Abstract
The aim of this study was to evaluate the association between semen quality and the secondary sex ratio (SSR), defined as the ratio of male to female live births. Our study cohort comprised 227 male partners who were enrolled prior to conception in Michigan and Texas between 2005 and 2009, and prospectively followed through delivery of a singleton birth. The male partners provided a baseline and a follow-up semen sample a month apart. Semen analysis was conducted to assess 27 parameters including five general characteristics, six sperm head measures, 14 morphology measures, and two sperm chromatin stability assay measures. Modified Poisson regression models with a robust error variance were used to estimate the relative risk (RR) and 95% confidence interval (95% CI) of a male birth for each semen parameter, after adjusting for potential confounders. Of the 27 semen parameters, only the percentage of bicephalic sperm was significantly associated with the SSR (2nd vs 1st quartile, RR, 0.65, 95% CI, 0.45–0.95, P = 0.03; 4th vs 1st quartile, RR, 0.61, 95% CI, 0.38–1.00, P < 0.05 before rounding to two decimal places), suggestive of a higher percentage of bicephalic sperm being associated with an excess of female births. Given the exploratory design of the present study, this preconception cohort study suggests no clear signal that human semen quality is associated with offspring sex determination.
Keywords: fertility, prospective studies, reproduction, semen analysis, sex ratio, sperm
INTRODUCTION
Semen analysis is a key component in population-based studies of male reproductive health, providing information on the functional status of the male reproductive system including the testes and accessory sex glands.1 In clinical practice, semen analysis is one of the initial tests performed to evaluate male fecundity or the biologic capacity for reproduction,2 despite its inherent limitations in classifying men by fertility potential.3,4,5 With its fifth edition of the laboratory manual for the examination and processing of human semen, the World Health Organization (WHO) provided reference distributions of semen parameters derived from over 4500 fertile men in 14 countries with a retrospectively reported or prospectively measured time-to-pregnancy (TTP) of ≤12 months.6,7 In this edition of the WHO manual for semen analysis, the fifth centile values were newly proposed as the lower cutoff limits for normality, serving as the source of much controversy.1,8 The lower reference limits of sperm counts have been decreasing in recent decades, in parallel with longstanding debates on a global decline in human semen quality with equivocal findings and no emerging consensus.9,10,11,12,13 Along with other reported adverse trends in male reproductive health including increasing rates of testicular cancer and genitourinary malformations,14,15 the testicular dysgenesis syndrome has been proposed as a conceptual paradigm for assessing the effect of environmental and genetic influences on male fecundity.16
The secondary sex ratio (SSR) is the ratio of male to female singleton live births, whereas the primary sex ratio is the ratio of male to female conceptions.2 Given that there is no available biomarker for conceptions at the population level, the SSR has been used as a potential population health and fertility indicator.17,18 Motivation for measurement and analysis of the SSR has arisen from multiple hypotheses across social biology, environmental, medical and behavioral science, demography, and epidemiology.19 Despite debates on its meaningfulness, the SSR has been used as a simple and noninvasive way to monitor population health and fertility, with its strengths being easy to measure, frequently recorded, and rarely subject to recall bias.20 The expected range of the SSR is from 1.05 to 1.07 in the United States and worldwide, indicative of a slight excess of male births.21,22 Recently, a decline in the SSR or the proportion of male births has been reported in the United States, Canada, Japan, and some Northern and Western European countries.22,23,24 The SSR has reportedly been varied by endogenous and exogenous factors, such as parental ages,22,25,26 birth order,22,27 race/ethnicity,22,23 length of the follicular phase,28,29time of conception within the menstrual cycle,29,30 coital rate,29,30 endocrine and immunological effects,18,31 stress,32,33 smoking,34,35 anthropometric parameters,36,37 and other environmental factors.20 One of the prevailing hypotheses that may explain a possible decline in male reproductive health is exposure to endocrine-disrupting chemicals.16,38 Several studies on the association between endocrine-disrupting chemicals and the SSR have demonstrated possible roles of paternal rather than maternal exposure to select chemicals in offspring sex determination,20 underscoring the need for investigation into the effect of paternal factors including male fertility on the SSR.
Previous research focusing on the association between human semen quality and the SSR is sparse, especially for population-based cohorts. A study of 15 218 Danish men who sought infertility evaluation between 1963 and 1993 observed no significant association of the SSR with sperm concentration (0–20 × 106 ml−1 vs ≥20 × 106 ml−1), sperm motility (poor vs good), and the percentage of morphologically abnormal spermatozoa (75%–100% vs 0%–75%).39 A study of 46 men undergoing assisted reproductive technologies (ARTs) showed that men with male offspring had slower sperm curvilinear (mean ± standard error of the mean [s.e.m.], 44.2 ± 1.8 μm s−1 vs 49.9 ± 2.7 μm s−1) and average path velocities (mean ± s.e.m., 32.4 ± 1.2 μm s−1 vs 36.3 ± 1.7 μm s−1) in seminal plasma than did men with female offspring.40 A follow-up expanded study of 187 men corroborated earlier findings on curvilinear and average path velocities in seminal plasma and offspring sex.41 Although not directly tested for semen quality, the effect of male fertility on the SSR has been evaluated in relation to TTP,30,42,43 resulting in equivocal findings. The SSR has been also compared by fertility status or infertility etiology such as male or female factor infertility, without evidence supporting an association between infertility and the SSR.44,45 In other respects, given that the Y chromosome is the sex-determining chromosome in men, the sperm Y:X chromosome ratio has been assessed relative to semen quality to capture the paternal role in offspring sex determination. In an infertile cohort of 185 men undergoing a semen fluorescence in situ hybridization (FISH) test from 2003 to 2010 in the United States, poor semen quality, which was reflected by semen volume, sperm concentration, and total motile sperm count, significantly decreased the odds of having a Y chromosome-bearing sperm.46 With increasing speculation that semen quality may affect the SSR,32,39,40,44 the present study aimed to evaluate a spectrum of semen parameters quantified in men participating in a population-based prospective cohort in relation to the SSR.
