 18 children conceived via ICSI. Hum Reprod. 2002;17:2813–24. doi: 10.1093/humrep/17.11.2813. [DOI] [PubMed] [Google Scholar]
- 53.Peterlin B, Kunej T, Sinkovec J, Gligorievska N, Zorn B. Screening for Y chromosome microdeletions in 226 Slovenian subfertile men. Hum Reprod. 2002;17:17–24. doi: 10.1093/humrep/17.1.17. [DOI] [PubMed] [Google Scholar]
- 54.Liu X, Qiao J, Li R, Yan L, Chen L. Y chromosome AZFc microdeletion may not affect the outcomes of ICSI for infertile males with fresh ejaculated sperm. J Assist Reprod Genet. 2013;30:813–9. doi: 10.1007/s10815-013-0009-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park SH, Lee HS, Choe JH, Lee JS, Seo JT. Success rate of microsurgical multiple testicular sperm extraction and sperm presence in the ejaculate in Korean men with Y chromosome microdeletions. Korean J Urol. 2013;54:536–40. doi: 10.4111/kju.2013.54.8.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Golde RJ, Wetzels AM, de Graaf R, Tuerlings JH, Braat DD, et al. Decreased fertilization rate and embryo quality after ICSI in oligozoospermic men with microdeletions in the azoospermia factor c region of the Y chromosome. Hum Reprod. 2001;16:289–92. doi: 10.1093/humrep/16.2.289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Embryological and clinical outcomes of patients with Y-chromosome microdeletions (TESE)
Newborn outcomes of patients with Y-chromosome microdeletions
Embryological and clinical outcomes of patients with Y-chromosome microdeletions (TESE-C)
Embryological and clinical outcomes of patients with Y-chromosome microdeletions: Ejaculated sperm