MATERIALS AND METHODS
Study population
Our study cohort comprised male partners who participated in the Longitudinal Investigation of Fertility and the Environment (LIFE) Study,47 provided that they had a singleton birth during the follow-up period. The LIFE study recruited 501 couples discontinuing contraception and attempting pregnancy from 16 counties in Michigan and Texas between 2005 and 2009. Couples were followed until pregnant or through 12 months of trying to conceive and through delivery for those becoming pregnant. Of the 501 couples, 237 couples (47.3%) had a live birth during the follow-up period, including two with multiple births. Of the 235 male partners with a singleton birth, our analysis was restricted to 227 male partners (96.6%) who provided a baseline semen sample. The eligibility criteria for participation were as follows: (a) men in a committed relationship; (b) 18 years of age; (c) no sterilization procedures or physician-diagnosed infertility; and (d) men able to communicate in English or Spanish.
Data collection
Upon enrollment, a pregnancy test was administered to ensure the absence of a preexisting pregnancy. All study participants completed baseline interviews, which were conducted separately with each partner of the couple in the couples’ home. Research assistants obtained information on sociodemographic and lifestyle factors and medical and reproductive histories from all male partners, followed by the completion of standardized anthropometric assessments to ascertain height (in centimeters) and weight (in kilograms). Blood was collected for the quantification of serum cotinine using liquid chromatography-isotope dilution tandem mass spectrometry.48 Serum cotinine concentrations were reported in nanograms per milliliter and used to differentiate active smokers (≥40.35 ng ml−1) from non- or passive smokers (<40.35 ng ml−1).49 Couples who had a live birth during the follow-up period were asked to report information on date of birth, sex of the infant, birth size, and delivery mode after delivery. This study was performed in adherence with the guidelines of the Declaration of Helsinki and approved by the Institutional Review Boards at all collaborating institutions. All study participants provided written informed consent before any data collection.
Semen collection and analysis
Male partners were asked to provide a baseline and a follow-up semen sample approximately 1 month apart, irrespective of pregnancy status. Of the 227 male partners with a first semen sample, 200 male partners (88.1%) provided a second semen sample. Semen samples were collected at home via masturbation without the use of any lubricant after 2 days of suggested abstinence. A thermometer was attached to the glass collection jar for monitoring temperature in light of our reliance on next day analysis. In addition, men were instructed to cut one end of a specially prepared glass straw filled with hyaluronic acid (VitroTubes™ #3520; VitroCom, Mountain Lakes, NJ, USA) and place it into the semen to assess a global marker of motility. This step was taken in light of at home collection, a method for population-based research. The duration of abstinence, time and date of collection, and other information regarding collection including any loss or spillage were recorded on labels. Semen samples were returned using insulated shipping containers (Hamilton Thorne Biosciences, Beverly, MA, USA) and freezer packs to foster the preservation of sperm integrity.50 Semen samples were shipped overnight to the study's andrology laboratory for semen analysis.
Next day semen analysis was conducted using established laboratory protocols inclusive of an ongoing quality assurance and quality control procedures. The distance traveled by the vanguard sperm (millimeters) in the sperm migration straw was measured with a microscope upon removal from the jar. The IVOS system (Hamilton Thorne Biosciences, Beverly, MA, USA) with the IDENT stain was used to determine sperm concentration.51 Semen smears were prepared for the evaluation of sperm morphometry and morphology. Sperm morphometric analysis was conducted using the IVOS METRIX system, and sperm morphology was assessed using both the traditional criteria with differential classification and the strict criteria.6,52,53 Sperm viability was assessed by the hypo-osmotic swelling (HOS) test.54 For the sperm chromatin stability assay (SCSA) analysis, an aliquot of semen was diluted in sodium chloride-Tris-EDTA buffer with glycerol and kept frozen at −70°C until analysis.55 The analysis was conducted by SCSA Diagnostics (Brookings, SD, USA) using a Coulter Epics Elite Flow Cytometer. We assessed a total of 27 parameters, including five general characteristics, six sperm head measures, 14 morphology measures, and two SCSA measures. Although eight computer-aided sperm analysis (CASA) motility parameters (i.e., average path velocity, straight line velocity, curvilinear velocity, amplitude head displacement, beat cross frequency, straightness, linearity, and percent motility) were assessed using the HTM-IVOS CASA system, these parameters were not included in the current analysis, given that 24-h semen quality analysis is not ideal for time-sensitive endpoints. Analysis of the second sample was restricted to semen volume, sperm concentration, total sperm count, hypo-osmotic swelling, and sperm head measures to affirm any azoospermia in the first sample.
Statistical analysis
In the descriptive phase of analysis, distributions were summarized as means ± standard deviations for continuous variables and categorically for other variables. Differences in baseline characteristics of male partners by infant sex were assessed using the nonparametric Wilcoxon test for continuous variables and Fisher's exact test for categorical variables. We calculated means, standard deviations, and the 5th and 95th percentiles for semen parameters by infant sex and assessed any significant differences in semen parameters using the nonparametric Wilcoxon test. Differences in semen parameters between the first and second semen samples were also evaluated using the nonparametric Wilcoxon test. Proportion tests were used to assess differences in the SSRs by semen parameters (≤ median vs > median; the median values were calculated using the first semen samples).
In the analytic phase, modified Poisson regression models with a robust error variance were used to estimate the relative risk (RR) and 95% confidence interval (95% CI) of a male birth for each semen parameter.56 Fixed and mixed effects models were used for the analysis of semen parameters with one and two measurements, respectively. We modeled each semen parameter both as a continuous variable and as a categorical variable. Specifically, continuous values were rescaled by dividing the original values by 103 (sperm concentration and total sperm count) or 10 (the remaining 25 endpoints) to aid in the interpretation of results in terms of clinical significance, allowing for the estimation of risk per 103 or 10 unit increase in semen parameters. Additionally, each semen parameter was categorized into quartiles for analysis. Separate models were run for each semen parameter, consistent with our aim to fully explore the multiple facets of semen quality relative to infant sex. We adjusted a priori for factors thought to be related to semen quality and the SSR, consistent with the definition of confounding, based upon our review of the literature: paternal age (years), body mass index (BMI, kg m−2), and smoking (non- or passive smoker/active smoker). Significance was initially set at P < 0.05, given the exploratory design of this study. In addition, considering the number of semen parameters examined (n = 27), we subsequently assessed the significance at the 0.002 (approximately equal to 0.05/27) level. All statistical analyses were performed by the SAS Version 9.3 (SAS Institute Inc., Cary, NC, USA).
RESULTS
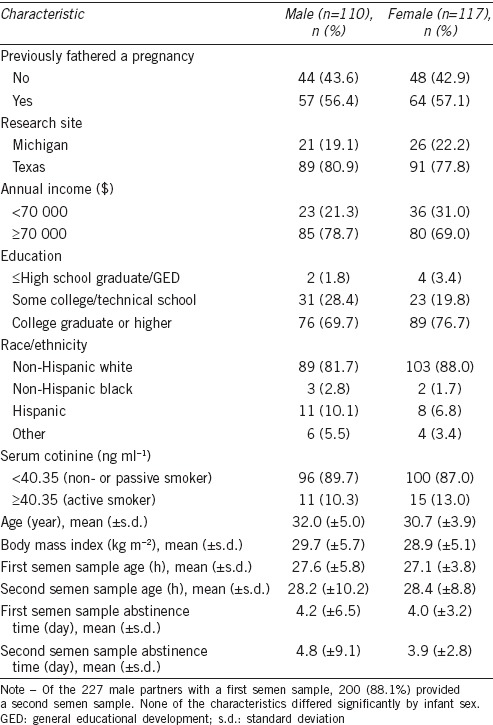
Of the 227 singleton live births, 110 (48.5%) were males and 117 (51.5%) were females. The overall SSR was 0.94, indicative of a female excess. The mean (±s.d.) age of the male partners was 31.3 (±4.5) years. More than half of the male partners had previously fathered a pregnancy upon enrollment. Non-Hispanic white and college-educated male partners comprised the majority of the study participants. Active smokers comprised 11.7% of the study participants. The mean (±s.d.) BMI of the male partners was 29.3 (±5.4) kg m−2. None of the baseline characteristics differed significantly by infant sex (Table 1).
Table 1.
Baseline characteristics of male partners with a singleton birth by infant sex (2005–2009)
The distributions of semen parameters for the male partners by infant sex are presented in Table 2. Of note, there were no statistically significant differences in semen parameters between the first and the second semen samples. In general, the distributions of gross semen parameters such as volume, sperm concentration, total sperm count, and % normal sperm morphology (the strict criteria) were comparable to normative values reported by WHO.6 The sperm migration distance measured using the first semen samples was significantly different between fathers of male infants (9.8 ± 6.8 mm) and fathers of female infants (11.6 ± 6.7 mm; P < 0.05 before rounding to two decimal places). For the second semen samples, a lower percentage of hypo-osmotic swelling (i.e., viability) was observed for fathers of female infants (65.3% ± 9.6%) versus male infants (68.2% ± 10.1%; P = 0.02). No other semen parameters differed significantly by infant sex (Table 2). The differences in the SSRs by semen parameters are presented in Table 3. Statistically significant differences in the SSRs were observed for select dichotomous semen parameters, such as straw distance (≤ median, 0.57; > median, 0.37; P = 0.01), sperm head length (≤ median, 0.46; > median, 0.56; P < 0.05 before rounding to two decimal places), and sperm head perimeter (≤ median, 0.44; > median, 0.55; P = 0.03) (Table 3).
Table 2.
Distributions of semen parameters by infant sex, 2005–2009 (n=227)
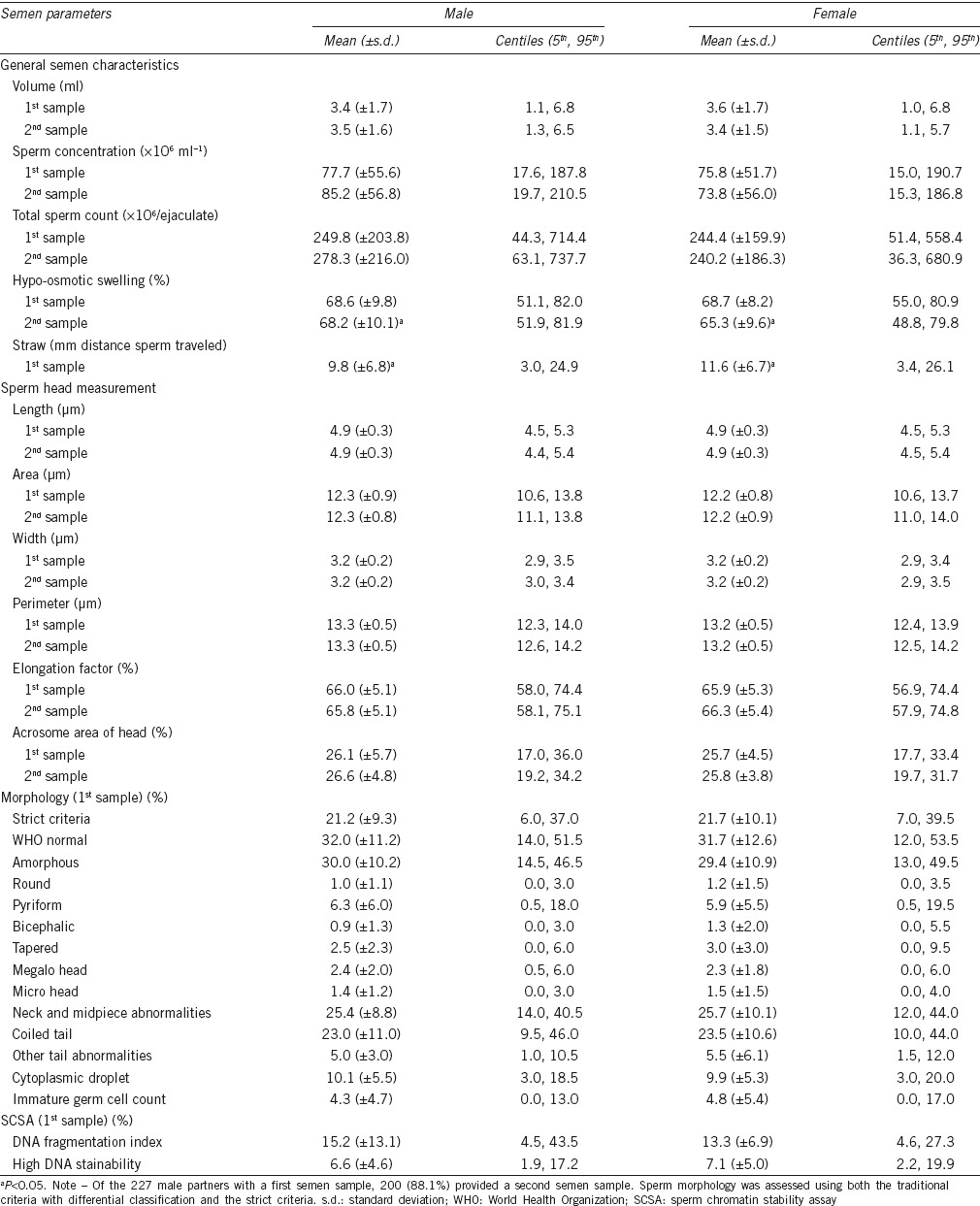
Table 3.
Differences in the SSRs by semen parameters, 2005–2009 (n=227)
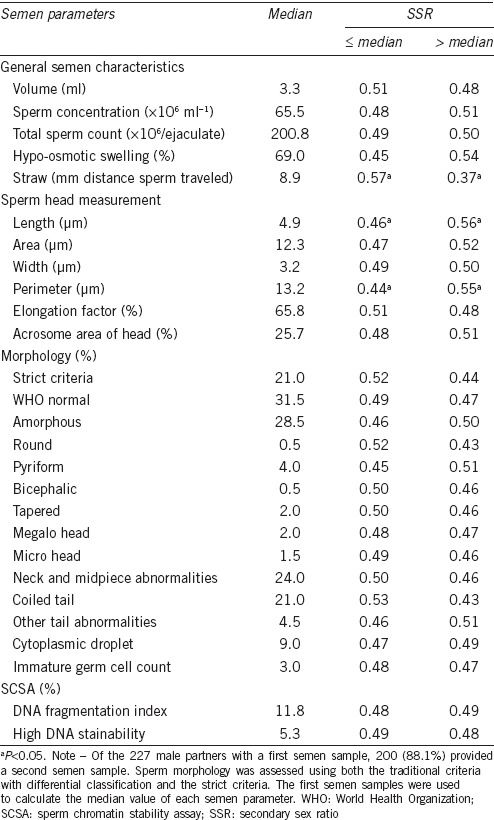
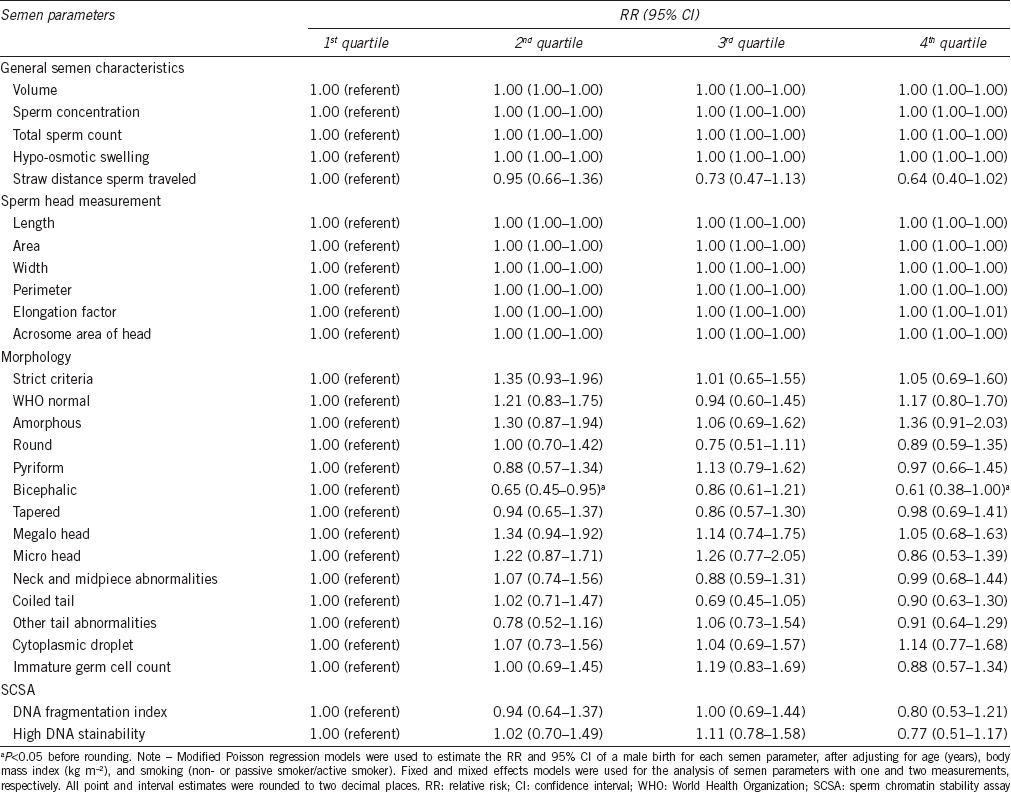
Table 4 presents the RRs of a male birth for each semen parameter. When analyzing semen parameters continuously, only DNA fragmentation index (DFI) was significantly associated with the SSR. An excess of male births was observed with a higher DFI in the unadjusted models (unadjusted RR, 1.08; 95% CI, 1.00–1.16; P < 0.05 before rounding to two decimal places), though not in the adjusted model. The adjusted RRs of a male birth for categorized semen parameters are presented in Table 5. When analyzing semen parameters categorically, only the percentage of bicephalic sperm was significantly associated with the SSR (2nd vs 1st quartile, adjusted RR, 0.65, 95% CI, 0.45–0.95, P = 0.03; 4th vs 1st quartile, adjusted RR, 0.61, 95% CI, 0.38–1.00, P < 0.05 before rounding to two decimal places), suggestive of a higher percentage of bicephalic sperm being associated with an excess of female births. None of the other semen parameters were significantly associated with an excess of male or female births. When the significance was assessed at the 0.002 level, the association observed for the percentage of bicephalic sperm no longer remained significant.
Table 4.
Semen parameters and the relative risks of a male birth, 2005–2009 (n=227)
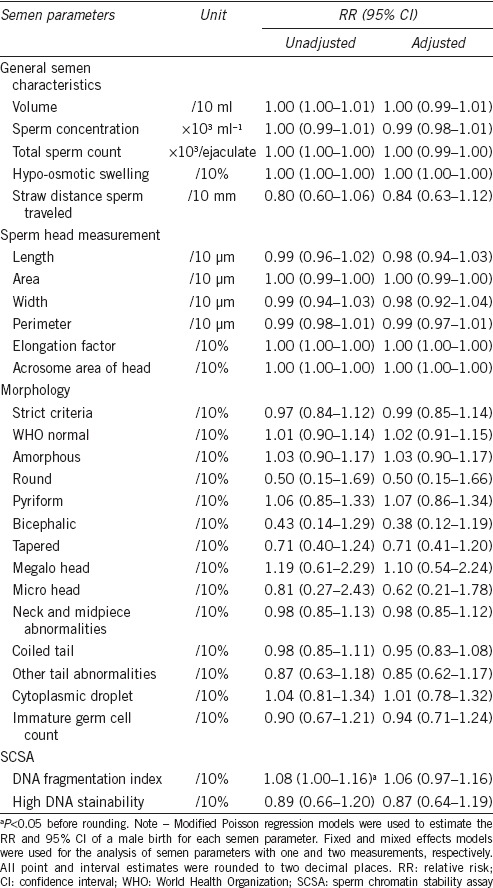
Table 5.
Categorized semen parameters and the adjusted relative risks of a male birth, 2005–2009 (n=227)
DISCUSSION
In our comprehensive analysis of preconception semen quality parameters and the SSR, we found no clear signal that human semen quality is associated with the SSR. In fact, our exploratory analysis identified only one semen parameter being associated with alterations in the SSR, when analyzing semen parameters categorically and controlling for potential confounders. Specifically, a higher percentage of bicephalic sperm was associated with a decreased SSR (i.e., an excess of female births). This study is the first known to us to report such an association between the percentage of bicephalic sperm and the SSR. However, when we adjusted for multiple comparisons, the association observed for the percentage of bicephalic sperm no longer remained significant, possibly reflecting a weak or null association of this parameter with the SSR. Furthermore, discrepancy in the significant findings across different statistical methods or categorizations may imply an uncertain association between semen quality and the SSR. Provided that there was no evidence for a nonlinear association between semen parameters and the risk of a male birth (data not shown), we did not use any nonlinear approaches to estimate the risk of a male relative to female birth. Given the lack of prior research findings, the association between a specific sperm morphology parameter and the SSR noted in the present study needs to be corroborated through further investigation.
It has been hypothesized that human semen quality plays a role in sex selection. For instance, men exposed to acute stress resulting from the Kobe earthquake in 1995 were reported to have a decrease in sperm motility and, subsequently, diminished fertility and SSR.32,57 The association between sperm motility and the SSR has been explored in other studies including a study of 46 men undergoing ART.40 Men with male offspring had slower sperm in seminal plasma, in terms of curvilinear and average path velocities, in comparison with men with female offspring. These findings on curvilinear and average path velocities in seminal plasma and offspring sex were confirmed in an expanded study of 187 men.41 In contrast, although not directly tested for the SSR, in a study of 500 men attending an andrology laboratory in the United Kingdom evaluating sibling sex composition relative to CASA sperm motility measures, men with female-biased siblings had significantly slower sperm than men from male-biased siblings.58 Meanwhile, a Danish study of 15 218 men seeking infertility evaluation reported no evidence for an association between sperm motility and the SSR.39 With regard to sperm morphology, this Danish study also found no evidence for an association between % morphologically abnormal spermatozoa (the traditional criteria) and the SSR, without providing any results on differential classification.39
As derived from the most extensive evaluation of semen quality parameters in relation to the SSR to date, comparison of our findings with prior research focusing on the association between semen quality and the SSR requires caution, in that much of the past research has relied on men seeking infertility evaluation or treatment, and whose semen quality may differ from fertile men.39,40,41 A Danish study assessed only three semen parameters (sperm concentration, motility, and % morphologically abnormal spermatozoa) that were dichotomized for analysis with the SSR, and observed no associations.39 Also of note is that the results were generated from single semen analysis that had been conducted before or after having one or more children, preventing the authors from being able to speak to semen quality around time of conception.39 In studies evaluating sperm motility relative to SSR, sperm in seminal plasma rather than ejaculated sperm were assessed in men undergoing ART.40,41 Prior research on the association between semen quality and sperm Y:X chromosome ratio may, in part, explain the effect of semen quality on the SSR, given that the SSR is affected by not only sperm Y:X chromosome ratio but also other factors, such as sperm selection within the female reproductive tract and differential implantation and survival rates of embryos.46,59,60
In spite of research efforts to date, the effect of male fertility including semen quality on the SSR remains elusive. There has been conflicting evidence for an association between TTP and the SSR from large European and Australian datasets.30,42,43 In a retrospective cohort of 30 448 women who sought infertility evaluation or treatment in California between 1990 and 1998, no significant difference was found for the SSR in comparison with matched fertile women derived from vital statistics records, irrespective of infertility etiology (male or female factor infertility).44 In a study of 15 164 singleton births from the Society for Assisted Reproductive Technology Outcomes Reporting System (SART CORS) database for 2005 in the United States, a diagnosis of male factor infertility was not associated with an altered SSR,45 although the reliability of the male factor infertility diagnosis in SART CORS is uncertain.61 Some previous studies have suggested that siring male offspring may be linked to male reproductive potential. A study of 185 American men undergoing a semen FISH test showed that there was a positive relationship between the production of Y chromosome-bearing sperm and sperm production, as an indicator of male fertility.46 In a study of 14 red deer stags, a positive relationship between the percentage of morphologically normal spermatozoa and the proportion of male offspring was observed.62 However, our study did not clearly demonstrate such a link between semen parameters and male reproductive potential.
One might also postulate that sperm motility or morphology may serve as a possible predictor of infant sex, in accordance with an existing theory focusing on the difference in sperm swimming velocity or size between Y chromosome-bearing sperm and X chromosome-bearing sperm as a potential influence of human sex selection.63 Specifically, the “over-ripeness ovopathy” concept hypothesizes that Y chromosome-bearing sperm are more suitable for navigating nonoptimally liquefied cervical mucus accompanied by nonoptimally matured oocytes than are X chromosome-bearing sperm, as Y chromosome-bearing sperm are smaller than X chromosome-bearing sperm in terms of the length, perimeter, and area of sperm head, and the length of sperm neck and tail.64 As a consequence, the preferential fertilization of nonoptimally matured oocytes by Y chromosome-bearing sperm may contribute to disproportional loss of male embryos and fetuses, resulting in alterations in the SSR. Intrinsic differences in sperm motility, viability, and fertility potential between Y- and X-chromosome bearing sperm have been suggested to alter the SSR on the paternal side,65,66 whereas the condition of the reproductive tract, including sex-specific transcriptomic responses of the oviduct, and the penetrability of the oocyte's zona pellucida have been suggested as sex-biasing mechanisms controlled by the mother.65,67 Still, biological mechanisms underlying the impact of a specific sperm morphology parameter on infant sex observed in our study remain unclear.
Our study is strengthened by several unique features, including the most extensive evaluation of semen parameters in relation to the SSR as well as the preconception measurement of semen quality. We also used data from both the first and second semen samples for analysis, while addressing biologic variability of semen parameters and correlations between the two semen samples. However, caution should be exercised when interpreting our results. In our study, 24-h semen quality analysis was used, which is not a conventional clinical or diagnostic evaluation tool. As such, our findings are not directly comparable to those derived from the clinical gold standard. Due to our inability to measure conceptions, we could not evaluate the primary sex ratio and possible disproportional male losses following conception to birth.2 Given the uncertainty as to factors affecting the SSR, we cannot eliminate residual confounding or model misclassification in the interpretation of our results. In light of our sampling on couples planning pregnancies, our findings may not be generalizable to the general population or among couples with unplanned pregnancy. Finally, given the likely small changes in the SSR that have been reported in the literature, it is conceivable that the present study is underpowered to detect all associations between semen quality and the SSR. For this reason, we refrain from in-depth discussion about the observed nonsignificant associations.
CONCLUSION
In a population-based preconception cohort, we found no clear signal that semen quality was associated with sex selection as measured by the SSR. With the need to identify novel surrogate markers of male reproductive health by applying new technologies,4,68 emphasis should be placed on determining possible interrelatedness of potential biomarkers of male fertility and offspring sex determination, such as hormonal profiles, semen quality, sperm Y:X chromosome ratio, and genetic and epigenetic sperm abnormalities.69,70 More comprehensive investigation incorporating these factors may help elucidate the underlying biological mechanisms of male fertility and human sex selection.
AUTHOR CONTRIBUTIONS
GMBL supervised cohort recruitment and data collection. All authors designed the study. SK and ZC analyzed the data. All authors interpreted the data. JB and SK drafted the manuscript. ZC, MLE and GMBL provided critical revisions to the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This study was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (contracts #N01-HD-3-3355, N01-HD-3-3356, N01-HD-3-3358). JB was supported by the Korea-US Visiting Scientist Training Award (VSTA) of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (award #VFTB057303). The authors acknowledge the Reproductive Health Assessment Team, Biomonitoring and Health Assessment Branch, National Institute for Occupational Safety and Health, for the analysis of semen samples.
REFERENCES
- 1.Esteves SC, Zini A, Aziz N, Alvarez JG, Sabanegh ES, Jr, et al. Critical appraisal of World Health Organization's new reference values for human semen characteristics and effect on diagnosis and treatment of subfertile men. Urology. 2012;79:16–22. doi: 10.1016/j.urology.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Buck Louis GM, Platt RW. Reproductive and Perinatal Epidemiology. New York: Oxford University Press; 2011. [Google Scholar]
- 3.Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, et al. Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med. 2001;345:1388–93. doi: 10.1056/NEJMoa003005. [DOI] [PubMed] [Google Scholar]
- 4.Kovac JR, Pastuszak AW, Lamb DJ. The use of genomics, proteomics, and metabolomics in identifying biomarkers of male infertility. Fertil Steril. 2013;99:998–1007. doi: 10.1016/j.fertnstert.2013.01.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Practice Committee of the American Society of Reproductive Medicine. The clinical utility of sperm DNA integrity testing: a guideline. Fertil Steril. 2013;99:673–7. doi: 10.1016/j.fertnstert.2012.12.049. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva: World Health Organization Press; 2010. [Google Scholar]
- 7.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–45. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 8.Yerram N, Sandlow JI, Brannigan RE. Clinical implications of the new 2010 WHO reference ranges for human semen characteristics. J Androl. 2012;33:289–90. doi: 10.2164/jandrol.111.014472. [DOI] [PubMed] [Google Scholar]
- 9.Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305:609–13. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisch H. Declining worldwide sperm counts: disproving a myth. Urol Clin North Am. 2008;35:137–46. doi: 10.1016/j.ucl.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Rolland M, Le Moal J, Wagner V, Royère D, De Mouzon J. Decline in semen concentration and morphology in a sample of 26,609 men close to general population between 1989 and 2005 in France. Hum Reprod. 2013;28:462–70. doi: 10.1093/humrep/des415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swan SH, Elkin EP, Fenster L. The question of declining sperm density revisited: an analysis of 101 studies published 1934-1996. Environ Health Perspect. 2000;108:961–6. doi: 10.1289/ehp.00108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swan SH, Elkin EP, Fenster L. Have sperm densities declined? A reanalysis of global trend data. Environ Health Perspect. 1997;105:1228–32. doi: 10.1289/ehp.971051228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paulozzi LJ. International trends in rates of hypospadias and cryptorchidism. Environ Health Perspect. 1999;107:297–302. doi: 10.1289/ehp.99107297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F. International testicular cancer incidence trends: generational transitions in 38 countries 1900-1990. Cancer Causes Control. 2015;26:151–8. doi: 10.1007/s10552-014-0486-z. [DOI] [PubMed] [Google Scholar]
- 16.Skakkebaek NE, Rajpert-DeMeyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16:972–8. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- 17.Davis DL, Gottlieb MB, Stampnitzky JR. Reduced ratio of male to female births in several industrial countries: a sentinel health indicator? JAMA. 1998;279:1018–23. doi: 10.1001/jama.279.13.1018. [DOI] [PubMed] [Google Scholar]
- 18.James WH. Evidence that mammalian sex ratios at birth are partially controlled by parental hormone levels around the time of conception. J Endocrinol. 2008;198:3–15. doi: 10.1677/JOE-07-0446. [DOI] [PubMed] [Google Scholar]
- 19.McDonald E, Watterson A, Tyler AN, McArthur J, Scott EM. Multi-factorial influences on sex ratio: a spatio-temporal investigation of endocrine disruptor pollution and neighborhood stress. Int J Occup Environ Health. 2014;20:235–46. doi: 10.1179/2049396714Y.0000000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terrell ML, Hartnett KP, Marcus M. Can environmental or occupational hazards alter the sex ratio at birth. Emerg Health Threats J. 2011;4:7109. doi: 10.3402/ehtj.v4i0.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Central Intelligence Agency. The World Factbook. [Last accessed on 2015 Nov 23]. Available from: https://www.cia.gov/library/publications/resources/the-world-factbook/index.html .
- 22.Mathews TJ, Hamilton BE. Trend analysis of the sex ratio at birth in the United States. Natl Vital Stat Rep. 2005;53:1–17. [PubMed] [Google Scholar]
- 23.Davis DL, Webster P, Stainthorpe H, Chilton J, Jones L, et al. Declines in sex ratio at birth and fetal deaths in Japan, and in U.S. whites but not African Americans. Environ Health Perspect. 2007;115:941–6. doi: 10.1289/ehp.9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grech V, Vassallo-Agius P, Savona-Ventura C. Secular trends in sex ratios at birth in North America and Europe over the second half of the 20th century. J Epidemiol Community Health. 2003;57:612–5. doi: 10.1136/jech.57.8.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobsen R, Møller H, Mouritsen A. Natural variation in the human sex ratio. Hum Reprod. 1999;14:3120–5. doi: 10.1093/humrep/14.12.3120. [DOI] [PubMed] [Google Scholar]
- 26.Juntunen KS, Kvist AP, Kauppila AJ. A shift from a male to a female majority in newborns with the increasing age of grand grand multiparous women. Hum Reprod. 1997;12:2321–3. doi: 10.1093/humrep/12.10.2321. [DOI] [PubMed] [Google Scholar]
- 27.Biggar RJ, Wohlfahrt J, Westergaard T, Melbye M. Sex ratios, family size, and birth order. Am J Epidemiol. 1999;150:957–62. doi: 10.1093/oxfordjournals.aje.a010104. [DOI] [PubMed] [Google Scholar]
- 28.Weinberg CR, Baird DD, Wilcox AJ. The sex of the baby may be related to the length of the follicular phase in the conception cycle. Hum Reprod. 1995;10:304–7. doi: 10.1093/oxfordjournals.humrep.a135932. [DOI] [PubMed] [Google Scholar]
- 29.Martin JF. Length of the follicular phase, time of insemination, coital rate, and the sex of offspring. Hum Reprod. 1997;12:611–6. doi: 10.1093/humrep/12.3.611. [DOI] [PubMed] [Google Scholar]
- 30.James WH. The variations of human sex ratio at birth with time of conception within the cycle, coital rate around the time of conception, duration of time taken to achieve conception, and duration of gestation: a synthesis. J Theor Biol. 2008;255:199–204. doi: 10.1016/j.jtbi.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 31.Ober C. The maternal-fetal relationship in human pregnancy: an immunogenetic perspective. Exp Clin Immunogenet. 1992;9:1–14. [PubMed] [Google Scholar]
- 32.Fukuda M, Fukuda K, Shimizu T, Moller H. Decline in sex ratio at birth after Kobe earthquake. Hum Reprod. 1998;13:2321–2. doi: 10.1093/humrep/13.8.2321. [DOI] [PubMed] [Google Scholar]
- 33.Zorn B, Sucur V, Stare J, Meden-Vrtovec H. Decline in sex ratio at birth after 10-day war in Slovenia: brief communication. Hum Reprod. 2002;17:3173–7. doi: 10.1093/humrep/17.12.3173. [DOI] [PubMed] [Google Scholar]
- 34.Beratis NG, Asimacopoulou A, Varvarigou A. Association of secondary sex ratio with smoking and parity. Fertil Steril. 2008;89:662–7. doi: 10.1016/j.fertnstert.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 35.Fukuda M, Fukuda K, Shimizu T, Andersen CY, Byskov AG. Parental periconceptional smoking and male:female ratio of newborn infants. Lancet. 2002;359:1407–8. doi: 10.1016/S0140-6736(02)08362-9. [DOI] [PubMed] [Google Scholar]
- 36.Abu-Rmeileh NM, Watt G, Lean ME. Sex distribution of offspring-parents obesity: Angel's hypothesis revisited. Hum Biol. 2011;83:523–30. doi: 10.3378/027.083.0406. [DOI] [PubMed] [Google Scholar]
- 37.Cagnacci A, Renzi A, Arangino S, Alessandrini C, Volpe A. Influences of maternal weight on the secondary sex ratio of human offspring. Hum Reprod. 2004;19:442–4. doi: 10.1093/humrep/deh071. [DOI] [PubMed] [Google Scholar]
- 38.Bonde JP, Ramlau-Hansen CH, Olsen J. Trends in sperm counts: the saga continues. Epidemiology. 2011;22:617–9. doi: 10.1097/EDE.0b013e318223442c. [DOI] [PubMed] [Google Scholar]
- 39.Jacobsen R, Bostofte E, Skakkebaek NE, Hansen J, Moller H. Offspring sex ratio of subfertile men and men with abnormal sperm characteristics. Hum Reprod. 2000;15:2369–70. doi: 10.1093/humrep/15.11.2369. [DOI] [PubMed] [Google Scholar]
- 40.Balli KS, Patton WC, Jacobson JD, Corselli J, King A, et al. Sperm velocity in seminal plasma and the association with gender of offspring. Arch Androl. 2004;50:37–40. doi: 10.1080/01485010490250560. [DOI] [PubMed] [Google Scholar]
- 41.Maligaya ML, Chan CA, Jacobson JD, Patton WC, Corselli J, et al. A follow-up expanded study of the correlation of sperm velocity in seminal plasma and offspring gender. Arch Androl. 2006;52:39–44. doi: 10.1080/01485010500301982. [DOI] [PubMed] [Google Scholar]
- 42.Joffe M, Bennett J, Best N, Jensen TK. Sex ratio and time to pregnancy: analysis of four large European population surveys. BMJ. 2007;334:524. doi: 10.1136/bmj.39097.508426.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smits LJ, de Bie RA, Essed GG, van den Brandt PA. Time to pregnancy and sex of offspring: cohort study. BMJ. 2005;331:1437–8. doi: 10.1136/bmj.331.7530.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eisenberg ML, Schembri M, Croughan MS, Walsh TJ. Fecundity and sex ratio of offspring in an infertile cohort. Fertil Steril. 2011;96:833–6. doi: 10.1016/j.fertnstert.2011.07.1141. [DOI] [PubMed] [Google Scholar]
- 45.Luke B, Brown MB, Grainger DA, Baker VL, Ginsburg E, et al. The sex ratio of singleton offspring in assisted-conception pregnancies. Fertil Steril. 2009;92:1579–85. doi: 10.1016/j.fertnstert.2008.08.107. [DOI] [PubMed] [Google Scholar]
- 46.Eisenberg ML, Murthy L, Hwang K, Lamb DJ, Lipshultz LI. Sperm counts and sperm sex ratio in male infertility patients. Asian J Androl. 2012;14:683–6. doi: 10.1038/aja.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buck Louis GM, Schisterman EF, Sweeney AM, Wilcosky TC, Gore-Langton RE, et al. Designing prospective cohort studies for assessing reproductive and developmental toxicity during sensitive windows of human reproduction and development – The LIFE study. Paediatr Perinat Epidemiol. 2011;25:413–24. doi: 10.1111/j.1365-3016.2011.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bernert JT, Jr, Turner WE, Pirkle JL, Sosnoff CS, Akins JR, et al. Development and validation of sensitive method for determination of serum cotinine in smokers and nonsmokers by liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Clin Chem. 1997;43:2281–91. [PubMed] [Google Scholar]
- 49.Jeemon P, Agarwal S, Ramakrishnan L, Gupta R, Snehi U, et al. Validation of self-reported smoking status by measuring serum cotinine levels: an Indian perspective. Natl Med J India. 2010;23:134–6. [PubMed] [Google Scholar]
- 50.Royster MO, Lobdell DT, Mendola P, Perreault SD, Selevan SG, et al. Evaluation of a container for collection and shipment of semen with potential uses in population-based, clinical, and occupational settings. J Androl. 2000;21:478–84. [PubMed] [Google Scholar]
- 51.Zinaman MJ, Uhler ML, Vertuno E, Fisher SG, Clegg ED. Evaluation of computer-assisted semen analysis (CASA) with IDENT stain to determine sperm concentration. J Androl. 1996;17:288–92. [PubMed] [Google Scholar]
- 52.World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. 3rd ed. Cambridge: Cambridge University Press; 1992. [Google Scholar]
- 53.Rothmann SA, Bort AM, Quickley J, Pillow R. Sperm morphology classification: A rational method for schemes adopted by the World Health Organization. In: Carrell DT, Aston KI, editors. Spermatogenesis: Methods and Protocols. New York: Humana Press; 2013. [DOI] [PubMed] [Google Scholar]
- 54.Jeyendran RS, Van der Ven HH, Perez-Palaez M, Crabo BG, Zaneveld LJ. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J Reprod Fertil. 1984;70:219–28. doi: 10.1530/jrf.0.0700219. [DOI] [PubMed] [Google Scholar]
- 55.Evenson DP, Larson KL, Jost LK. Sperm chromatin structure assay: its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. J Androl. 2002;23:25–43. doi: 10.1002/j.1939-4640.2002.tb02599.x. [DOI] [PubMed] [Google Scholar]
- 56.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 57.Fukuda M, Fukuda K, Shimizu T, Yomura W, Shimizu S. Kobe earthquake and reduced sperm motility. Hum Reprod. 1996;11:1244–6. doi: 10.1093/oxfordjournals.humrep.a019365. [DOI] [PubMed] [Google Scholar]
- 58.Mossman JA, Slate J, Birkhead TR, Moore HD, Pacey AA. Sperm speed is associated with sex bias of siblings in a human population. Asian J Androl. 2013;15:152–4. doi: 10.1038/aja.2012.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Graffelman J, Fugger EF, Keyvanfar K, Schulman JD. Human live birth and sperm-sex ratios compared. Hum Reprod. 1999;14:2917–9. doi: 10.1093/humrep/14.11.2917. [DOI] [PubMed] [Google Scholar]
- 60.Irving R, Bittles A, Peverall J, Murch A, Matson P. The ratio of X- and Y-bearing sperm in ejaculates of men with three or more children of the same sex. J Assist Reprod Genet. 1999;16:492–4. doi: 10.1023/A:1020555101059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nangia AK, Luke B, Smith JF, Mak W, Stern JE, et al. National study of factors influencing assisted reproductive technology outcomes with male factor infertility. Fertil Steril. 2011;96:609–14. doi: 10.1016/j.fertnstert.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 62.Gomendio M, Malo AF, Soler AJ, Fernández-Santos MR, Esteso MC, et al. Male fertility and sex ratio at birth in red deer. Science. 2006;314:1445–7. doi: 10.1126/science.1133064. [DOI] [PubMed] [Google Scholar]
- 63.Jongbloet PH. Over-ripeness ovopathy: a challenging hypothesis for sex ratio modulation. Hum Reprod. 2004;19:769–74. doi: 10.1093/humrep/deh136. [DOI] [PubMed] [Google Scholar]
- 64.Cui KH. Size differences between human X and Y spermatozoa and prefertilization diagnosis. Mol Hum Reprod. 1997;3:61–7. doi: 10.1093/molehr/3.1.61. [DOI] [PubMed] [Google Scholar]
- 65.Almiñana C, Caballero I, Heath PR, Maleki-Dizaji S, Parrilla I, et al. The battle of the sexes starts in the oviduct: modulation of oviductal transcriptome by X and Y-bearing spermatozoa. BMC Genomics. 2014;15:293. doi: 10.1186/1471-2164-15-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shettles LB. Sperm morphology and sex ratios. J Urol. 1961;86:450–5. doi: 10.1016/S0022-5347(17)65197-8. [DOI] [PubMed] [Google Scholar]
- 67.Dominko T, First NL. Relationship between the maturational state of oocytes at the time of insemination and sex ratio of subsequent early bovine embryos. Theriogenology. 1997;47:1041–50. doi: 10.1016/s0093-691x(97)00061-7. [DOI] [PubMed] [Google Scholar]
- 68.Isobe T. New method to estimate the possibility of natural pregnancy using computer-assisted sperm analysis. Syst Biol Reprod Med. 2012;58:339–47. doi: 10.3109/19396368.2012.700759. [DOI] [PubMed] [Google Scholar]
- 69.Emery BR, Carrell DT. The effect of epigenetic sperm abnormalities on early embryogenesis. Asian J Androl. 2006;8:131–42. doi: 10.1111/j.1745-7262.2006.00127.x. [DOI] [PubMed] [Google Scholar]
- 70.James WH. Evolution and the variation of mammalian sex ratios at birth: reflections on Trivers and Willard (1973) J Theor Biol. 2013;334:141–8. doi: 10.1016/j.jtbi.2013.06.023. [DOI] [PubMed] [Google Scholar]


